Prediction of the Outcome of Cochlear Implantation in the Patients with Congenital Cytomegalovirus Infection based on Magnetic Resonance Imaging Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Audiological Evaluation
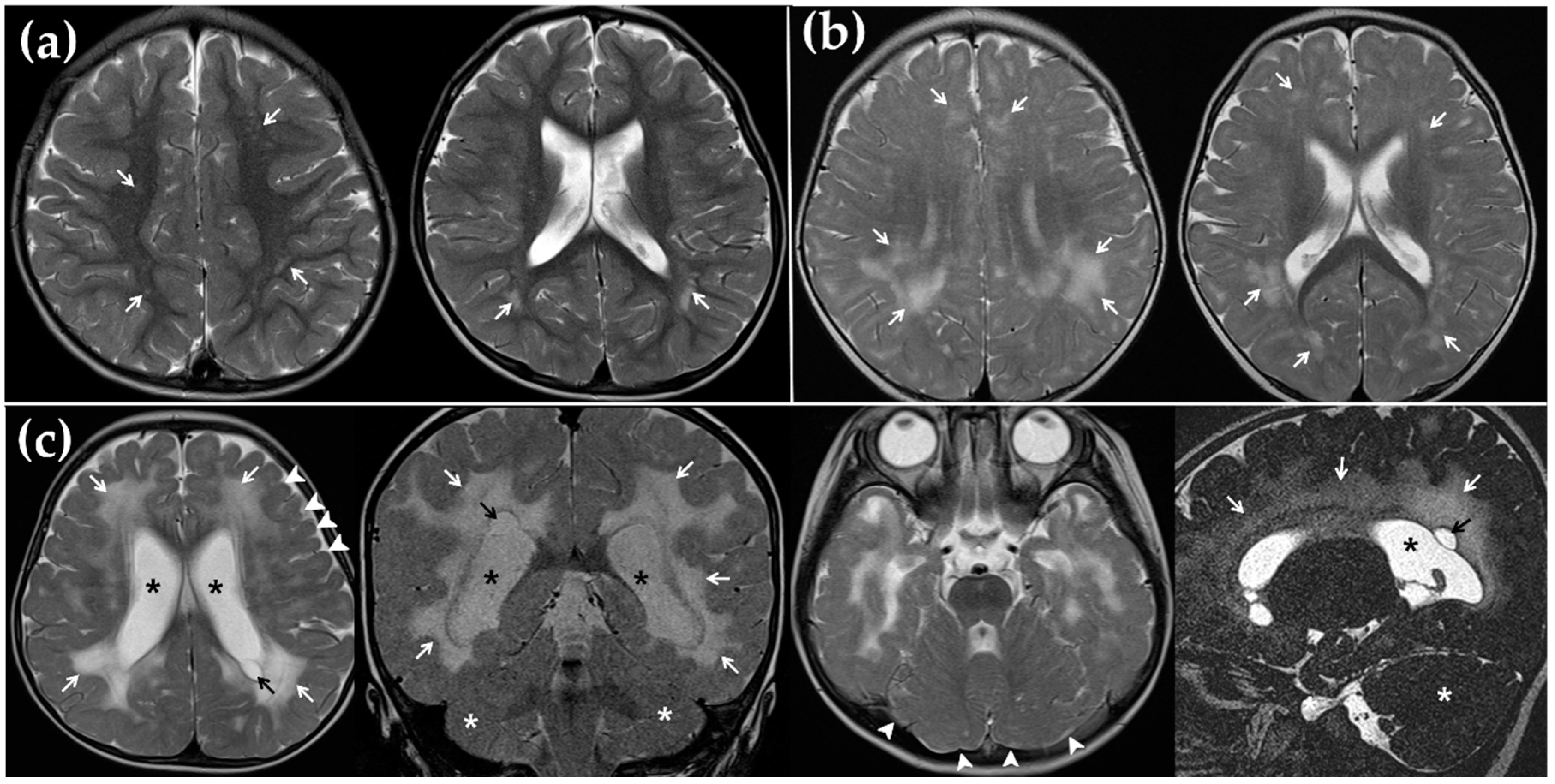
2.3. Magnetic Resonance Imaging (MRI)
2.4. Statistical Analysis
3. Results
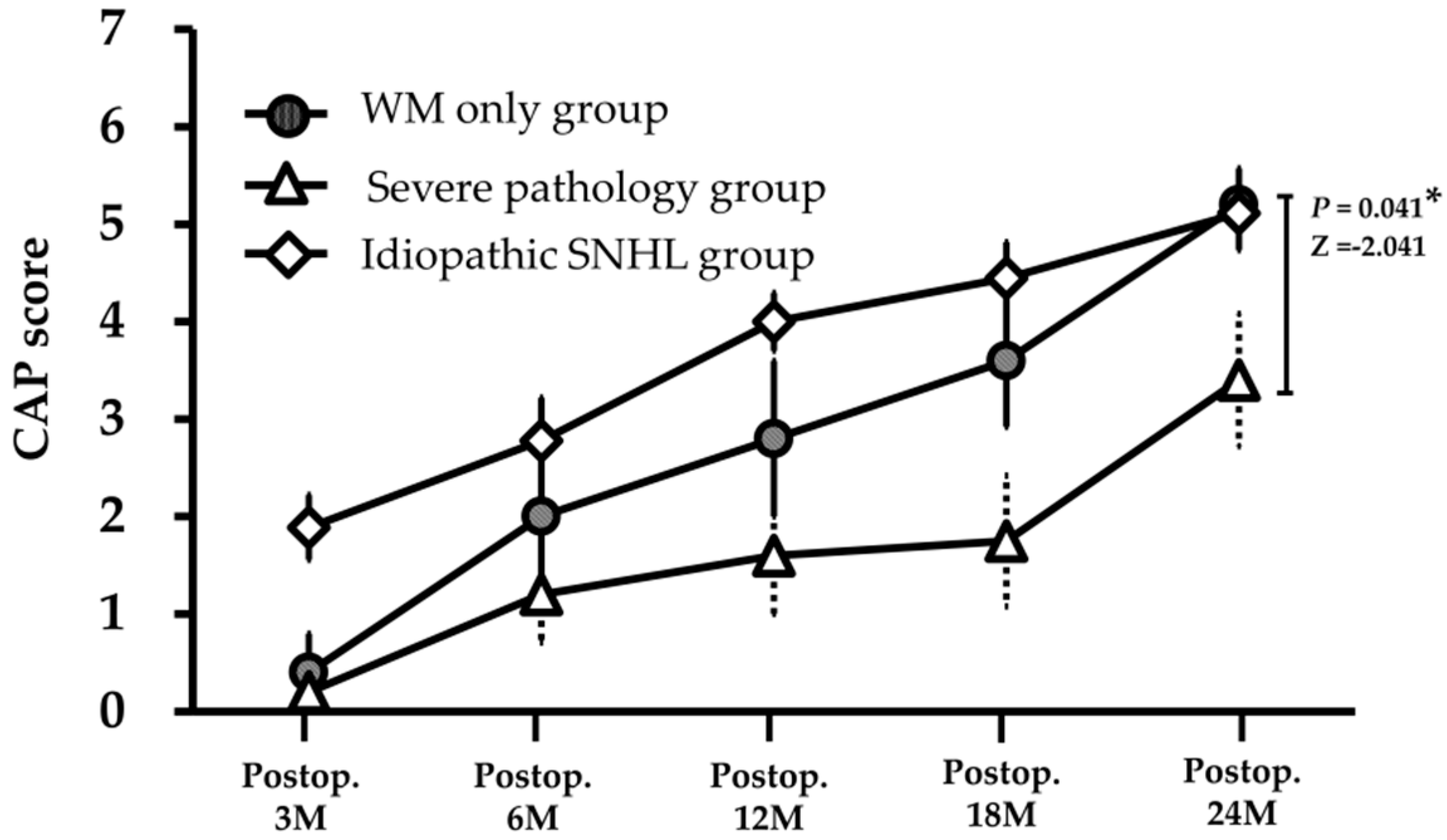
3.1. Speech Perception after CI
3.2. MRI Findings and Its Correlation with CI Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kenneson, A.; Cannon, M.J. Review and Meta-Analysis of the Epidemiology of Congenital Cytomegalovirus (Cmv) Infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Peckham, C.S.; Stark, O.; Dudgeon, J.A.; Martin, J.A.; Hawkins, G. Congenital Cytomegalovirus Infection: A Cause of Sensorineural Hearing Loss. Arch. Dis. Child. 1987, 62, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Pass, R.F.; Britt, W.J.; Stagno, S.; Alford, C.A. Symptomatic Congenital Cytomegalovirus Infection: Neonatal Morbidity and Mortality. Pediatr. Infect. Dis. J. 1992, 11, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.B.; Boppana, S.B.; Fowler, K.B.; Britt, W.J.; Stagno, S.; Pass, R.F. Predictors of Hearing Loss in Children with Symptomatic Congenital Cytomegalovirus Infection. Pediatrics 2002, 110, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; McCollister, F.P.; Dahle, A.J.; Boppana, S.; Britt, W.J.; Pass, R.F. Progressive and Fluctuating Sensorineural Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus Infection. J. Pediatr. 1997, 130, 624–630. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Williamson, W.D.; Demmler, G.J.; Percy, A.K.; Catlin, F.I. Progressive Hearing Loss in Infants with Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 1992, 90, 862–866. [Google Scholar]
- Kim, B.J.; Han, J.J.; Shin, S.H.; Kim, H.S.; Yang, H.R.; Choi, E.H.; Chang, M.Y.; Lee, S.Y.; Suh, M.W.; Koo, J.W.; et al. Characterization of Detailed Audiological Features of Cytomegalovirus Infection: A Composite Cohort Study from Groups with Distinct Demographics. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef]
- Ramirez Inscoe, J.M.; Nikolopoulos, T.P. Cochlear Implantation in Children Deafened by Cytomegalovirus: Speech Perception and Speech Intelligibility Outcomes. Otol. Neurotol. 2004, 25, 479–482. [Google Scholar] [CrossRef]
- Iwasaki, S.; Nakanishi, H.; Misawa, K.; Tanigawa, T.; Mizuta, K. Cochlear Implant in Children with Asymptomatic Congenital Cytomegalovirus Infection. Audiol. Neurootol. 2009, 14, 146–152. [Google Scholar] [CrossRef]
