Effectiveness and Safety of Direct Oral Anticoagulant for Secondary Prevention in Asians with Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Covariates
2.3. Study Outcomes and Follow-Up
2.4. Statistical Analyses
2.5. Subgroup and Sensitivity Analyses
3. Results
3.1. Patients’ Baseline Characteristics
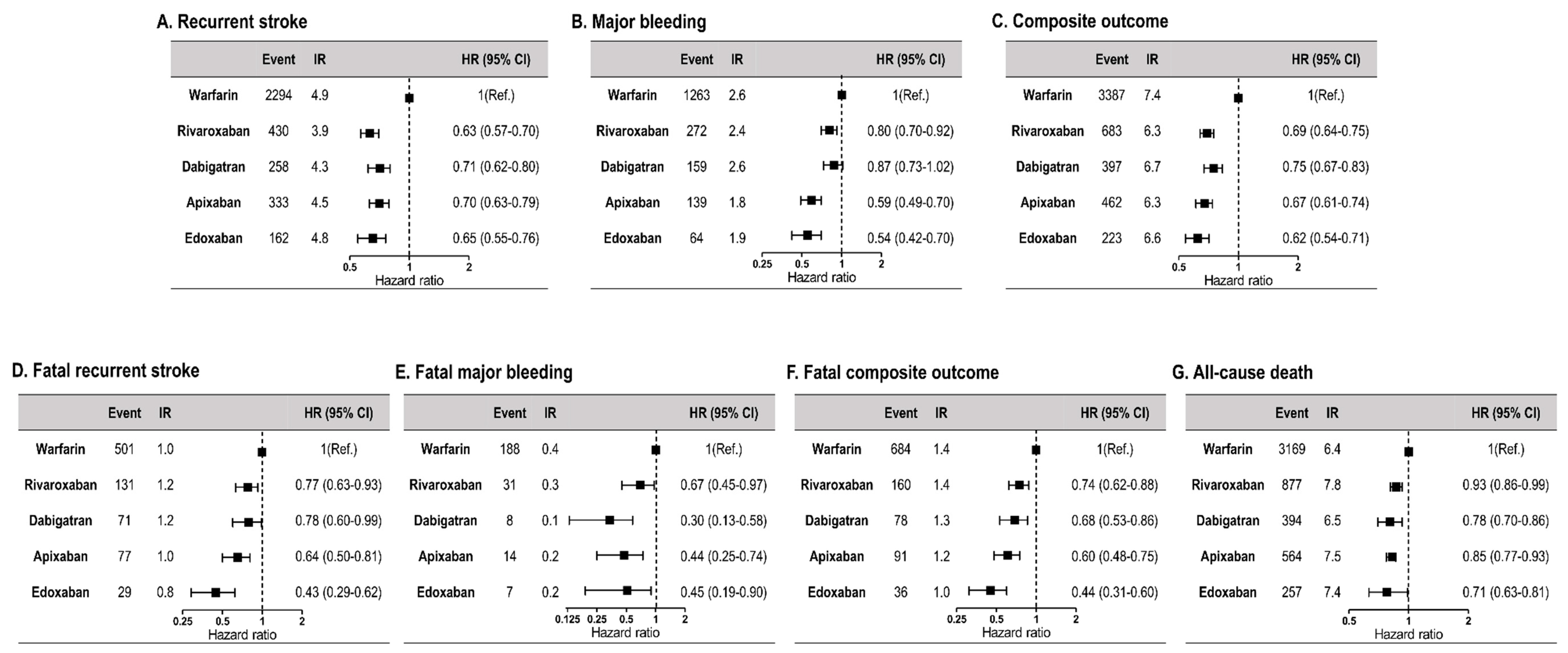
3.2. Recurrent Stroke, Major Bleeding, Composite Outcome, Death from Each Clinical Outcome, and All-Cause Death
3.3. Comparison between Warfarin and Individual Non-Vitamin K Oral Anticoagulants
3.4. The Number Needed to Treat for Clinical Outcomes in the Non-Vitamin K Oral Anticoagulant and Warfarin Groups
3.5. Subgroup Analyses
3.6. Severe, Disabling, and Recent Stroke
3.7. Sensitivity Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dulli, D.A.; Stanko, H.; Levine, R.L. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003, 22, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef]
- Group SRiAFW. Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology 2007, 69, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Giugliano, R.P.; Ruff, C.T.; Murphy, S.A.; Crompton, A.E.; Norden, A.D.; Silverman, S.; Singhal, A.B.; Nicolau, J.C.; SomaRaju, B.; et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: Findings from ENGAGE AF-TIMI 48 (effective anticoagulation with factor xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Stroke 2016, 47, 2075–2082. [Google Scholar] [CrossRef]
- Hankey, G.J.; Patel, M.R.; Stevens, S.R.; Becker, R.C.; Breithardt, G.; Carolei, A.; Diener, H.C.; Donnan, G.A.; Halperin, J.L.; Mahaffey, K.W.; et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: A subgroup analysis of ROCKET AF. Lancet Neurol. 2012, 11, 315–322. [Google Scholar] [CrossRef]
- Easton, J.D.; Lopes, R.D.; Bahit, M.C.; Wojdyla, D.M.; Granger, C.B.; Wallentin, L.; Alings, M.; Goto, S.; Lewis, B.S.; Rosenqvist, M.; et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: A subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012, 11, 503–511. [Google Scholar] [CrossRef]
- Diener, H.C.; Connolly, S.J.; Ezekowitz, M.D.; Wallentin, L.; Reilly, P.A.; Yang, S.; Xavier, D.; Di Pasquale, G.; Yusuf, S.; Group R-Ls. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: A subgroup analysis of the RE-LY trial. Lancet Neurol. 2010, 9, 1157–1163. [Google Scholar] [CrossRef]
