Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases
Abstract
1. Introduction
2. Patients and methods
2.1. Patients
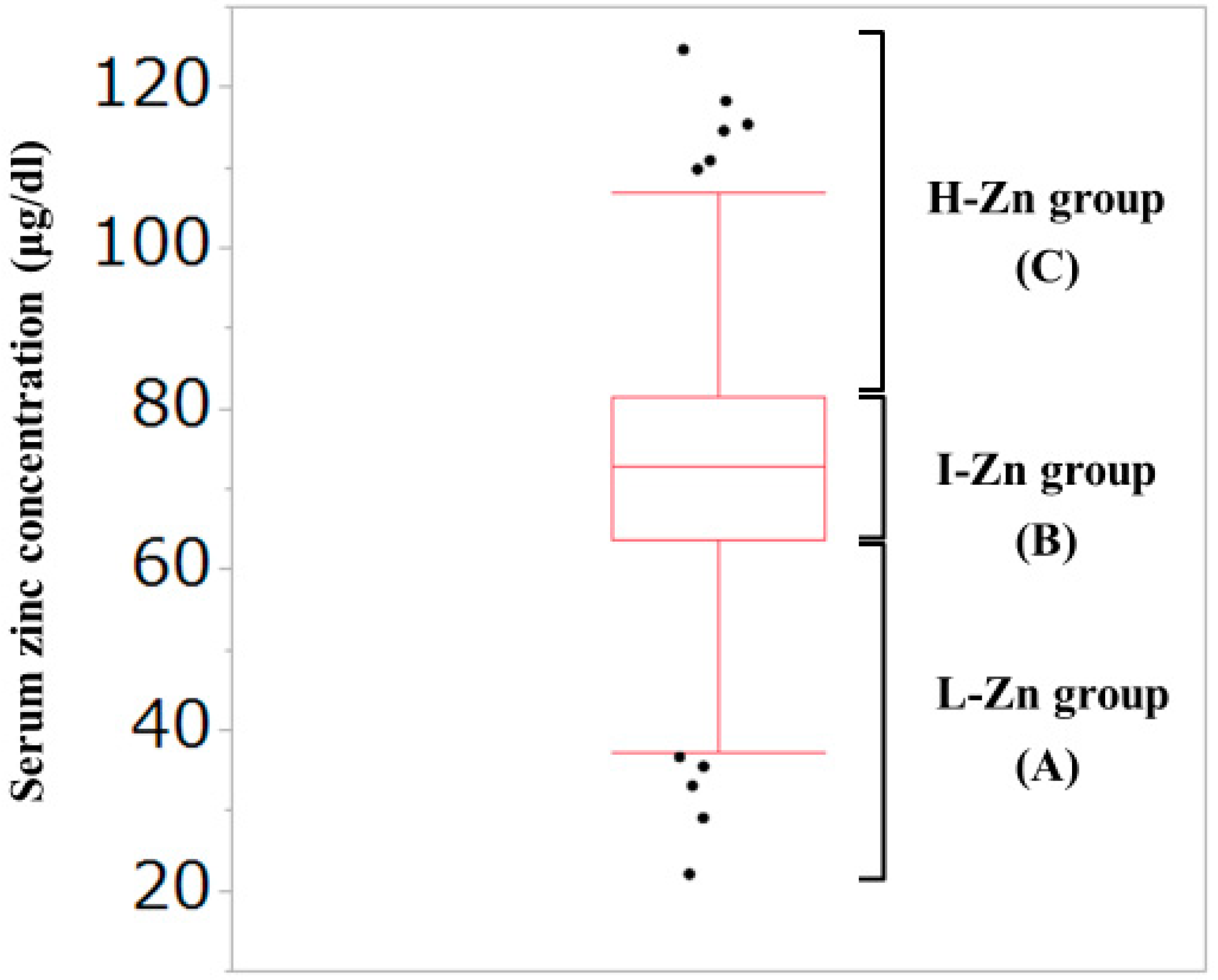
2.2. Our Classification Based on the Serum Zn Concentration
2.3. Measurement of GpS and SMI for Evaluating Sarcopenia
2.4. Statistical Considerations
3. Results
3.1. Patient Baseline Characteristics
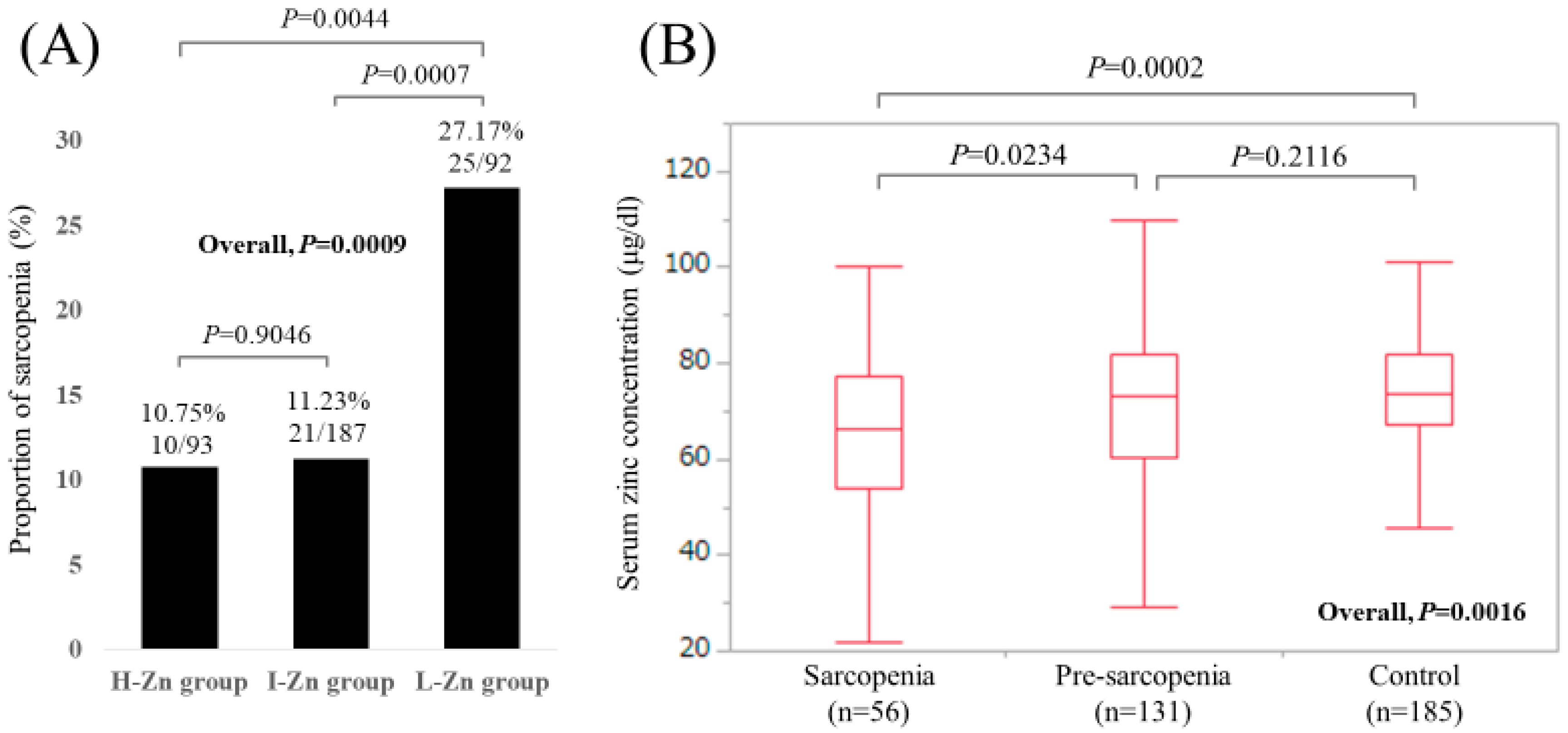
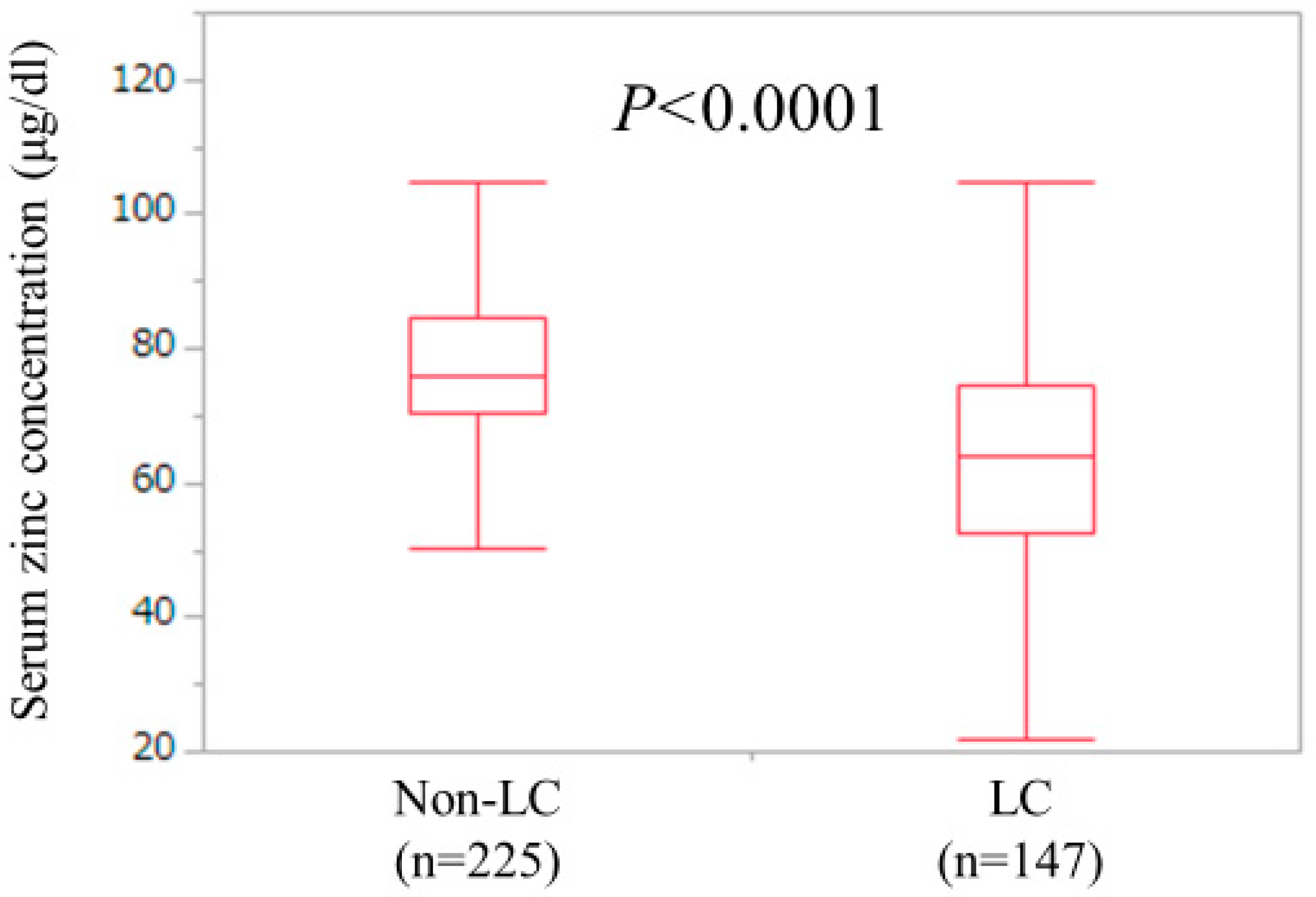
3.2. Analyses According to the LC Status
3.3. Uni- and Multivariate Analyses of Factors Associated with the Presence of Sarcopenia for All Cases (n = 372)
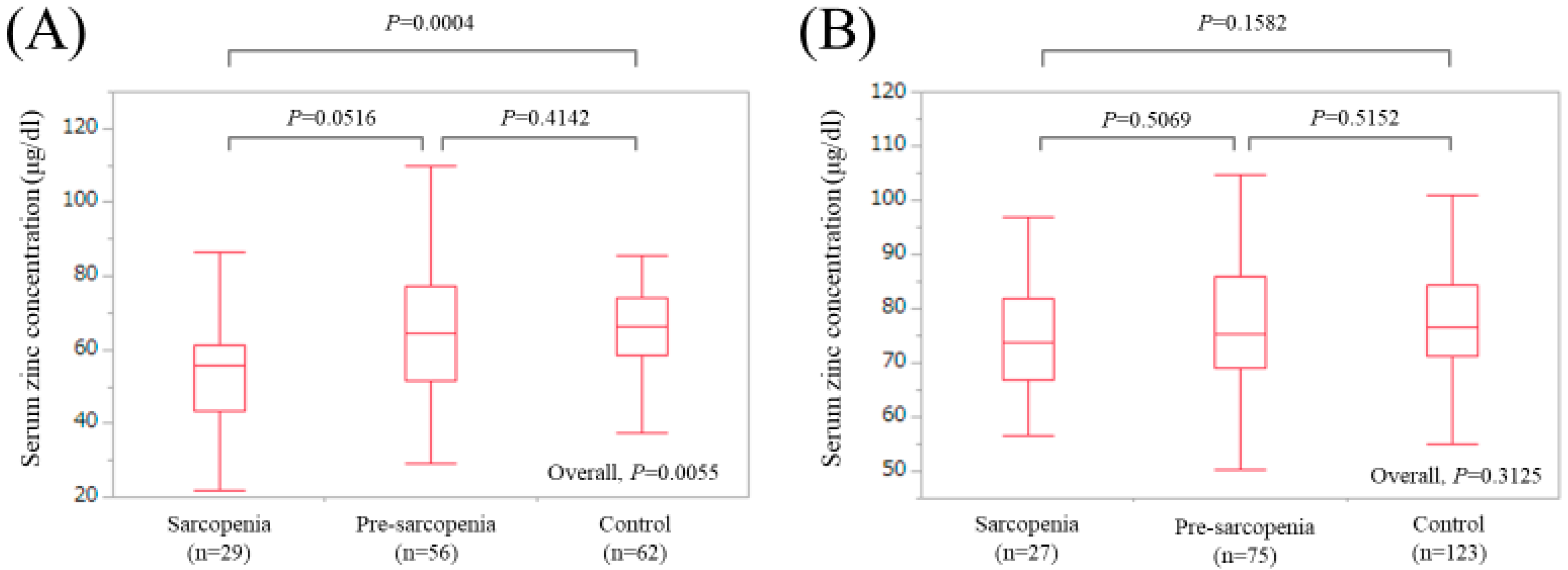
3.4. Uni- and Multivariate Analyses of Factors Associated with the Presence of Sarcopenia for LC Cases (n = 147)
3.5. Uni- and Multivariate Analyses of Factors Associated with the Presence of Sarcopenia for Non-LC Cases (n = 225)
3.6. Correlation between Serum Zinc Concentration and Baseline Characteristics
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Guo, L.; Yoo, C.; Stewart, T.S. Zinc and autophagy. Biometals 2014, 27, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Vidot, H.; Carey, S.; Allman-Farinelli, M.; Shackel, N. Systematic review: The treatment of muscle cramps in patients with cirrhosis. Aliment. Pharmacol. Ther. 2014, 40, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Koop, A.H.; Mousa, O.Y.; Pham, L.E.; Corral-Hurtado, J.E.; Pungpapong, S.; Keaveny, A.P. An Argument for Vitamin D, A, and Zinc Monitoring in Cirrhosis. Ann. Hepatol. 2018, 17, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, M.; Rueda-Robles, A.; Nogueras-López, F.; Villalón-Mir, M.; Oliveras-López, M.J.; Navarro-Alarcón, M. Serum zinc and copper concentrations and ratios in cirrhotic patients: Correlation with severity index. Nutr. Hosp. 2018, 35, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Skalnaya, M.G.; Grabeklis, A.R.; Skalnaya, A.A.; Tinkov, A.A. Zinc deficiency as a mediator of toxic effects of alcohol abuse. Eur. J. Nutr. 2018, 57, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K. Branched Amino Acids and Zinc in the Nutrition of Liver Cirrhosis. J. Clin. Exp. Hepatol. 