- Matsui, T.; Ogawa, H.; Yamada, N.; Baba, Y.; Suzuki, Y.; Nomoto, M.; Suzutani, T.; Inoue, N.; Omori, K. Outcome of Cochlear Implantation in Children with Congenital Cytomegalovirus Infection or Gjb2 Mutation. Acta Otolaryngol. 2012, 132, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.; Maes, L.K.; Keppler, H.; Dhooge, I. Cochlear Implants in Children Deafened by Congenital Cytomegalovirus and Matched Connexin 26 Peers. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Bovo, R.; Trevisi, P.; Bianchini, C.; Arboretti, R.; Martini, A. Rehabilitation and Outcome of Severe Profound Deafness in a Group of 16 Infants Affected by Congenital Cytomegalovirus Infection. Eur. Arch. Otorhinolaryngol. 2009, 266, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Bruce, I.A.; Broomfield, S.J.; Henderson, L.; Green, K.M.; Ramsden, R.T. Outcome of Cochlear Implantation in Asymptomatic Congenital Cytomegalovirus Deafened Children. Laryngoscope 2011, 121, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lustig, L.; Sampson, M.; Chinnici, J.; Niparko, J.K. Effects of Cytomegalovirus (Cmv) Related Deafness on Pediatric Cochlear Implant Outcomes. Otolaryngol. Head Neck Surg. 2005, 133, 900–905. [Google Scholar] [CrossRef]
- Viccaro, M.; Filipo, R.; Bosco, E.; Nicastri, M.; Mancini, P. Long-Term Follow-up of Implanted Children with Cytomegalovirus-Related Deafness. Audiol. Neurootol. 2012, 17, 395–399. [Google Scholar] [CrossRef]
- Yoshida, H.; Takahashi, H.; Kanda, Y.; Kitaoka, K.; Hara, M. Long-Term Outcomes of Cochlear Implantation in Children with Congenital Cytomegalovirus Infection. Otol. Neurotol. 2017, 38, e190–e194. [Google Scholar] [CrossRef]
- Laccourreye, L.; Ettienne, V.; Prang, I.; Couloigner, V.; Garabedian, E.N.; Loundon, N. Speech Perception, Production and Intelligibility in French-Speaking Children with Profound Hearing Loss and Early Cochlear Implantation after Congenital Cytomegalovirus Infection. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 317–320. [Google Scholar] [CrossRef]
- Lyutenski, S.; Gotz, F.; Giourgas, A.; Majdani, O.; Bultmann, E.; Lanfermann, H.; Lenarz, T.; Giesemann, A.M. Does Severity of Cerebral Mri Lesions in Congenital Cmv Infection Correlates with the Outcome of Cochlear Implantation? Eur. Arch. Otorhinolaryngol. 2017, 274, 1397–1403. [Google Scholar] [CrossRef]
