Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus
Abstract
1. Introduction: The Current Status of Type 1 Diabetes Mellitus
2. What are Adipose Tissue-Derived Mesenchymal Stromal Cells?
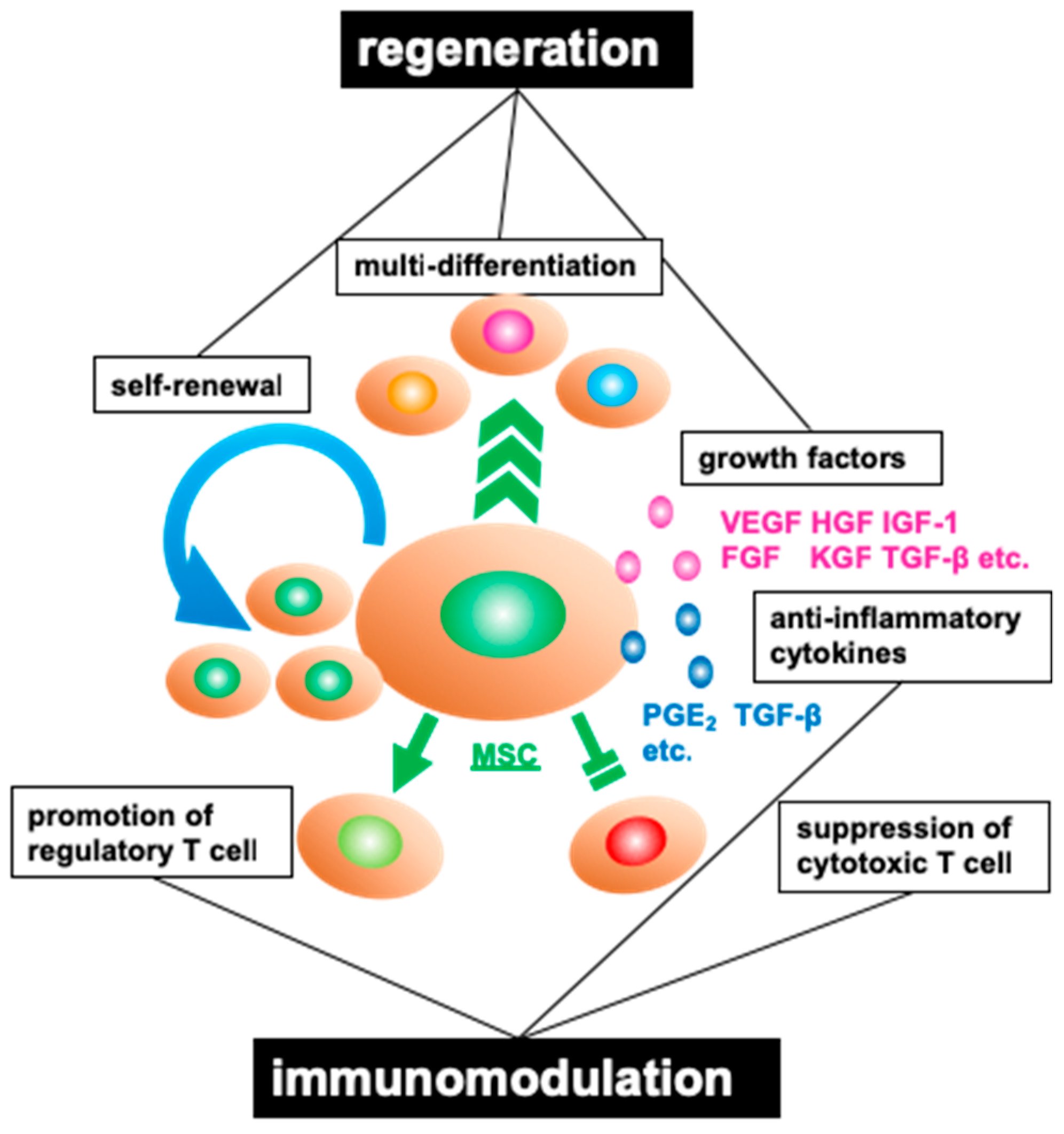
2.1. Properties of Mesenchymal Stromal Cells
2.2. Characteristics of Adipose Tissue-Derived Mesenchymal Stromal Cells
3. Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Insulin-Dependent Type 1 Diabetes Mellitus
3.1. Outline of Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells
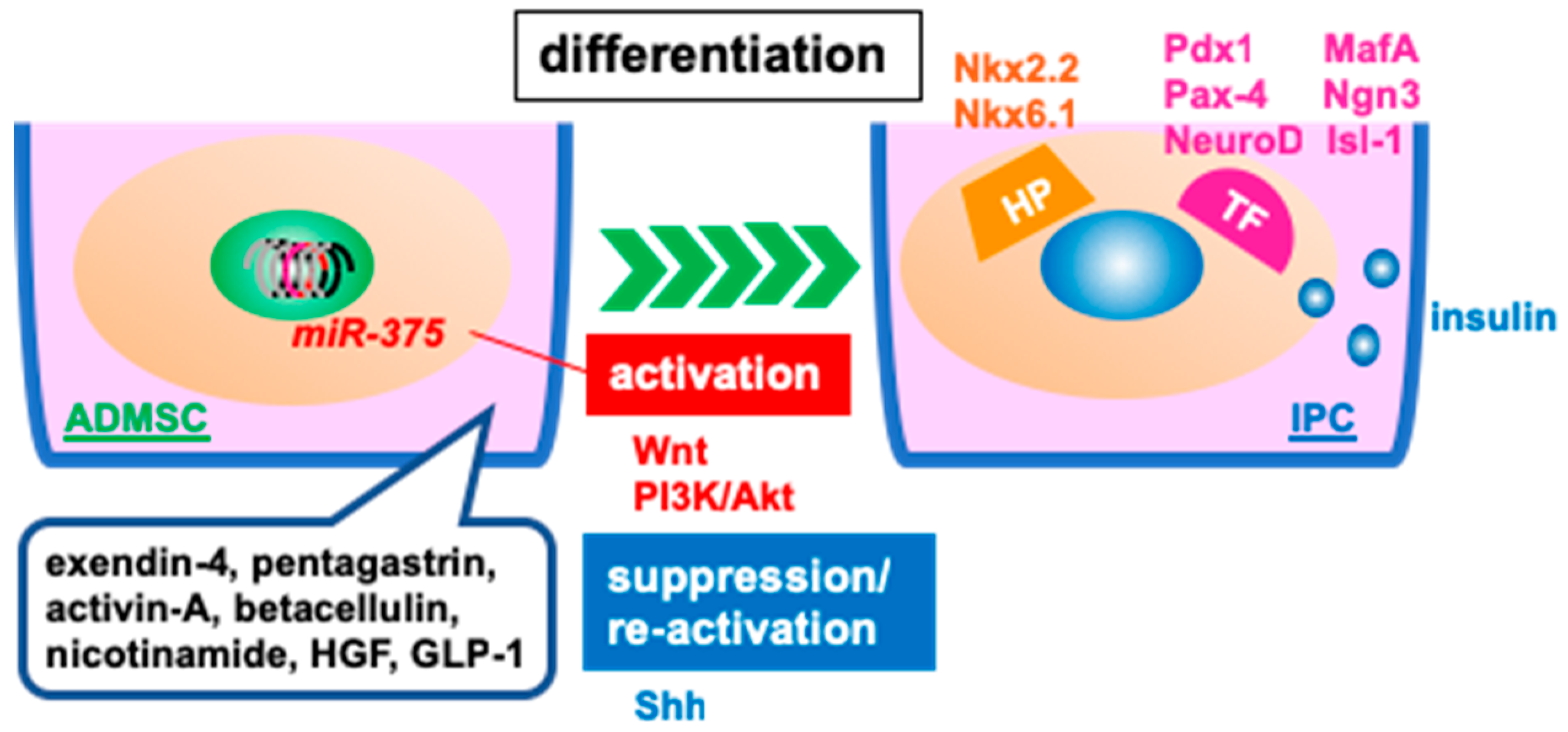
3.2. Differentiated, Insulin-Producing Cells for Transplantation
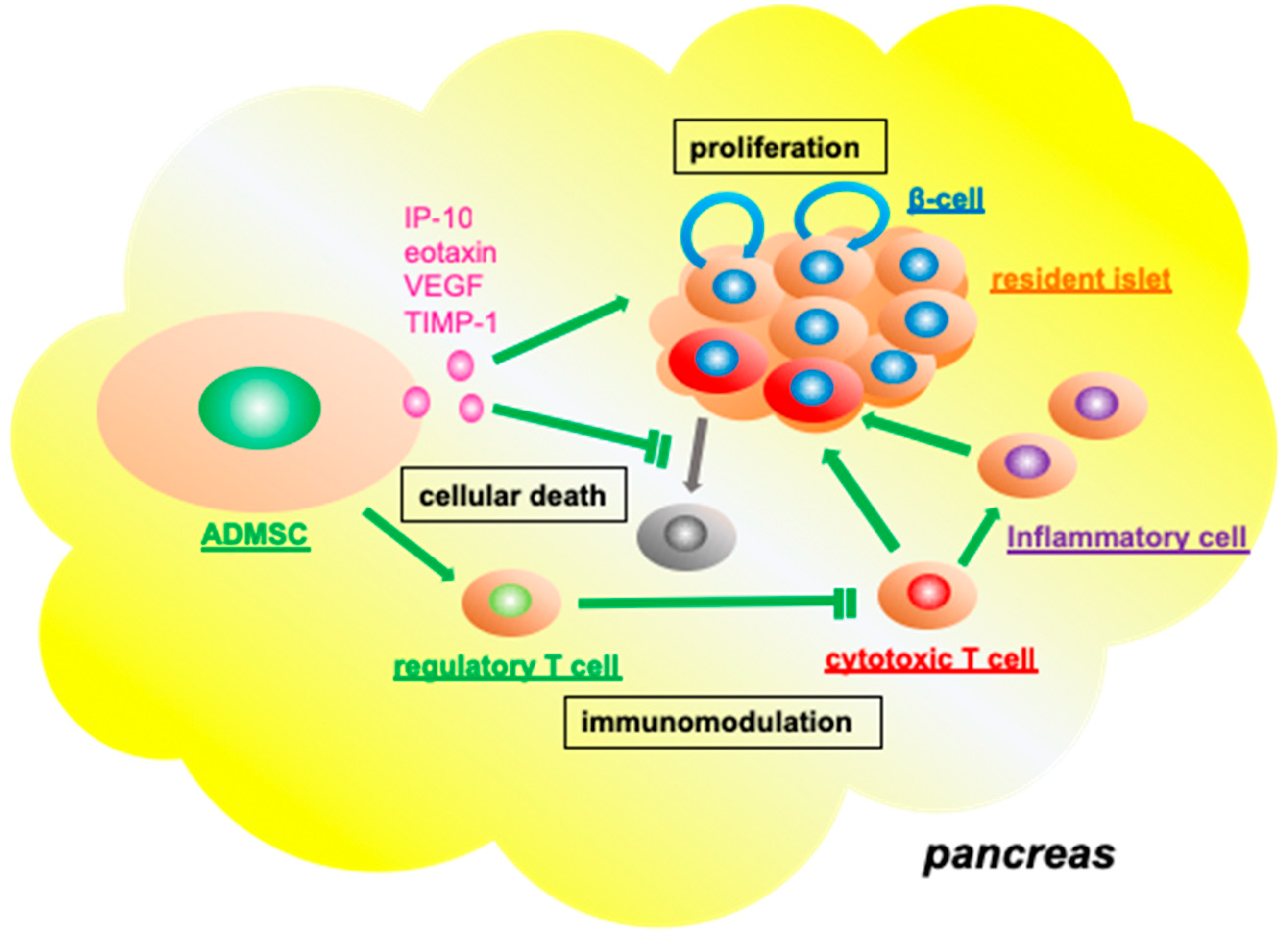
3.3. Functional Role of Adipose Tissue-Derived Mesenchymal Stromal Cells in the Resident Pancreatic Islets
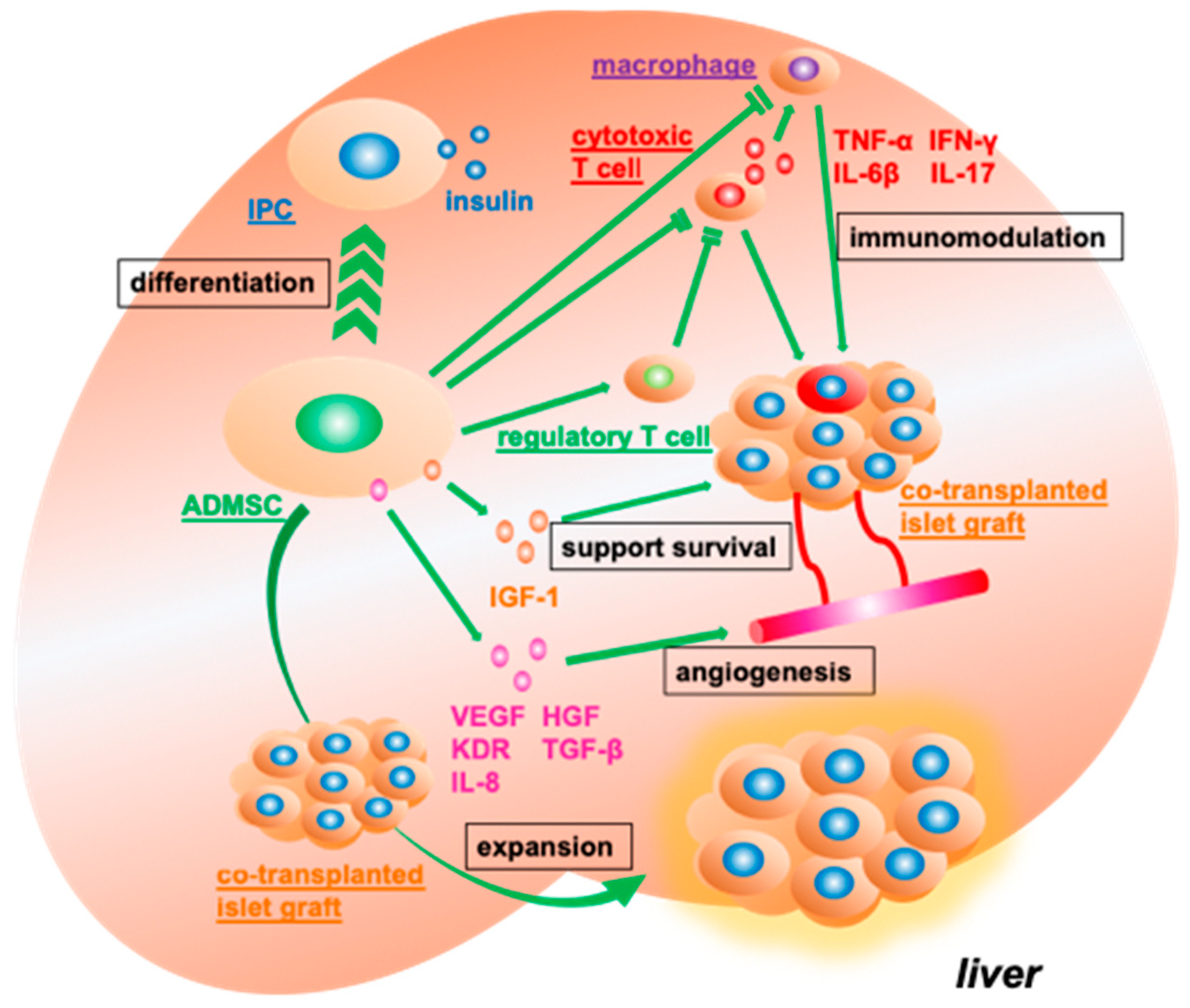
3.4. Supporting the Function and Engraftment of Co-Transplanted Islet Grafts
3.5. In Vitro Co-Culture of Syngeneic Islets Graft with Adipose Tissue-Derived Mesenchymal Stromal Cells Ameliorates Transplantation Efficiency
4. Towards a Suitable Adipose Tissue-Derived Mesenchymal Stromal Cells
4.1. Sources of Adipose Tissue-Derived Mesenchymal Stromal Cells
4.2. Method for the Preparation of Adipose Tissue-Derived Mesenchymal Stromal Cell
4.3. Various Factors Influencing the Quality of Adipose Tissue-Derived Mesenchymal Stromal Cells
4.4. The Origin and Malignant Potential of Adipose Tissue-Derived Mesenchymal Stromal Cells
4.5. Optimal Transplant Site for Adipose Tissue-Derived Mesenchymal Stromal Cells in T1DM Cellular Therapy
5. Clinical Trials on Adipose Tissue-Derived Mesenchymal Stromal Cell Therapy for T1DM
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
References
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pamoukian, V.N.; Rubino, F.; Iraci, J.C. Review and case report of idiopathic lower extremity compartment syndrome and its treatment in diabetic patients. Diabetes Metab. 2000, 26, 489–492. [Google Scholar] [PubMed]
- Chan, L.; Terashima, T.; Fujimiya, M.; Kojima, H. Chronic diabetic complications: The body’s adaptive response to hyperglycemia gone awry? Trans. Am. Clin. Climatol. Assoc. 2006, 117, 341–351. [Google Scholar] [PubMed]
- Eisenbarth, G.S. Type I diabetes mellitus: A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef]
- Chhabra, P.; Brayman, K.L. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl. Med. 2013, 2, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Kitabchi, A.E. Diabetic ketoacidosis: Risk factors and management strategies. Treat. Endocrino.l 2003, 2, 95–108. [Google Scholar] [CrossRef]
- Misso, M.L.; Egberts, K.J.; Page, M.; O’Connor, D.; Shaw, J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 1, CD005103. [Google Scholar] [CrossRef]
- Tauschmann, M.; Thabit, H.; Bally, L.; Allen, J.M.; Hartnell, S.; Wilinska, M.E.; Ruan, Y.; Sibayan, J.; Kollman, C.; Cheng, P.; et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: A multicentre, 12-week randomised trial. Lancet 2018, 392, 1321–1329. [Google Scholar] [CrossRef]
- Tauschmann, M.; Hovorka, R. Technology in the management of type 1 diabetes mellitus—Current status and future prospects. Nat. Rev. Endocrinol. 2018, 14, 464–475. [Google Scholar] [CrossRef]
- Hirshberg, B.; Rother, K.I.; Digon, B.J., 3rd; Lee, J.; Gaglia, J.L.; Hines, K.; Read, E.J.; Chang, R.; Wood, B.J.; Harlan, D.M. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: The National Institutes of Health experience. Diabetes Care 2003, 26, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Rickels, M.R.; Peleckis, A.J.; Markmann, E.; Dalton-Bakes, C.; Kong, S.M.; Teff, K.L.; Naji, A. Long-Term Improvement in Glucose Control and Counterregulation by Islet Transplantation for Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Yoshimatsu, G.; Kodama, S. The Spleen as an Optimal Site for Islet Transplantation and a Source of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1391. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Radosevich, D.M.; Bellin, M.D.; Hering, B.J.; Beilman, G.J.; Dunn, T.B.; Chinnakotla, S.; Vickers, S.M.; Bland, B.; Balamurugan, A.N.; et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J. Am. Coll. Surg. 2012, 214, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Strom, T.B. Clinical islet transplantation: Advances and immunological challenges. Nat. Rev. Immunol. 2004, 4, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Barton, F.B.; Rickels, M.R.; Alejandro, R.; Hering, B.J.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.S.; Garfinkel, M.R.; Levy, M.; et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef]
- Sakata, N.; Goto, M.; Yoshimatsu, G.; Egawa, S.; Unno, M. Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J. Gastroenterol. 2011, 17, 5150–5155. [Google Scholar] [CrossRef]