2018, 8, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Hosui, A.; Kimura, E.; Abe, S.; Tanimoto, T.; Onishi, K.; Kusumoto, Y.; Sueyoshi, Y.; Matsumoto, K.; Hirao, M.; Yamada, T.; et al. Long-Term Zinc Supplementation Improves Liver Function and Decreases the Risk of Developing Hepatocellular Carcinoma. Nutrients 2018, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Presser, H.J.; Franke, D.; Lössner, B.; Kleine, F.D. Correlations between zinc, amino acids and ammonia in liver cirrhosis. Z. Gastroenteriol. 1989, 27, 731–735. [Google Scholar]
- Katayama, K.; Saito, M.; Kawaguchi, T.; Endo, R.; Sawara, K.; Nishiguchi, S.; Kato, A.; Kohgo, H.; Suzuki, K.; Sakaida, I.; et al. Effect of zinc on liver cirrhosis with hyperammonemia: A preliminary randomized, placebo-controlled double-blind trial. Nutrition 2014, 30, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Wroblewski, K.; Aronsohn, A.; Reau, N.; Reddy, K.G.; Jensen, D.; Te, H. Screening for Zinc Deficiency in Patients with Cirrhosis: When Should We Start? Dig. Dis. Sci. 2015, 60, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Kazunari, I.; Hirayuki, E.; Shuhei, N.; Nobuhiro, A.; Yoshiyuki, S.; Yoshinori, I.; Hironori, T.; Naoto, I.; Tomoyuki, T.; Masaki, S.; et al. Serum zinc value in patients with hepatitis virus-related chronic liver disease: Association with the histological degree of liver fibrosis and with the severity of varices in compensated cirrhosis. J. Clin. Biochem. Nutr. 2014, 55, 147–152. [Google Scholar]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and Its Impact on Quality of Life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J. Clinical relevance of sarcopenia in patients with cirrhosis. World J. Gastroenterol. 