- Archbold, S.; Lutman, M.E.; Marshall, D.H. Categories of Auditory Performance. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 166, 312–314. [Google Scholar]
- van der Knaap, M.S.; Vermeulen, G.; Barkhof, F.; Hart, A.A.; Loeber, J.G.; Weel, J.F. Pattern of White Matter Abnormalities at Mr Imaging: Use of Polymerase Chain Reaction Testing of Guthrie Cards to Link Pattern with Congenital Cytomegalovirus Infection. Radiology 2004, 230, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Cannie, M.M.; Devlieger, R.; Leyder, M.; Claus, F.; Leus, A.; de Catte, L.; Cossey, V.; Foulon, I.; van der Valk, E.; Foulon, W.; et al. Congenital Cytomegalovirus Infection: Contribution and Best Timing of Prenatal Mr Imaging. Eur. Radiol. 2016, 26, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- Manara, R.; Balao, L.; Baracchini, C.; Drigo, P.; D’Elia, R.; Ruga, E.M. Brain Magnetic Resonance Findings in Symptomatic Congenital Cytomegalovirus Infection. Pediatr. Radiol. 2011, 41, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Issakainen, J.; Kewitz, G.; Kikinis, R.; Martin, E.; Boltshauser, E. Magnetic Resonance Imaging of the Brain in Congenital Cytomegalovirus Infection. Pediatr. Radiol. 1989, 19, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. White Matter in Learning, Cognition and Psychiatric Disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; van der Knaap, M.S. An Mri-Based Approach to the Diagnosis of White Matter Disorders. Neurology 2009, 72, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Woodward, L.J.; Clark, C.A.; Bora, S.; Inder, T.E. Neonatal White Matter Abnormalities an Important Predictor of Neurocognitive Outcome for Very Preterm Children. PLoS ONE 2012, 7, e51879. [Google Scholar] [CrossRef]
- Skiold, B.; Vollmer, B.; Bohm, B.; Hallberg, B.; Horsch, S.; Mosskin, M.; Lagercrantz, H.; Aden, U.; Blennow, M. Neonatal Magnetic Resonance Imaging and Outcome at Age 30 Months in Extremely Preterm Infants. J. Pediatr. 2012, 160, 559–566. [Google Scholar] [CrossRef]
- Woodward, L.J.; Anderson, P.J.; Austin, N.C.; Howard, K.; Inder, T.E. Neonatal Mri to Predict Neurodevelopmental Outcomes in Preterm Infants. N. Engl. J. Med. 2006, 355, 685–694. [Google Scholar] [CrossRef]
- Fazekas, F.; Barkhof, F.; Wahlund, L.O.; Pantoni, L.; Erkinjuntti, T.; Scheltens, P.; Schmidt, R. Ct and Mri Rating of White Matter Lesions. Cerebrovasc. Dis. 2002, 13, 31–36. [Google Scholar] [CrossRef]