- Mundra, V.; Gerling, I.C.; Mahato, R.I. Mesenchymal stem cell-based therapy. Mol. Pharm. 2013, 10, 77–89. [Google Scholar] [CrossRef]
- Watt, F.M.; Hogan, B.L. Out of Eden: Stem cells and their niches. Science 2000, 287, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.J.; Kroeze, K.L.; Waaijman, T.; Breetveld, M.; Sampat-Sardjoepersad, S.C.; Niessen, F.B.; Middelkoop, E.; Scheper, R.J.; Gibbs, S. Differential response of human adipose tissue-derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: Potential role of skin-specific chemokine CCL27. Tissue Eng. Part. A 2014, 20, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanalakshmi, G.; Arfuso, F.; Kumar, A.P.; Dharmarajan, A.; Warrier, S. Epigenetic reprogramming converts human Wharton’s jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Res. Ther. 2017, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Vykoukal, J.; Abdelsalam, M.; Recio-Boiles, A.; Huang, Q.; Qiao, Y.; Singhana, B.; Wallace, M.; Avritscher, R.; Melancon, M.P. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology 2014, 25, 405101. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, P.; Ma, X.L.; Wang, J.; Zhao, L.J.; Du, L.; Wang, L.Y.; Wang, X.R.; Liu, K.D. Effect of bone marrow stromal cell transplantation on neurologic function and expression of VEGF in rats with focal cerebral ischemia. Mol. Med. Rep. 2014, 10, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Zolocinska, A. The expression of marker genes during the differentiation of mesenchymal stromal cells. Adv. Clin. Exp. Med. 2018, 27, 717–723. [Google Scholar] [CrossRef]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef]
- Prevosto, C.; Zancolli, M.; Canevali, P.; Zocchi, M.R.; Poggi, A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 2007, 92, 881–888. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Mabuchi, Y.; Niibe, K.; Suzuki, S.; Nagoshi, N.; Sunabori, T.; Shimmura, S.; Nagai, Y.; Nakagawa, T.; Okano, H.; et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys Res. Commun. 2009, 379, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Steens, J.; Klein, D. Current Strategies to Generate Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2018, 2018, 6726185. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Kodama, S.; Kuhtreiber, W.; Fujimura, S.; Dale, E.A.; Faustman, D.L. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 2003, 302, 1223–1227. [Google Scholar] [CrossRef]
- Seeberger, K.L.; Dufour, J.M.; Shapiro, A.M.J.; Lakey, J.R.T.; Rajotte, R.V.; Korbutt, G.S. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab. Invest. 2006, 86, 141–153. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Akatsuka, A.; Okada, Y.; Matsuzaki, Y.; Okano, H.; Kimura, M. Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp. Cell Res. 2003, 291, 83–90. [Google Scholar] [CrossRef]
- Li, C.D.; Zhang, W.Y.; Li, H.L.; Jiang, X.X.; Zhang, Y.; Tang, P.H.; Mao, N. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005, 15, 539–547. [Google Scholar] [CrossRef]
- Gan, E.H.; Robson, W.; Murphy, P.; Pickard, R.; Pearce, S.; Oldershaw, R. Isolation of a multipotent mesenchymal stem cell-like population from human adrenal cortex. Endocr. Connect. 2018, 7, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, A.; Niezgoda, P.; Nowowiejska, L.; Bialecka, A.; Mecinska-Jundzill, K.; Adamska, U.; Czajkowski, R. Properties of skin stem cells and their potential clinical applications in modern dermatology. Eur. J. Dermatol. 2017, 27, 227–236. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Bateman, M.E.; Strong, A.L.; Gimble, J.M.; Bunnell, B.A. Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells 2018. [Google Scholar] [CrossRef]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.F.; Hedayati, V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell 2010, 42, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, R.I.; Minullina, I.R.; Bilibina, A.A.; Tarasova, O.V.; Anisimov, S.V.; Zaritskey, A.Y. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle 2012, 11, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.; Blanco, B.; Yanez, R.; Vazquez, M.; Herrero Sanchez, C.; Fernandez-Garcia, M.; Rodriguez Serrano, C.; Pescador, D.; Blanco, J.F.; Hernando-Rodriguez, M.; et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy 2016, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Bassi, E.J.; Moraes-Vieira, P.M.; Moreira-Sa, C.S.; Almeida, D.C.; Vieira, L.M.; Cunha, C.S.; Hiyane, M.I.; Basso, A.S.; Pacheco-Silva, A.; Camara, N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012, 61, 2534–2545. [Google Scholar] [CrossRef]