2014, 20, 8061–8071. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Review article: Sarcopenia in cirrhosis—aetiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Takata, R.; Hasegawa, K.; Nakano, C.; et al. Development of a simple predictive model for decreased skeletal muscle mass in patients with compensated chronic liver disease. Hepatol. Res. 2017, 47, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Okumura, S.; Yao, S.; Yagi, S.; Kamo, N.; Taura, K.; Okajima, H.; et al. Preoperative Low Muscle Mass and Low Muscle Quality Negatively Impact on Pulmonary Function in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 76–89. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P.; Jegatheesan, P.; Tennoune-El-Hafaia, N. Muscle Loss in Chronic Liver Diseases: The Example of Nonalcoholic Liver Disease. Nutrients 2018, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Kozuka, A.; Hirai, T.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; Goto, H. Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Laube, R.; Wang, H.; Park, L.; Heyman, J.K.; Vidot, H.; Majumdar, A.; Strasser, S.I.; McCaughan, G.W.; Liu, K. Frailty in advanced liver disease. Liver Int. 2018, 38, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.; Wagner, D.; Homfeld, N.; Müller, H.; Kniepeiss, D.; Schemmer, P. Both sarcopenia and frailty determine suitability of patients for liver transplantation—A systematic review and meta-analysis of the literature. Clin. Transplant. 2018, 32, e13226. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K. Zinc in liver disease. J. Trace Elem. Exp. Med. 2002, 15, 67–78. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nagao, Y.; Abe, K.; Imazeki, F.; Honda, K.; Yamasaki, K.; Miyanishi, K.; Taniguchi, E.; Kakuma, T.; Kato, J.; et al. Effects of branched-chain amino acids and zinc-enriched nutrients on prognosticators in HCV-infected patients: A multicenter randomized controlled trial. Mol. Med. Rep. 2015, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Asakawa, A.; Li, J.B.; Tsai, M.; Amitani, H.; Ohinata, K.; Komai, M.; Inui, A. Zinc as an appetite stimulator - the possible role of zinc in the progression of diseases such as cachexia and sarcopenia. Recent Pat. Food Nutr. Agric. 2011, 3, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Vainshelboim, B.; Ferreira, A.P.; Neri, S.G.R.; Bottaro, M.; Lima, R.M. Stages of sarcopenia and the incidence of falls in older women: A prospective study. Arch. Gerontol. Geriatr. 2018, 79, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, V.; Rockabrand, E.; Mocchegiani, E.; Sensi, S.L. Oxidative stress and brain aging: Is zinc the link? Biogerontology 2006, 7, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Troche, C.; Kim, J.; Kim, M.H.; Teran, O.Y.; Leeuwenburgh, C.; Cousins, R.J. Aging amplifies multiple phenotypic defects in mice with zinc transporter Zip14 (Slc39a14) deletion. Exp. Gerontol. 2016, 85, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Osaki, Y.; Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 2015, 45, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kamo, N.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Uemoto, S. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones (Athens) 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Cases (n = 372) |
|---|---|
| Age (years) | 65 (54, 71.75) |
| Gender, male/female | 171/201 |
| HBV/HCV/HBV and HCV/NBNC | 53/224/8/87 |
| Presence of LC, yes/no | 147/225 |
| Body mass index (kg/m2) | 22.6 (20.4, 25.3) |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.1) |
| Serum albumin (g/dL) | 4.1 (3.8, 4.5) |
| Prothrombin time (%) | 88.5 (78.3, 96.5) |
| Platelet count (×104/mm3) | 16.5 (11.35, 20.7) |
| Total cholesterol (mg/dL) | 179 (151, 206) |
| AST (IU/L) | 28 (21, 42) |