- Suzuki, Y.; Toribe, Y.; Mogami, Y.; Yanagihara, K.; Nishikawa, M. Epilepsy in Patients with Congenital Cytomegalovirus Infection. Brain Dev. 2008, 30, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, V.M.; Dakovic, I.; Duranovic, V.; Lujic, L.; Krakar, G.; Marn, B. Malformations of Cortical Development in Children with Congenital Cytomegalovirus Infection—A Study of Nine Children with Proven Congenital Cytomegalovirus Infection. Coll. Antropol. 2011, 35, 229–234. [Google Scholar] [PubMed]
- Pyman, B.; Blamey, P.; Lacy, P.; Clark, G.; Dowell, R. The Development of Speech Perception in Children Using Cochlear Implants: Effects of Etiologic Factors and Delayed Milestones. Am. J. Otol. 2000, 21, 57–61. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bonasoni, M.P.; Santini, D.; Piccirilli, G.; Chiereghin, A.; Guerra, B.; Landini, M.P.; Capretti, M.G.; Lanari, M.; Lazzarotto, T. Human Fetal Inner Ear Involvement in Congenital Cytomegalovirus Infection. Acta Neuropathol. Commun. 2013, 1, 63. [Google Scholar] [CrossRef]
- Katano, H.; Sato, Y.; Tsutsui, Y.; Sata, T.; Maeda, A.; Nozawa, N.; Inoue, N.; Nomura, Y.; Kurata, T. Pathogenesis of Cytomegalovirus-Associated Labyrinthitis in a Guinea Pig Model. Microbes Infect. 2007, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Yoshikawa, T.; Nishiyama, Y.; Morishita, Y.; Sato, E.; Hattori, T.; Nakashima, T. Detection of Human Cytomegalovirus DNA in Perilymph of Patients with Sensorineural Hearing Loss Using Real-Time Pcr. J. Med. Virol. 2003, 69, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.W.; Parizi-Robinson, M.; Roland, P.S.; Yegappan, S. Cytomegalovirus in the Perilymphatic Fluid. Laryngoscope 2005, 115, 223–225. [Google Scholar] [CrossRef]
- Wang, Y.; Patel, R.; Ren, C.; Taggart, M.G.; Firpo, M.A.; Schleiss, M.R.; Park, A.H. A Comparison of Different Murine Models for Cytomegalovirus-Induced Sensorineural Hearing Loss. Laryngoscope 2013, 123, 2801–2806. [Google Scholar] [CrossRef]
- Carraro, M.; Almishaal, A.; Hillas, E.; Firpo, M.; Park, A.; Harrison, R.V. Cytomegalovirus (Cmv) Infection Causes Degeneration of Cochlear Vasculature and Hearing Loss in a Mouse Model. J. Assoc. Res. Otolaryngol. 2017, 18, 263–273. [Google Scholar] [CrossRef]
- Fortunato, E.A.; Dell’Aquila, M.L.; Spector, D.H. Specific Chromosome 1 Breaks Induced by Human Cytomegalovirus. Proc. Natl. Acad. Sci. USA 2000, 97, 853–858. [Google Scholar] [CrossRef]