- Coleman, C.I.; Peacock, W.F.; Bunz, T.J.; Alberts, M.J. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke 2017, 48, 2142–2149. [Google Scholar] [CrossRef]
- Wang, K.L.; Lip, G.Y.; Lin, S.J.; Chiang, C.E. Non-vitamin k antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: Meta-analysis. Stroke 2015, 46, 2555–2561. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Cho, S.K.; Kim, J.B.; Joung, B.; Kim, C. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Korean Circ. J. 2019, 49, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.Y.; Kwon, H.S.; Cha, B.S.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Lee, B.W. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Edoxaban in Asian patients with atrial fibrillation: Effectiveness and safety. J. Am. Coll. Cardiol. 2018, 72, 838–853. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Park, C.S.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J. Am. Coll. Cardiol. 2019, 73, 919–931. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, H.J.; Choi, E.K.; Han, K.D.; Jung, J.H.; Cha, M.J.; Oh, S.; Lip, G.Y.H. Direct oral anticoagulants in patients with atrial fibrillation and liver disease. J. Am. Coll. Cardiol. 2019, 73, 3295–3308. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, Y.S.; Park, J.S.; Cha, M.J.; Kim, T.H.; Park, J.; Park, J.K.; Lee, J.M.; Kang, K.W.; Shim, J.; et al. Label adherence for non-vitamin k antagonist oral anticoagulants in a prospective cohort of Asian patients with atrial fibrillation. Yonsei Med. J. 2019, 60, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Papavasileiou, V.; Diener, H.C.; Makaritsis, K.; Michel, P. Nonvitamin-k-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: An updated systematic review and meta-analysis of randomized controlled trials. Int. J. Stroke 2017, 12, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Skjoth, F.; Nielsen, P.B.; Kjaeldgaard, J.N.; Lip, G.Y. Comparative effectiveness and safety of non-vitamin k antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Lee, H.F.; See, L.C.; Tu, H.T.; Chao, T.F.; Yeh, Y.H.; Wu, L.S.; Kuo, C.T.; Chang, S.H.; Lip, G.Y.H. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest 2019, 156, 529–543. [Google Scholar] [CrossRef]