| ALT (IU/L) | 23 (15, 40) |
| ALP (IU/L) | 243 (193, 318.25) |
| GGT (IU/L) | 28 (17, 50) |
| eGFR (mL/min/1.73 m2) | 83 (70, 96) |
| HbA1c (NGSP) | 5.6 (5.4, 6.0) |
| Serum sodium (mmol/L) | 140 (139, 142) |
| Serum zinc concentration (μg/dL) | 72.85 (63.7, 81.45) |
| BTR | 5.63 (4.2375, 6.65) |
| FIB-4 index | 2.39 (1.60, 4.07) |
| APRI | 0.52 (0.33, 1.15) |
| Variables | Sarcopenia (n = 56) | Non-Sarcopenia (n = 316) | P Value |
|---|---|---|---|
| Age (years) | 72.5 (67.25, 76.75) | 63 (52, 70) | <0.0001 |
| Gender, male/female | 27/29 | 144/172 | 0.7144 |
| HBV/HCV/HBV and HCV/NBNC | 6/31/2/17 | 47/193/6/70 | 0.4195 |
| Body mass index (kg/m2) | 20.6 (19.7, 22.7) | 22.95 (20.7, 26) | <0.0001 |
| Presence of LC, yes/no | 29/27 | 118/198 | 0.0416 |
| Total bilirubin (mg/dL) | 0.7 (0.5, 0.95) | 0.8 (0.6, 1.1) | 0.0276 |
| Serum albumin (g/dL) | 3.95 (3.225, 4.3) | 4.2 (3.8, 4.5) | 0.0008 |
| Prothrombin time (%) | 85.3 (74.45, 98.825) | 88.7 (78.45, 96.475) | 0.6971 |
| Platelet count (×104/mm3) | 15.1 (10.2, 18.6) | 16.75 (11.525, 20.8) | 0.2303 |
| AST (IU/L) | 32 (22, 49) | 27 (21, 40.75) | 0.3978 |
| ALT (IU/L) | 26 (15, 42) | 23 (16, 40) | 0.8374 |
| ALP (IU/L) | 287 (206, 372) | 237 (192, 301) | 0.0143 |
| GGT (IU/L) | 30 (18.5, 52) | 28 (17, 50) | 0.5899 |
| Total cholesterol (mg/dL) | 179 (142, 212) | 179 (154, 205.75) | 0.3933 |
| eGFR (mL/min/1.73 m2) | 76 (57, 92) | 83 (71, 96.75) | 0.0178 |
| Serum sodium (mmol/L) | 140 (138, 141) | 140 (139, 142) | 0.0164 |
| HbA1c (NGSP) | 5.85 (5.2, 6.1) | 5.6 (5.4, 6.0) | 0.4177 |
| Serum zinc concentration (High/Intermediate/Low) | 10/21/25 | 83/166/67 | 0.0009 |
| BTR | 5.19 (3.6, 7.21) | 5.64 (4.34, 6.625) | 0.5460 |
| FIB-4 index | 3.09 (2.15, 5.09) | 2.28 (1.49, 4.01) | 0.1409 |
| APRI | 0.61 (0.35, 1.42) | 0.51 (0.32, 1.09) | 0.8192 |
| Multivariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | |
| Age (per one year) | 1.075 | 1.032–1.120 | 0.0005 |