- Nystad, M.; Fagerheim, T.; Brox, V.; Fortunato, E.A.; Nilssen, O. Human Cytomegalovirus (Hcmv) and Hearing Impairment: Infection of Fibroblast Cells with Hcmv Induces Chromosome Breaks at 1q23.3, between Loci Dfna7 and Dfna49—Both Involved in Dominantly Inherited, Sensorineural, Hearing Impairment. Mutat. Res. 2008, 637, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.H.; Hannemann, H.; Kulkarni, A.S.; Schwartz, P.H.; O’Dowd, J.M.; Fortunato, E.A. Human Cytomegalovirus Infection Causes Premature and Abnormal Differentiation of Human Neural Progenitor Cells. J. Virol. 2010, 84, 3528–3541. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kosugi, I.; Meguro, S.; Iwashita, T. Pathogenesis of Developmental Anomalies of the Central Nervous System Induced by Congenital Cytomegalovirus Infection. Pathol. Int. 2017, 67, 72–82. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | Sex | UNHS Rt/Lt | Hearing Thresholds † Rt/Lt (dB) | Age at 1st CI (year) | Age at 2nd CI (year) | Deaf Duration (years) | DD | CAP Score | White Matter Involvement | Myelination Delay | Ventriculomegaly | Hippocampal Dysplasia | Cerebellar Hypoplasia | Migration Disorder | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 0.5 | 1 | 2 | 3 | Distribution | Periventricular | Deep WM | Arcuate Fiber | Frontal Lobe | Parietal Lobe | Occipital Lobe | Temporal Lobe | |||||||||||||
| Severe pathology group | |||||||||||||||||||||||||
| CH-1 | M | R/R | 85/90 | 3.0 | 3.0 | ● | 0 | 1 | 1 | 1 | 1 | Df | ● | - | - | - | ● | - | - | ● | ● | - | ● | - | |
| CH-2 | M | R/R | 115/110 | 1.2 | 2.4 | 1.2 | - | 0 | 1 | 2 | 3 | Ex | ● | ● | ● | ● | ● | - | ● | - | ● | - | - | ● | |
| CH-3 | F | R/R | 100/100 | 1.8 | 1.8 | ● | 0 | 1 | 1 | 4 | 4 | Df | ● | ● | ● | ● | ● | ● | ● | ● | ● | - | ● | ● | |
| CH-5 | M | R/R | 115/115 | 1.2 | 2.3 | 1.2 | ● | 0 | 1 | 1 | 4 | 4 | Ex | ● | ● | - | ● | ● | - | - | - | ● | - | - | - |
| CH-8 | M | R/R | 100/110 | 1.3 | 1.3 | 1.3 | - | 1 | 4 | 4 | 5 | Mf | ● | ● | - | ● | ● | ● | ● | - | ● | - | - | - | |
| Mild pathology group | |||||||||||||||||||||||||
| CH-4 | M | P/P | 100/100 | 2.6 | 0.6 | - | 0 | 0 | 1 | 4 | Mf | - | ● | - | ● | ● | - | - | - | - | - | - | - | ||
| CH-6 | M | R/R | 100/110 | 1.2 | 1.2 | - | 0 | 2 | 4 | 5 | 6 | Mf | ● | - | - | - | ● | - | - | - | - | - | - | - | |
| CH-7 | M | R/R | 85/100 | 2.0 | 2.0 | 2.0. | - | 0 | 4 | 5 | 5 | Ex | ● | ● | ● | ● | ● | ● | ● | - | - | - | - | - | |
| CH-9 | M | R/R | 100/100 | 1.1 | 1.1 | 1.1 | - | 0 | 4 | 4 | 6 | 7 | Ex | ● | ● | ● | ● | ● | ● | ● | - | - | - | - | - |
| CH-10 | F | R/R | 95/105 | 6.4 | 12.4 | 6.4 | - | 2 | 4 | 4 | 6 | none | - | - | - | - | - | - | - | - | - | - | - | - | |