- Cho, M.S.; Yun, J.E.; Park, J.J.; Kim, Y.J.; Lee, J.; Kim, H.; Park, D.W.; Nam, G.B. Outcomes after use of standard- and low-dose non-vitamin k oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2019, 50, 110–118. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Cha, M.J.; Oh, S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int. J. Cardiol. 2017, 236, 226–231. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, H.J.; Kim, T.H.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Appropriate doses of non-vitamin k antagonist oral anticoagulants in high-risk subgroups with atrial fibrillation: Systematic review and meta-analysis. J. Cardiol. 2018, 72, 284–291. [Google Scholar] [CrossRef]
- Hong, K.S.; Kim, Y.K.; Bae, H.J.; Nam, H.S.; Kwon, S.U.; Bang, O.Y.; Cha, J.K.; Yoon, B.W.; Rha, J.H.; Lee, B.C.; et al. Quality of anticoagulation with warfarin in Korean patients with atrial fibrillation and prior stroke: A multicenter retrospective observational study. J. Clin. Neurol. 2017, 13, 273–280. [Google Scholar] [CrossRef]
- Li, Y.G.; Lee, S.R.; Choi, E.K.; Lip, G.Y. Stroke prevention in atrial fibrillation: Focus on Asian patients. Korean Circ. J. 2018, 48, 665–684. [Google Scholar] [CrossRef]

| Warfarin | DOAC | ASD | Rivaroxaban | Dabigatran | Apixaban | Edoxaban | ASD | |
|---|---|---|---|---|---|---|---|---|
| (n = 28,839) | (n = 32,729) | (n = 12,311) | (n = 6293) | (n = 8837) | (n = 5288) | |||
| Age | ||||||||
| Mean ± SD, years | 73.1 ± 9.4 | 75.2 ± 9.1 | 0.233 | 75.4 ± 8.8 | 73.9 ± 9.5 | 75.9 ± 9.1 | 75.4 ± 9.0 | 0.253 |
| <65 years | 4757 (16.5) | 3974 (12.1) | 1389 (11.3) | 956 (15.2) | 981 (11.1) | 648 (12.3) | ||
| 65–74 years | 10,255 (35.6) | 9775 (29.9) | 3717 (30.2) | 2035 (32.3) | 2462 (27.9) | 1561 (29.5) | ||
| 75≤ years | 13,827 (48.0) | 18,980 (58.0) | 7205 (58.5) | 3302 (52.5) | 5394 (61.0) | 3079 (58.2) | ||
| Male | 15,338 (53.2) | 16,703 (51.0) | 0.043 | 6037 (49.0) | 3453 (54.9) | 4427 (50.1) | 2786 (52.7) | 0.083 |
| CHA2DS2-VASc score | ||||||||
| Mean ± SD | 5.8 ± 1.5 | 5.9 ± 1.4 | 0.123 | 6.0 ± 1.4 | 5.8 ± 1.5 | 6.02 ± 1.41 | 5.9 ± 1.42 | 0.143 |
| HAS-BLED score | ||||||||
| Mean ± SD | 4.2 ± 1.1 | 4.2 ± 1.1 | 0.029 | 4.3 ± 1.1 | 4.15 ± 1.1 | 4.24 ± 1.11 | 4.1 ± 1.11 | 0.020 |
| ≥3 | 27,534 (95.5) | 30,997 (94.7) | 11,715 (95.2) | 5922 (94.1) | 8417 (95.3) | 4943 (93.5) | ||
| CCI | ||||||||
| Mean ± SD | 5.2 ± 2.5 | 4.8 ± 2.5 | 0.125 | 4.8 ± 2.5 | 4.8 ± 2.5 | 5.0 ± 2.5 | 4.5 ± 2.5 | 0.124 |
| ≥3 | 24,466 (84.8) | 26,808 (81.9) | 10,085 (81.9) | 5176 (82.3) | 7432 (84.1) | 4115 (77.8) | ||
| Time lapse from previous stroke | ||||||||