- Ivanova-Todorova, E.; Bochev, I.; Mourdjeva, M.; Dimitrov, R.; Bukarev, D.; Kyurkchiev, S.; Tivchev, P.; Altunkova, I.; Kyurkchiev, D.S. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol. Lett. 2009, 126, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Osaki, M.; Kawamata, M.; Kato, T.; Okochi, H.; Ochiya, T. IFATS collection: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 2008, 26, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Kocan, B.; Maziarz, A.; Tabarkiewicz, J.; Ochiya, T.; Banas-Zabczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017, 2017, 1653254. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.A.; Baregundi Subbarao, R.; Lee, Y.M.; Lee, J.H.; Jeon, R.H.; Lee, S.L.; Park, J.K.; Hwang, S.C.; Rho, G.J. Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int. 2016, 2016, 9581350. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Wang, R.; Zhao, F.; Shi, P.; Jiang, Y.; Pang, X. Direct comparison of the potency of human mesenchymal stem cells derived from amnion tissue, bone marrow and adipose tissue at inducing dermal fibroblast responses to cutaneous wounds. Int. J. Mol. Med. 2013, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Semon, J.A.; Maness, C.; Zhang, X.; Sharkey, S.A.; Beuttler, M.M.; Shah, F.S.; Pandey, A.C.; Gimble, J.M.; Zhang, S.; Scruggs, B.A.; et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, Y.; Zhang, H.; Min, S.; Yu, B.; He, B.; Jin, A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013, 15, 434–448. [Google Scholar] [CrossRef]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef]
- Burt, R.K.; Oyama, Y.; Traynor, A.; Kenyon, N.S. Hematopoietic stem cell therapy for type 1 diabetes: Induction of tolerance and islet cell neogenesis. Autoimmun. Rev. 2002, 1, 133–138. [Google Scholar] [CrossRef]
- Hess, D.; Li, L.; Martin, M.; Sakano, S.; Hill, D.; Strutt, B.; Thyssen, S.; Gray, D.A.; Bhatia, M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 2003, 21, 763–770. [Google Scholar] [CrossRef]
- Roy, S.S.; Mukherjee, M.; Bhattacharya, S.; Mandal, C.N.; Kumar, L.R.; Dasgupta, S.; Bandyopadhyay, I.; Wakabayashi, K. A new cell secreting insulin. Endocrinology 2003, 144, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Bhonde, R.R.; Sheshadri, P.; Sharma, S.; Kumar, A. Making surrogate beta-cells from mesenchymal stromal cells: Perspectives and future endeavors. Int. J. Biochem. Cell Biol. 2014, 46, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, C.; Anazawa, T.; Cross, S.E.; Labriola, L.; Meier, R.P.H.; Redfield, R.R., 3rd; Scholz, H.; Stock, P.G.; Zammit, N.W.; Committee, I.Y.Y.I. beta Cell Replacement Therapy: The Next 10 Years. Transplantation 2018, 102, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Seboek, D.; Eberhardt, M.; Linscheid, P.; Christ-Crain, M.; Keller, U.; Muller, B.; Zulewski, H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys Res. Commun. 2006, 341, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Komoda, H.; Fumimoto, Y.; Lee, C.M.; Nishida, T.; Sawa, Y.; Matsuyama, A. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J. Artif. Organs 2009, 12, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, J.; Park, S.; Kim, J.; Kim, H.; Kim, K.S.; Lee, E.J.; Seo, S.I.; Kang, S.G.; Lee, J.E.; et al. Insulin-secreting cells from human eyelid-derived stem cells alleviate type I diabetes in immunocompetent mice. Stem Cells 2009, 27, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Hamazaki, T.S.; Tokuhara, M.; Masui, S.; Okabayashi, K.; Ohnuma, K.; Yabe, S.; Yasuda, K.; Ishiura, S.; Okochi, H.; et al. Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int. J. Dev. Biol. 2010, 54, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Swetha, G.; Muthyala, S.; Jaiswal, A.K.; Bellare, J.R.; Nair, P.D.; Bhonde, R.R. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PloS ONE 2011, 6, e20615. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.S.; Ko, E.S.; Lim, S.M.; Lee, C.W.; Kim, D.I. Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J. Biosci. Bioeng. 2012, 113, 771–777. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.C.; Kim, S.J.; Lee, H.; Jung, E.J.; Jung, S.H.; Han, D.J. Differentiation of human adipose tissue-derived stem cells into aggregates of insulin-producing cells through the overexpression of pancreatic and duodenal homeobox gene-1. Cell Transplant. 2013, 22, 1053–1060. [Google Scholar] [CrossRef]
- Nam, J.S.; Kang, H.M.; Kim, J.; Park, S.; Kim, H.; Ahn, C.W.; Park, J.O.; Kim, K.R. Transplantation of insulin-secreting cells differentiated from human adipose tissue-derived stem cells into type 2 diabetes mice. Biochem. Biophys Res. Commun. 2014, 443, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Liu, T.J.; Li, L.; Tang, W.; Zou, J.J.; Chen, X.F.; Zheng, J.Y.; Jiang, B.G.; Shi, Y.Q. Transplantation of betatrophin-expressing adipose-derived mesenchymal stem cells induces beta-cell proliferation in diabetic mice. Int. J. Mol. Med. 2017, 39, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.G.; Embaby, A.S.; Karam, R.A.; Amer, M.G. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene 2018, 654, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fazili, A.; Gholami, S.; Minaie Zangi, B.; Seyedjafari, E.; Gholami, M. In Vivo Differentiation of Mesenchymal Stem Cells into Insulin Producing Cells on Electrospun Poly-L-Lactide Acid Scaffolds Coated with Matricaria chamomilla L. Oil. Cell J. 2016, 18, 310–321. [Google Scholar] [PubMed]