| BMI (per one kg/m2) | 0.790 | 0.695–0.898 | 0.0003 |
| Presence of LC | 1.541 | 0.645–3.690 | 0.3306 |
| Serum albumin (per one g/dL) | 0.208 | 0.081–0.533 | 0.0011 |
| Total bilirubin (per one mg/dL) | 0.439 | 0.176–1.093 | 0.0769 |
| ALP (per one IU/L) | 1.002 | 0.999–1.005 | 0.1273 |
| eGFR (per one mL/min/1.73 m2) | 1.001 | 0.985–1.018 | 0.8715 |
| Serum sodium (per one mmol/L) | 0.899 | 0.791–1.022 | 0.1061 |
| Classification of serum zinc level | |||
| Intermediate-Zn group | 1.000 | Reference | |
| High-Zn group | 1.812 | 0.722–4.545 | 0.2053 |
| Low-Zn group | 3.030 | 1.160–7.937 | 0.0236 |
| Variables | Sarcopenia (n = 29) | Non-Sarcopenia (n = 118) | P Value |
|---|---|---|---|
| Age (years) | 71 (67, 76) | 67 (59, 72) | 0.0026 |
| Gender, male/female | 15/54 | 65/53 | 0.7448 |
| HBV/HCV/HBV and HCV/NBNC | 4/15/1/9 | 15/73/0/30 | 0.1897 |
| Body mass index (kg/m2) | 20.4 (19.8, 23.0) | 23.05 (20.7, 26.275) | 0.0010 |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.2) | 1.1 (0.8, 1.625) | 0.0582 |
| Serum albumin (g/dL) | 3.4 (2.95, 3.9) | 3.8 (3.4, 4.125) | 0.0035 |
| Prothrombin time (%) | 81.9 (58.55, 87.95) | 78.25 (63.175, 88.3) | 0.8211 |
| Platelet count (×104/mm3) | 10.4 (8.05, 15.7) | 10.85 (7.4, 14.525) | 0.6432 |
| AST (IU/L) | 45 (29.75, 56.75) | 37.5 (25, 53) | 0.2028 |
| ALT (IU/L) | 32.5 (21.75, 48) | 28.5 (17.75, 43.25) | 0.3652 |
| ALP (IU/L) | 339.5 (234, 499.75) | 264 (219, 402) | 0.1226 |
| GGT (IU/L) | 41 (22, 74) | 37 (21.5, 67) | 0.6964 |
| Total cholesterol (mg/dL) | 144 (120.75, 188.5) | 155.5 (137, 180) | 0.4466 |
| eGFR (mL/min/1.73 m2) | 80 (66, 93.75) | 82 (68, 99) | 0.4683 |
| Serum sodium (mmol/L) | 139 (137, 140) | 140 (138, 142) | 0.0574 |
| HbA1c (NGSP) | 5.2 (5.0, 6.0) | 5.6 (5.2, 6.15) | 0.3641 |
| Serum zinc classification High/Intermediate/Low | 2/4/23 | 15/53/50 | 0.0016 |
| BTR | 3.7 (2.9875, 4.935) | 4.245 (3.2675, 5.64) | 0.1690 |