| Mean ± SD, years | 1.5 ± 1.9 | 2.6 ± 2.8 | 2.7 ± 2.7 | 2.2 ± 2.6 | 2.6 ± 2.9 | 3.0 ± 3.0 | ||
| Median (IQR), years | 0.6 (0.1–2.5) | 1.4 (0.1–4.7) | 1.7 (0.2–4.8) | 0.9 (0.1–3.9) | 1.2 (0.1–4.7) | 2.0 (0.1–5.4) | ||
| Hypertension | 26,072 (90.4) | 29,265 (89.4) | 0.033 | 11,056 (89.8) | 5578 (88.6) | 7945 (89.9) | 4686 (88.6) | 0.020 |
| Diabetes mellitus | 9869 (34.2) | 10,467 (32.0) | 0.048 | 3885 (31.6) | 1971 (31.3) | 3036 (34.4) | 1575 (29.8) | 0.057 |
| Dyslipidemia | 18,774 (65.1) | 21,899 (66.9) | 0.038 | 7890 (64.1) | 4484 (71.3) | 6062 (68.6) | 3463 (65.5) | 0.021 |
| Congestive heart failure | 11,972 (41.5) | 14,780 (45.2) | 0.074 | 5389 (43.8) | 2751 (43.7) | 4175 (47.2) | 2465 (46.6) | 0.046 |
| Myocardial infarction | 1836 (6.4) | 1829 (5.6) | 0.033 | 705 (5.7) | 325 (5.2) | 553 (6.3) | 246 (4.7) | 0.027 |
| Peripheral arterial disease | 7668 (26.6) | 9075 (27.7) | 0.026 | 3510 (28.5) | 1816 (28.9) | 2269 (25.7) | 1480 (28.0) | 0.043 |
| COPD | 4080 (14.2) | 3994 (12.2) | 0.058 | 1620 (13.2) | 687 (10.9) | 1158 (13.1) | 529(10) | 0.029 |
| Cancer | 2000 (6.9) | 2541 (7.8) | 0.032 | 970 (7.9) | 449 (7.1) | 763 (8.6) | 359 (6.8) | 0.036 |
| Previous Intracranial hemorrhage | 1530 (5.3) | 1959 (6.0) | 0.029 | 719 (5.8) | 404 (6.4) | 548 (6.2) | 288 (5.5) | 0.023 |
| Previous gastrointestinal bleeding | 5039 (17.5) | 7678 (23.5) | 0.149 | 2878 (23.4) | 1396 (22.2) | 2117 (24.0) | 1287 (24.3) | 0.147 |
| Antiplatelets | 0.440 | 0.431 | ||||||
| No antiplatelets | 12,731 (44.2) | 21,454 (65.6) | 8016 (65.1) | 4097 (65.1) | 5705 (64.6) | 3636 (68.8) | ||
| Aspirin only | 7990 (27.7) | 5183 (15.8) | 2006 (16.3) | 1021 (16.2) | 1410 (16.0) | 746 (14.1) | ||
| Clopidogrel only | 2816 (9.8) | 3209 (9.8) | 1199 (9.7) | 621 (9.9) | 870 (9.8) | 519 (9.8) | ||
| Dual antiplatelets | 5302 (18.4) | 2883 (8.8) | 1090 (8.9) | 554 (8.8) | 852 (9.6) | 387 (7.3) | ||
| NSAID | 13,940 (48.3) | 14,321 (43.8) | 0.092 | 5799 (47.1) | 2902 (46.1) | 3617 (40.9) | 2003 (37.9) | 0.025 |
| Proton pump inhibitors | 9090 (31.5) | 12,073 (36.9) | 0.113 | 4429 (36.0) | 2516 (40.0) | 3336 (37.8) | 1792 (33.9) | 0.094 |
| Outcome | Warfarin | DOAC | *HR (95% CI) | p-Value |
|---|---|---|---|---|
| Event (IR) | Event (IR) | |||
| Recurrent stroke | 2294 (4.9) | 1184 (4.2) | 0.67 (0.62–0.72) | <0.001 |
| Major bleeding | 1263 (2.6) | 633 (2.2) | 0.73 (0.66–0.80) | <0.001 |