- Dayer, D.; Tabar, M.H.; Moghimipour, E.; Tabandeh, M.R.; Ghadiri, A.A.; Bakhshi, E.A.; Orazizadeh, M.; Ghafari, M.A. Sonic hedgehog pathway suppression and reactivation accelerates differentiation of rat adipose-derived mesenchymal stromal cells toward insulin-producing cells. Cytotherapy 2017, 19, 937–946. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, X.; Shi, X.; Zhao, J.; Chen, Y.; Yao, Q.; Sun, C.; Yang, J. Regulatory Mechanisms of the Wnt/beta-Catenin Pathway in Diabetic Cutaneous Ulcers. Front. Pharmacol. 2018, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Li, X. MiR-375, a microRNA related to diabetes. Gene 2014, 533, 1–4. [Google Scholar] [CrossRef]
- Liu, Z.; Habener, J.F. Wnt signaling in pancreatic islets. Adv. Exp. Med. Biol. 2010, 654, 391–419. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Hu, X.; Ma, M.; Wang, X.; Liang, H.; Liu, D. Effect of Wnt Signaling on the Differentiation of Islet beta-Cells from Adipose-Derived Stem Cells. Biomed. Res. Int. 2017, 2017, 2501578. [Google Scholar] [CrossRef]
- Anjum, M.S.; Mehmood, A.; Mahmood, F.; Ali, M.; Tarrar, M.N.; Khan, S.N.; Riazuddin, S. In vitro preconditioning of insulin-producing cells with growth factors improves their survival and ability to release insulin. J. Biosci. 2018, 43, 649–659. [Google Scholar] [CrossRef]
- Piran, M.; Enderami, S.E.; Piran, M.; Sedeh, H.S.; Seyedjafari, E.; Ardeshirylajimi, A. Insulin producing cells generation by overexpression of miR-375 in adipose-derived mesenchymal stem cells from diabetic patients. Biologicals 2017, 46, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Poy, M.N.; Hausser, J.; Trajkovski, M.; Braun, M.; Collins, S.; Rorsman, P.; Zavolan, M.; Stoffel, M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl. Acad. Sci. 2009, 106, 5813–5818. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Abdel-Rahman, E.A.; Reda, A.M.; Ali, S.S.; Khater, S.M.; Ashamallah, S.A.; Ismail, A.M.; Ismail, H.E.A.; et al. From Human Mesenchymal Stem Cells to Insulin-Producing Cells: Comparison between Bone Marrow- and Adipose Tissue-Derived Cells. Biomed. Res. Int. 2017, 2017, 3854232. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, E.; Okcu, A.; Unal, Z.S.; Subasi, C.; Saglam, O.; Duruksu, G. Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy 2013, 15, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Reppel, L.; Leger, L.; Schenowitz, C.; Huselstein, C.; Bensoussan, D.; Carosella, E.D.; Han, Z.C.; Rouas-Freiss, N. Mesenchymal Stem Cells Derived from Human Bone Marrow and Adipose Tissue Maintain Their Immunosuppressive Properties After Chondrogenic Differentiation: Role of HLA-G. Stem Cells Dev. 2016, 25, 1454–1469. [Google Scholar] [CrossRef]
- Kono, T.M.; Sims, E.K.; Moss, D.R.; Yamamoto, W.; Ahn, G.; Diamond, J.; Tong, X.; Day, K.H.; Territo, P.R.; Hanenberg, H.; et al. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: Analysis of hASC-derived paracrine effectors. Stem Cells 2014, 32, 1831–1842. [Google Scholar] [CrossRef]
- Navaei-Nigjeh, M.; Moloudizargari, M.; Baeeri, M.; Gholami, M.; Lotfibakhshaiesh, N.; Soleimani, M.; Vasheghani-Farahani, E.; Ai, J.; Abdollahi, M. Reduction of marginal mass required for successful islet transplantation in a diabetic rat model using adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2018. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.T.; Bui, A.N.; Le-Thanh Nguyen, C.; Truong, N.C.; Bui, A.T.; Kim, N.P.; Truong, K.D.; Van Pham, P. Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells to Treat Type 1 Diabetic Mellitus in Mice: An Evaluation of Grafted Cell Doses. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Caballero, S.; Sengupta, N.; Afzal, A.; Chang, K.H.; Li Calzi, S.; Guberski, D.L.; Kern, T.S.; Grant, M.B. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007, 56, 960–967. [Google Scholar] [CrossRef]
- Solari, M.G.; Srinivasan, S.; Boumaza, I.; Unadkat, J.; Harb, G.; Garcia-Ocana, A.; Feili-Hariri, M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J. Autoimmun. 2009, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.; Cornolti, R.; Perico, N.; Rota, C.; Morigi, M.; Remuzzi, G.; Remuzzi, A.; Benigni, A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant. Proc. 2009, 41, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Itakura, S.; Todorov, I.; Rawson, J.; Asari, S.; Shintaku, J.; Nair, I.; Ferreri, K.; Kandeel, F.; Mullen, Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 2010, 89, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Willman, M.A.; Han, D.; Kleiner, G.; Kenyon, N.M.; Cabrera, O.; Karl, J.A.; Wiseman, R.W.; O’Connor, D.H.; Bartholomew, A.M.; et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 2010, 59, 2558–2568. [Google Scholar] [CrossRef]