| Abnormal Findings | Involved Area | Ratio of Positive Finding | Positive (CAP Score) | Negative (CAP Score) | Z | P |
|---|---|---|---|---|---|---|
| Involvement of WM abnormality | Periventricular | 7/9 (78%) | 4.6 ± 1.0 | 5.0 ± 1.4 | −0.457 | 0.648 |
| Deep WM | 7/9 (78%) | 4.4 ± 1.0 | 5.5 ± 0.7 | −1.370 | 0.171 | |
| Arcuate fiber | 4/9 (44%) | 4.5 ± 1.3 | 4.8 ± 0.8 | −0.382 | 0.702 | |
| Frontal lobe | 7/9 (78%) | 4.4 ± 1.0 | 5.5 ± 0.7 | −1.370 | 0.171 | |
| Parietal lobe | 8/9 (89%) | 4.5 ± 0.9 | 6.0 | −1.409 | 0.159 | |
| Occipital lobe | 4/9 (44%) | 5.0 ± 0.8 | 4.4 ± 1.1 | −0.891 | 0.373 | |
| Temporal lobe | 5/9 (56%) | 4.6 ± 1.1 | 4.8 ± 1.0 | −0.127 | 0.899 | |
| Myelination delay | 2/10 (20%) | 2.5 ± 2.1 | 4.8 ± 1.0 | −1.611 | 0.107 | |
| Ventriculomegaly | 5/10 (50%) | 3.4 ± 1.5 | 5.2 ± 0.8 | −2.041 | 0.041 * | |
| Cerebellar hypoplasia | 2/10 (20%) | 2.5 ± 2.1 | 4.8 ± 1.0 | −1.611 | 0.107 | |
| Migration disorder | 2/10 (20%) | 3.5 ± 0.7 | 4.5 ± 1.6 | −1.343 | 0.179 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.J.; Bae, Y.J.; Song, S.K.; Song, J.-J.; Koo, J.-W.; Lee, J.H.; Oh, S.H.; Kim, B.J.; Choi, B.Y. Prediction of the Outcome of Cochlear Implantation in the Patients with Congenital Cytomegalovirus Infection based on Magnetic Resonance Imaging Characteristics. J. Clin. Med. 2019, 8, 136. https://doi.org/10.3390/jcm8020136
Han JJ, Bae YJ, Song SK, Song J-J, Koo J-W, Lee JH, Oh SH, Kim BJ, Choi BY. Prediction of the Outcome of Cochlear Implantation in the Patients with Congenital Cytomegalovirus Infection based on Magnetic Resonance Imaging Characteristics. Journal of Clinical Medicine. 2019; 8(2):136. https://doi.org/10.3390/jcm8020136
Chicago/Turabian StyleHan, Jae Joon, Yun Jung Bae, Seul Ki Song, Jae-Jin Song, Ja-Won Koo, Jun Ho Lee, Seung Ha Oh, Bong Jik Kim, and Byung Yoon Choi. 2019. "Prediction of the Outcome of Cochlear Implantation in the Patients with Congenital Cytomegalovirus Infection based on Magnetic Resonance Imaging Characteristics" Journal of Clinical Medicine 8, no. 2: 136. https://doi.org/10.3390/jcm8020136
APA StyleHan, J. J., Bae, Y. J., Song, S. K., Song, J.-J., Koo, J.-W., Lee, J. H., Oh, S. H., Kim, B. J., & Choi, B. Y. (2019). Prediction of the Outcome of Cochlear Implantation in the Patients with Congenital Cytomegalovirus Infection based on Magnetic Resonance Imaging Characteristics. Journal of Clinical Medicine, 8(2), 136. https://doi.org/10.3390/jcm8020136