| Composite outcome (Recurrent stroke + major bleeding) | 3387 (7.4) | 1765 (6.4) | 0.69 (0.65–0.73) | <0.001 |
| Fatal recurrent stroke | 501 (1.0) | 307 (1.1) | 0.69 (0.59–0.79) | <0.001 |
| Fatal major bleeding | 188 (0.4) | 60 (0.2) | 0.50 (0.37–0.68) | <0.001 |
| Fatal composite outcome | 684 (1.4) | 366 (1.3) | 0.65 (0.57–0.74) | <0.001 |
| All-cause death | 3169 (6.4) | 2092 (7.4) | 0.84 (0.80–0.89) | <0.001 |
| Outcome | Pooled DOAC | Rivaroxaban | Dabigatran | Apixaban | Edoxaban | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 year ARR | NNT | 1 year ARR | NNT | 1 year ARR | NNT | 1 year ARR | NNT | 1 year ARR | NNT | |
| Recurrent stroke | 1.5 | 65 | 1.7 | 60 | 1.3 | 78 | 1.0 | 100 | 2.3 | 44 |
| Major bleeding | 0.7 | 142 | 0.4 | 284 | 0.8 | 119 | 1.0 | 98 | 0.7 | 135 |
| Composite outcome (Recurrent stroke + major bleeding) | 2.1 | 48 | 1.9 | 54 | 2.0 | 49 | 1.8 | 54 | 2.9 | 35 |
| All-cause death | 1.1 | 94 | 0.6 | 180 | 1.3 | 77 | 0.6 | 179 | 2.7 | 38 |
| Fatal composite outcome | 0.5 | 190 | 0.6 | 175 | 0.4 | 271 | 0.3 | 287 | 0.9 | 118 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, S.-R.; Choi, E.-K.; Kwon, S.; Jung, J.-H.; Han, K.-D.; Cha, M.-J.; Ko, S.-B.; Oh, S.; Lip, G.Y.H. Effectiveness and Safety of Direct Oral Anticoagulant for Secondary Prevention in Asians with Atrial Fibrillation. J. Clin. Med. 2019, 8, 2228. https://doi.org/10.3390/jcm8122228
Park J, Lee S-R, Choi E-K, Kwon S, Jung J-H, Han K-D, Cha M-J, Ko S-B, Oh S, Lip GYH. Effectiveness and Safety of Direct Oral Anticoagulant for Secondary Prevention in Asians with Atrial Fibrillation. Journal of Clinical Medicine. 2019; 8(12):2228. https://doi.org/10.3390/jcm8122228
Chicago/Turabian StylePark, Jiesuck, So-Ryoung Lee, Eue-Keun Choi, Soonil Kwon, Jin-Hyung Jung, Kyung-Do Han, Myung-Jin Cha, Sang-Bae Ko, Seil Oh, and Gregory Y. H. Lip. 2019. "Effectiveness and Safety of Direct Oral Anticoagulant for Secondary Prevention in Asians with Atrial Fibrillation" Journal of Clinical Medicine 8, no. 12: 2228. https://doi.org/10.3390/jcm8122228
APA StylePark, J., Lee, S.-R., Choi, E.-K., Kwon, S., Jung, J.-H., Han, K.-D., Cha, M.-J., Ko, S.-B., Oh, S., & Lip, G. Y. H. (2019). Effectiveness and Safety of Direct Oral Anticoagulant for Secondary Prevention in Asians with Atrial Fibrillation. Journal of Clinical Medicine, 8(12), 2228. https://doi.org/10.3390/jcm8122228