- Ohmura, Y.; Tanemura, M.; Kawaguchi, N.; Machida, T.; Tanida, T.; Deguchi, T.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 2010, 90, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kojima, D.; Mera, T.; Matsumoto, M.; Yasunami, Y.; Yanase, T. Expansion of transplanted islets in mice by co-transplantation with adipose tissue-derived mesenchymal stem cells. Heliyon 2018, 4, e00632. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Ayenehdeh, J.; Niknam, B.; Rasouli, S.; Hashemi, S.M.; Rahavi, H.; Rezaei, N.; Soleimani, M.; Liaeiha, A.; Niknam, M.H.; Tajik, N. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol. Lett. 2017, 188, 21–31. [Google Scholar] [CrossRef]
- Cavallari, G.; Olivi, E.; Bianchi, F.; Neri, F.; Foroni, L.; Valente, S.; La Manna, G.; Nardo, B.; Stefoni, S.; Ventura, C. Mesenchymal stem cells and islet cotransplantation in diabetic rats: Improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012, 21, 2771–2781. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jung, M.J.; Shin, J.Y.; La, W.G.; Hwang, Y.H.; Kim, M.J.; Kim, B.S.; Lee, D.Y. Mutual effect of subcutaneously transplanted human adipose-derived stem cells and pancreatic islets within fibrin gel. Biomaterials 2013, 34, 7247–7256. [Google Scholar] [CrossRef]
- Arzouni, A.A.; Vargas-Seymour, A.; Rackham, C.L.; Dhadda, P.; Huang, G.C.; Choudhary, P.; Nardi, N.; King, A.J.F.; Jones, P.M. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. (Lond) 2017, 131, 2835–2845. [Google Scholar] [CrossRef]
- Golocheikine, A.; Tiriveedhi, V.; Angaswamy, N.; Benshoff, N.; Sabarinathan, R.; Mohanakumar, T. Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 2010, 90, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Rahavi, H.; Hashemi, S.M.; Soleimani, M.; Mohammadi, J.; Tajik, N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J. Diabetes Res. 2015, 2015, 878535. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, Z.; Kim, D.S.; Gou, W.; Strange, C.; Dong, H.; Cui, W.; Gilkeson, G.; Morgan, K.A.; Adams, D.B.; et al. Adipose stem cells from chronic pancreatitis patients improve mouse and human islet survival and function. Stem Cell Res. Ther. 2017, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shimada, M.; Utsunomiya, T.; Ikemoto, T.; Saito, Y.; Morine, Y.; Imura, S.; Mori, H.; Arakawa, Y.; Kanamoto, M.; et al. Trophic effect of adipose tissue-derived stem cells on porcine islet cells. J. Surg. Res. 2014, 187, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 31. [Google Scholar] [CrossRef] [PubMed]
- Rackham, C.L.; Dhadda, P.K.; Le Lay, A.M.; King, A.J.; Jones, P.M. Preculturing Islets With Adipose-Derived Mesenchymal Stromal Cells Is an Effective Strategy for Improving Transplantation Efficiency at the Clinically Preferred Intraportal Site. Cell Med. 2014, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, D.; Brandhorst, H.; Acreman, S.; Schive, S.W.; Bjornson Scholz, H.; Johnson, P.R.V. Hypoxia-Induced Damage in Human Islets Is Reduced With the Use of Mesenchymal Stem Cell-Preconditioned Medium. Transplant. Proc. 2017, 49, 2330–2332. [Google Scholar] [CrossRef]
- Schive, S.W.; Mirlashari, M.R.; Hasvold, G.; Wang, M.; Josefsen, D.; Gullestad, H.P.; Korsgren, O.; Foss, A.; Kvalheim, G.; Scholz, H. Human Adipose-Derived Mesenchymal Stem Cells Respond to Short-Term Hypoxia by Secreting Factors Beneficial for Human Islets In Vitro and Potentiate Antidiabetic Effect In Vivo. Cell Med. 2017, 9, 103–116. [Google Scholar] [CrossRef]
- Imamura, H.; Adachi, T.; Kin, T.; Ono, S.; Sakai, Y.; Adachi, T.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Shapiro, A.M.J.; et al. An engineered cell sheet composed of human islets and human fibroblast, bone marrow-derived mesenchymal stem cells, or adipose-derived mesenchymal stem cells: An in vitro comparison study. Islets 2018, 10, e1445948. [Google Scholar] [CrossRef]
- Prunet-Marcassus, B.; Cousin, B.; Caton, D.; Andre, M.; Penicaud, L.; Casteilla, L. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp. Cell Res. 2006, 312, 727–736. [Google Scholar] [CrossRef]
- Padoin, A.V.; Braga-Silva, J.; Martins, P.; Rezende, K.; Rezende, A.R.; Grechi, B.; Gehlen, D.; Machado, D.C. Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast. Reconstr. Surg. 2008, 122, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, W.J.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; Zandiehdoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.; van Milligen, F.J. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef]