| FIB-4 index | 4.83 (3.14, 7.56) | 4.27 (2.68, 6.80) | 0.9641 |
| APRI | 1.29 (0.70, 1.95) | 1.08 (0.60, 1.91) | 0.6814 |
| Multivariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | |
| Age (per one year) | 1.054 | 1.003–1.106 | 0.0363 |
| BMI (per one kg/m2) | 0.755 | 0.636–0.898 | 0.0014 |
| Serum albumin (per one g/dL) | 0.377 | 0.121–1.520 | 0.1890 |
| Classification of serum zinc level | |||
| Intermediate-Zn group | 1.000 | Reference | |
| High-Zn group | 2.500 | 0.377–16.667 | 0.3429 |
| Low-Zn group | 6.410 | 1.618–25.641 | 0.0082 |
| Variables | Sarcopenia (n = 27) | Non-Sarcopenia (n = 198) | P Value |
|---|---|---|---|
| Age (years) | 73 (67, 78) | 60 (48.75, 67) | <0.0001 |
| Gender, male/female | 12/15 | 79/119 | 0.6518 |
| HBV/HCV/HBV and HCV/NBNC | 2/16/1/8 | 32/120/6/40 | 0.5248 |
| Body mass index (kg/m2) | 20.9 (19.3, 22.3) | 22.9 (20.675, 25.725) | 0.0003 |
| Total bilirubin (mg/dL) | 0.6 (0.5, 0.7) | 0.8 (0.6, 1.0) | 0.0527 |
| Serum albumin (g/dL) | 4.2 (4.0, 4.5) | 4.3 (4.1, 4.5) | 0.1374 |
| Prothrombin time (%) | 93.5 (84.7, 99) | 92.5 (84.95, 100.025) | 0.5236 |
| Platelet count (×104/mm3) | 17.45 (14.85, 20.7) | 19.25 (15.65, 22.95) | 0.5282 |
| AST (IU/L) | 23 (18, 33) | 24 (19, 33) | 0.3645 |
| ALT (IU/L) | 15 (12, 32) | 20 (15, 35.25) | 0.3151 |
| ALP (IU/L) | 243 (177, 306) | 224.5 (178.75, 275.75) | 0.1149 |
| GGT (IU/L) | 25 (15.75, 42.5) | 23 (15, 44) | 0.9164 |
| Total cholesterol (mg/dL) | 195 (165, 218) | 193 (170.75, 219) | 0.8202 |
| eGFR (mL/min/1.73 m2) | 66 (56, 92) | 84 (72, 95.5) | 0.0063 |
| Serum sodium (mmol/L) | 140 (138, 142) | 141 (139, 142) | 0.3751 |
| HbA1c (NGSP) | 6.0 (5.6, 6.4) | 5.7 (5.4, 5.925) | 0.0091 |
| Serum zinc classification High/Intermediate/Low | 8/17/2 | 68/113/17 | 0.8444 |
| BTR | 6.92 (5.64, 7.72) | 6.25 (5.28, 7.05) | 0.0544 |
| FIB-4 index | 2.13 (1.64, 2.86) | 1.73 (1.13, 2.47) | 0.1066 |