- Kim, B.; Lee, B.; Kim, M.K.; Gong, S.P.; Park, N.H.; Chung, H.H.; Kim, H.S.; No, J.H.; Park, W.Y.; Park, A.K.; et al. Gene expression profiles of human subcutaneous and visceral adipose-derived stem cells. Cell Biochem. Funct. 2016, 34, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded mesenchymal stem cells for regenerative medicine: Yield in stromal vascular fraction from adipose tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgro, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Trojahn Kolle, S.F.; Oliveri, R.S.; Glovinski, P.V.; Elberg, J.J.; Fischer-Nielsen, A.; Drzewiecki, K.T. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J. Plast. Surg. Hand. Surg. 2012, 46, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Knapinska, A.M.; Amar, S.; He, Z.; Matosevic, S.; Zylberberg, C.; Fields, G.B. Matrix metalloproteinases as reagents for cell isolation. Enzyme Microb. Technol. 2016, 93-94, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Skurk, T.; Rohrig, K.; Lee, Y.M.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D. Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering. Cell Transplant. 2017, 26, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef] [PubMed]
- El-Ftesi, S.; Chang, E.I.; Longaker, M.T.; Gurtner, G.C. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast. Reconstr. Surg. 2009, 123, 475–485. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Lopa, S.; Arrigoni, E.; Sartori, M.F.; Baruffaldi Preis, F.W.; Brini, A.T. Human adipose-derived stem cells isolated from young and elderly women: Their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy 2009, 11, 793–803. [Google Scholar] [CrossRef]
- Sozer, S.O.; Basaran, K.; Alim, H. Abdominoplasty with Circumferential Liposuction: A Review of 1000 Consecutive Cases. Plast. Reconstr. Surg. 2018, 142, 891–901. [Google Scholar] [CrossRef]
- Perez, L.M.; Bernal, A.; de Lucas, B.; San Martin, N.; Mastrangelo, A.; Garcia, A.; Barbas, C.; Galvez, B.G. Altered metabolic and stemness capacity of adipose tissue-derived stem cells from obese mouse and human. PloS ONE 2015, 10, e0123397. [Google Scholar] [CrossRef]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef]
- Oliva-Olivera, W.; Moreno-Indias, I.; Coin-Araguez, L.; Lhamyani, S.; Alcaide Torres, J.; Fernandez-Veledo, S.; Vendrell, J.; Camargo, A.; El Bekay, R.; Tinahones, F.J. Different response to hypoxia of adipose-derived multipotent cells from obese subjects with and without metabolic syndrome. PloS ONE 2017, 12, e0188324. [Google Scholar] [CrossRef]
- Rennert, R.C.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Koci, Z.; Turnovcova, K.; Dubsky, M.; Baranovicova, L.; Holan, V.; Chudickova, M.; Sykova, E.; Kubinova, S. Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient’s distal limbs with critical ischemia. Cell Biochem. Funct. 2014, 32, 597–604. [Google Scholar] [CrossRef]
- Yaochite, J.N.; De Lima, K.W.; Caliari-Oliveira, C.; Palma, P.V.; Couri, C.E.; Simoes, B.P.; Covas, D.T.; Voltarelli, J.C.; Oliveira, M.C.; Donadi, E.A.; et al. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res. Ther. 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lin, Y.; Hauschka, P.V.; Grottkau, B.E. Adipose stem cells originate from perivascular cells. Biol. Cell 2011, 103, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Carrion, B.; Huang, C.P.; Ghajar, C.M.; Kachgal, S.; Kniazeva, E.; Jeon, N.L.; Putnam, A.J. Recreating the perivascular niche ex vivo using a microfluidic approach. Biotechnol. Bioeng. 2010, 107, 1020–1028. [Google Scholar] [CrossRef]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef]
- Maumus, M.; Peyrafitte, J.A.; D’Angelo, R.; Fournier-Wirth, C.; Bouloumie, A.; Casteilla, L.; Sengenes, C.; Bourin, P. Native human adipose stromal cells: Localization, morphology and phenotype. Int. J. Obes. (Lond) 2011, 35, 1141–1153. [Google Scholar] [CrossRef]
- Prockop, D.J.; Brenner, M.; Fibbe, W.E.; Horwitz, E.; Le Blanc, K.; Phinney, D.G.; Simmons, P.J.; Sensebe, L.; Keating, A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010, 12, 576–578. [Google Scholar] [CrossRef]
- Yoshida, Y.; Naito, M.; Yamada, T.; Aisu, N.; Kojima, D.; Mera, T.; Tanaka, T.; Naito, K.; Yasumoto, K.; Kamigaki, T.; et al. Clinical Study on the Medical Value of Combination Therapy Involving Adoptive Immunotherapy and Chemotherapy for Stage IV Colorectal Cancer (COMVI Study). Anticancer. Res. 2017, 37, 3941–3946. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PloS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.C.; Bui, K.H.; Van Pham, P. Characterization of Senescence of Human Adipose-Derived Stem Cells After Long-Term Expansion. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Schumacher, A.; Filipowicz, N.; Wardowska, A.; Zielinski, M.; Madanecki, P.; Nowicka, E.; Langa, P.; Deptula, M.; Zielinski, J.; et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci. Rep. 2018, 8, 11339. [Google Scholar] [CrossRef] [PubMed]
- Froelich, K.; Mickler, J.; Steusloff, G.; Technau, A.; Ramos Tirado, M.; Scherzed, A.; Hackenberg, S.; Radeloff, A.; Hagen, R.; Kleinsasser, N. Chromosomal aberrations and deoxyribonucleic acid single-strand breaks in adipose-derived stem cells during long-term expansion in vitro. Cytotherapy 2013, 15, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Rubio, D.; Garcia-Castro, J.; Martin, M.C.; de la Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous human adult stem cell transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, C.; Vaegler, M.; Gieseke, F.; Pfister, S.M.; Handgretinger, R.; Kerst, G.; Muller, I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010, 11, 11. [Google Scholar] [CrossRef]
- Nava, M.B.; Catanuto, G.; Pennati, A.E.; Rocco, N.; Spano, A.; Villa, R.; Daidone, M. Lack of activation of telomere maintenance mechanisms in human adipose stromal cells derived from fatty portion of lipoaspirates. Plast. Reconstr. Surg. 2015, 135, 114e–123e. [Google Scholar] [CrossRef]
- Lu, J.H.; Wei, H.J.; Peng, B.Y.; Chou, H.H.; Chen, W.H.; Liu, H.Y.; Deng, W.P. Adipose-Derived Stem Cells Enhance Cancer Stem Cell Property and Tumor Formation Capacity in Lewis Lung Carcinoma Cells Through an Interleukin-6 Paracrine Circuit. Stem Cells Dev. 2016, 25, 1833–1842. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Fornoff, F.; Adjan, M.; Solbach, C.; Yuan, J.; Louwen, F. Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget 2015, 6, 34475–34493. [Google Scholar] [CrossRef]
- Zhang, Y.; Nowicka, A.; Solley, T.N.; Wei, C.; Parikh, A.; Court, L.; Burks, J.K.; Andreeff, M.; Woodward, W.A.; Dadbin, A.; et al. Stromal Cells Derived from Visceral and Obese Adipose Tissue Promote Growth of Ovarian Cancers. PloS ONE 2015, 10, e0136361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Wang, L.; Liu, G.; Li, Y.; Wu, X.; Jing, Y.; Li, H.; Wang, G. Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell Biosci. 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Vanikar, A.V.; Dave, S.D.; Thakkar, U.G.; Trivedi, H.L. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010, 2010, 582382. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, U.G.; Trivedi, H.L.; Vanikar, A.V.; Dave, S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 2015, 17, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nishinakamura, H.; Kumano, K.; Takahashi, H.; Kodama, S. The Spleen Is an Ideal Site for Inducing Transplanted Islet Graft Expansion in Mice. PloS ONE 2017, 12, e0170899. [Google Scholar] [CrossRef] [PubMed]
- Yaochite, J.N.; Caliari-Oliveira, C.; de Souza, L.E.; Neto, L.S.; Palma, P.V.; Covas, D.T.; Malmegrim, K.C.; Voltarelli, J.C.; Donadi, E.A. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res. Ther. 2015, 6, 31. [Google Scholar] [CrossRef]
- Comella, K.; Parcero, J.; Bansal, H.; Perez, J.; Lopez, J.; Agrawal, A.; Ichim, T. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. J. Transl. Med. 2016, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef]
- De la Portilla, F.; Alba, F.; Garcia-Olmo, D.; Herrerias, J.M.; Gonzalez, F.X.; Galindo, A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int. J. Colorectal. Dis. 2013, 28, 313–323. [Google Scholar] [CrossRef]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Tran, T.D.; Nguyen, H.T.; Vu, H.T.; Le, P.T.; Phan, N.L.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Collawn, S.S.; Banerjee, N.S.; de la Torre, J.; Vasconez, L.; Chow, L.T. Adipose-derived stromal cells accelerate wound healing in an organotypic raft culture model. Ann. Plast. Surg. 2012, 68, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.L.; Vanikar, A.V.; Thakker, U.; Firoze, A.; Dave, S.D.; Patel, C.N.; Patel, J.V.; Bhargava, A.B.; Shankar, V. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant. Proc. 2008, 40, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.L.; Thakkar, U.G.; Vanikar, A.V.; Dave, S.D. Treatment of polyglandular autoimmune syndrome type 3 using co-transplantation of insulin-secreting mesenchymal stem cells and haematopoietic stem cells. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.D.; Vanikar, A.V.; Trivedi, H.L. Co-infusion of adipose tissue derived mesenchymal stem cell-differentiated insulin-making cells and haematopoietic cells with renal transplantation: A novel therapy for type 1 diabetes mellitus with end-stage renal disease. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, U.G.; Vanikar, A.V.; Trivedi, H.L. Co-infusion of autologous adipose tissue derived insulin-secreting mesenchymal stem cells and bone marrow derived hematopoietic stem cells: Viable therapy for type III.C. a diabetes mellitus. Biomed. J. 2013, 36, 304–307. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00703599?term=NCT00703599&rank=1 (accessed on 20 June 2018).