| APRI | 0.35 (0.28, 0.52) | 0.36 (0.29, 0.63) | 0.3683 |
| Multivariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | |
| Age (per one year) | 1.071 | 1.004–1.142 | 0.0249 |
| BMI (per one kg/m2) | 0.746 | 0.602–0.923 | 0.0021 |
| eGFR (per one mL/min/1.73 m2) | 0.985 | 0.955–1.015 | 0.3257 |
| HbA1c (per one) | 1.280 | 0.727–5.376 | 0.2127 |
| r | P Value | |
|---|---|---|
| Age | −0.186 | 0.0003 |
| Body mass index | −0.032 | 0.5424 |
| Total bilirubin | −0.355 | <0.0001 |
| Serum albumin | 0.654 | <0.0001 |
| Prothrombin time | 0.395 | <0.0001 |
| Platelets | 0.295 | <0.0001 |
| Serum sodium | 0.249 | 0.8923 |
| eGFR | −0.020 | 0.7029 |
| Total cholesterol | 0.266 | <0.0001 |
| AST | −0.230 | <0.0001 |
| ALT | −0.065 | 0.2126 |
| ALP | −0.245 | <0.0001 |
| GGT | −0.206 | <0.0001 |
| HbA1c (NSGP) | 0.133 | 0.0242 |
| BTR | 0.496 | <0.0001 |
| FIB-4 index | −0.360 | <0.0001 |
| APRI | −0.289 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. J. Clin. Med. 2019, 8, 336. https://doi.org/10.3390/jcm8030336
Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, et al. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. Journal of Clinical Medicine. 2019; 8(3):336. https://doi.org/10.3390/jcm8030336
Chicago/Turabian StyleNishikawa, Hiroki, Hirayuki Enomoto, Kazunori Yoh, Yoshinori Iwata, Yoshiyuki Sakai, Kyohei Kishino, Naoto Ikeda, Tomoyuki Takashima, Nobuhiro Aizawa, Ryo Takata, and et al. 2019. "Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases" Journal of Clinical Medicine 8, no. 3: 336. https://doi.org/10.3390/jcm8030336
APA StyleNishikawa, H., Enomoto, H., Yoh, K., Iwata, Y., Sakai, Y., Kishino, K., Ikeda, N., Takashima, T., Aizawa, N., Takata, R., Hasegawa, K., Ishii, N., Yuri, Y., Nishimura, T., Iijima, H., & Nishiguchi, S. (2019). Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. Journal of Clinical Medicine, 8(3), 336. https://doi.org/10.3390/jcm8030336