- Dave, S.D.; Trivedi, H.L.; Gopal, S.C.; Chandra, T. Combined therapy of insulin-producing cells and haematopoietic stem cells offers better diabetic control than only haematopoietic stem cells’ infusion for patients with insulin-dependent diabetes. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Ikemoto, T.; Feng, R.; Shimada, M.; Saito, Y.; Iwahashi, S.; Morine, Y.; Imura, S. A New 2-Step Acceleration Protocol Using a Histone Deacetylase Inhibitor to Generate Insulin-Producing Cells From Adipose-Derived Mesenchymal Stem Cells. Pancreas 2018, 47, 477–481. [Google Scholar] [CrossRef]
- Takeyama, H.; Mizushima, T.; Uemura, M.; Haraguchi, N.; Nishimura, J.; Hata, T.; Matsuda, C.; Takemasa, I.; Ikenaga, M.; Murata, K.; et al. Adipose-Derived Stem Cells Ameliorate Experimental Murine Colitis via TSP-1-Dependent Activation of Latent TGF-beta. Dig. Dis. Sci. 2017, 62, 1963–1974. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mauricio, G.; Moscoso, I.; Martin-Cancho, M.F.; Crisostomo, V.; Prat-Vidal, C.; Baez-Diaz, C.; Sanchez-Margallo, F.M.; Bernad, A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res. Ther. 2016, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Kim, B.J.; Arai, Y.; Han, I.B.; Moon, J.J.; Paulmurugan, R.; Park, H.; Lee, S.H. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials 2018, 185, 360–370. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Ref. | Donor of ADMSCs Species | Source | Procedure of Differentiation or Transplantation | Outcomes |
|---|---|---|---|---|---|---|
| Timper | (2007) | [74] | Human | Uncertain |
|
|
| Okura | (2009) | [75] | Human | Omentum |
|
|
| Kang | (2009) | [76] | Human | Eyelid |
|
|
| Kajiyama | (2010) | [77] | Mice | Inguinal fat |
|
|
| Chandra | (2011) | [78] | Human | Abdomen |
|
|
| Kim | (2012) | [79] | Human | Uncertain |
|
|
| Lee | (2013) | [80] | Human | Abdomen |
|
|
| Nam | (2014) | [81] | Human | Eyelid |
|
|
| Sun | (2017) | [82] | Human | Uncertain |
|
|
| Amer | (2018) | [83] | Rat | Abdomen |
|
|
| Authors | Year | Ref. | Donor of ADMSCs Species | Source | Number | Donor of Islets Species | Number | Procedure of Transplantation | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ohmura | (2010) | [105] | Mice | Inguinal fat | 2 × 105 cells | Mice | 200 islets |
|
|
| Cavallari | (2012) | [108] | Human | Subcutaneous fat | 2.5 × 104 cells | Rat | 500 islets |
|
|
| Karaoz | (2013) | [95] | Rat | Peritoneal fat | 1.0 × 106 cells | Rat | 500 islets |
|
|
| Bhang | (2013) | [109] | Human | Uncertain | 8 × 105 cells | Rat | 800 islets |
|
|
| Mohammadi | (2017) | [107] | Mice (C57BL/6) | Abdomen | 2 × 105 cells | Mice (BALB/c) | 200 islets |
|
|
| Song | (2017) | [113] | Human | Abdomen | 1 × 104 cells | Mice | 125–150 islets |
|
|
| Navaei | (2018) | [98] | Human | Epididymal | 6 × 106 cells | Rat | 1000 IEQs |
|
|
| Tanaka | (2018) | [106] | Mice | Inguinal fat | 1.0 × 105, 5.0 × 105 or 1.0 × 106 cells | Mice | 50 islets |
|
|
| Authors/(Year) | Number of Patients | Age | year | Disease Duration/(year) | Number of ADMSCs | Pre/Post Infusion | C-peptide/(ng/mL) | HbA1c/(%) | Insulin Requirement/(Units/day) | Follow-Up/(Months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanikar [163]/(2010) | 11 | 21.1 | (13–43) | 8.2/(1–24) | 3.0 × 106 | Pre | 0.10/(0.02–0.30) | 8.47/(6.22–10.30) | 1.14/kg BW/(0.42–2.10) | 7.3/(2.2–12.0) | |
| Post | 0.37/(0.1–1.8) | 7.39/(5.72–8.98) | 0.63/kg BW/(0.09–1.00) | ||||||||
| Thakkar [164]/(2015) | Auto- | 10 | 20.20 ± 6.90 | 8.1 ± 3.4 | 2.7 ± 0.8 × 102 (/μL) × 103.1 ± 28.3 (mL) | Pre | 0.220 ± 0.210 | 10.99 ± 2.10 | 63.90 ± 20.95 | 33.10 ± 18.38 | |
| Post (2y) | 0.930 ± 0.240 | 7.75 ± 1.05 | 39.66 ± 9.37 | ||||||||
| Allo- | 10 | 19.70 ± 9.96 | 9.9 ± 7.1 | 2.1 ± 0.7 × 102 (/μL) × 95.3 ± 14.2 (mL) | Pre | 0.028 ± 0.010 | 11.93 ± 1.90 | 57.55 ± 21.82 | 54.24 ± 15.75 | ||
| Post (2y) | 0.460 ± 0.290 | 8.01 ± 1.04 | 38.50 ± 13.34 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, H.; Sakata, N.; Yoshimatsu, G.; Hasegawa, S.; Kodama, S. Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. J. Clin. Med. 2019, 8, 249. https://doi.org/10.3390/jcm8020249
Takahashi H, Sakata N, Yoshimatsu G, Hasegawa S, Kodama S. Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. Journal of Clinical Medicine. 2019; 8(2):249. https://doi.org/10.3390/jcm8020249
Chicago/Turabian StyleTakahashi, Hiroyuki, Naoaki Sakata, Gumpei Yoshimatsu, Suguru Hasegawa, and Shohta Kodama. 2019. "Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus" Journal of Clinical Medicine 8, no. 2: 249. https://doi.org/10.3390/jcm8020249
APA StyleTakahashi, H., Sakata, N., Yoshimatsu, G., Hasegawa, S., & Kodama, S. (2019). Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue-Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. Journal of Clinical Medicine, 8(2), 249. https://doi.org/10.3390/jcm8020249





