The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alsfyani, M.A.; Golant, A.K.; Lebwohl, M. Psoriasis and the metabolic syndrome. Dermatol. Ther. 2010, 2, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Prodanovich, S.; Kirsner, R.S.; Kravetz, J.D.; Ma, F.; Martinez, L.; Federman, D.G. Association of psoriasis with coronary artery, cerebrovascular and peripheral vascular disease and mortality. Arch. Dermatol. 2009, 145, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Guerin, A.; Latremounille-Viau, D.; Yu, A.P.; Gupta, S.; Bao, Y.; Mulani, P. Coronary heart diseases and stroke risk in patients with psoriasis: Retrospective analysis. Am. J. Med. 2010, 123, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Abuabara, K.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Cause-specific mortality in patients with severe psoriasis: A population—Based cohort study in the U.K. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Boehncke, S.; Tobin, A.M.; Kirby, B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef]
- Beeneken, A.; Mahammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the FGF and FGFR gene families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.F.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef]
- Kurosu, H.; Choi, M.; Ogawa, Y.; Dickson, A.S.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Rosenblatt, K.P.; Kliewer, S.A.; Kuro-o, M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007, 282, 26687–26695. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of novel FGF, FGF21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Wente, W.; Efanov, A.M.; Brenner, M.; Kharitonenkov, A.; Köster, A.; Sandusky, G.E.; Sewing, S.; Treinies, I.; Zitzer, H.; Gromada, J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase ½ and Akt signaling pathways. Diabetes 2006, 55, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Bookout, A.L.; de Groot, M.H.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef]
- Adams, A.C.; Cheng, C.C.; Coskun, T. Kharitonenkov, A. FGF21 requires βklotho to act in vivo. PLoS ONE 2012, 7, 4064–4075. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.S. FGF21 as a stress hormone: The roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab. J. 2014, 38, 245–251. [Google Scholar] [CrossRef]
- Galman, C.; Lundasen, T.; Kharitonenkov, A.; Bina, H.A.; Eriksson, M.; Hafström, I.; Dahlin, M.; Åmark, P.; Angelin, B.; Rudling, M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008, 8, 169–174. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Moosa Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela–Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez–Chantar, M.L.; Maratos–Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Laeger, T.; Henagan, T.M.; Albrado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.S.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Heblinski, M.; Wahl, D.; McMahon, A.C.; Warren, A.; Durrant-Whyte, J.; Walters, K.A.; Krycer, J.R.; et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016, 24, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Raamos, D.; Aguilar-Salinas, C.A. Modulation of energy balance by fibroblast growth factor 21. Horm. Mol. Biol. Clin. Investig. 2016, 30. [Google Scholar] [CrossRef]
- Woo, Y.C.; Xu, Y.; Wang, Y.; Karen, S.L.; Lam, K.S.L.L. Fibroblast growth factor 21 as an emerging metabolic regulator: Clinical perspectives. Clin. Endocrinol. 2013, 78, 489–496. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y. Fibroblast growth factor 21 analogs for treating metabolic disorders. Front. Endocrinol. 2015, 6, 168. [Google Scholar] [CrossRef]
- Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012, 82, 737–747. [Google Scholar] [CrossRef]
- Gateva, A.; Assyov, Y.; Tsakova, A.; Kamenov, Z. Classical (adiponectin, leptin, resistin) and new (chemerin, vaspin, omentin) adipocytokines in patients with prediabetes. Horm. Mol. Biol. Clin. Investig. 2018, 34. [Google Scholar] [CrossRef]
- Wojcik, M.; Janus, D.; Dolezal-Oltarzewska, K.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. The association of FGF23 levels in obese adolescents with insulin sensitivity. J. Pediatr. Endocrinol. Metab. 2012, 25, 687–690. [Google Scholar] [CrossRef]
- Mirza, M.A.; Larsson, A.; Lind, L.; Larsson, T.E. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009, 205, 385–390. [Google Scholar] [CrossRef]
- Mehta, N.N.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the General Practice Research Database. Eur. Heart J. 2010, 31, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.Y.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.C.; Chow, W.S.; Tso, W.K.A.; Lam, K.S.L. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Misra, M.; Goldstein, M.; Miller, K.K.; Klibanski, A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J. Clin. Endocrinol. Metab. 2010, 95, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jeong, Y.T.; Oh, H.; Kim, S.H.; Cho, J.M.; Kim, Y.N.; Kim, S.S.; Kim, D.H.; Hur, K.Y.; Kim, H.K.; et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013, 19, 83–92. [Google Scholar] [CrossRef]
- Thiessen, S.E.; Vanhorebeek, I.; Derese, I.; Gunst, J.; Van den Berghe, G. FGF21 Response to Critical Illness: Effect of Blood Glucose Control and Relation with Cellular Stress and Survival. J. Clin. Endocrinol. Metab. 2015, 100, E1319–E1327. [Google Scholar] [CrossRef]
- Hanssen, M.J.W.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; van der Lans, A.A.J.J.; Cheng, C.C.; Adams, A.C.; van Marken Lichtenbelt, W.D.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef]
- DeSousa-Coelho, A.L.; Relat, J.; Hondares, E.; Pérez-Martí, A.; Ribas, F.; Villarroya, F.; Marrero, P.F.; Haro, D. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res. 2013, 54, 1786–1797. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Z.; Liu, Y.; Gong, Q.; Yan, X.; Xiao, J.; Wang, X.; Lin, S.; Feng, W.; Li, X. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS ONE 2011, 6, e18398. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Yin, X.; Liu, Y.; Yan, X.; Lin, S.; Xiao, J.; Wang, X.; Feng, W.; Li, X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE 2010, 5, e15534. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, X.; Zhou, J.; Pan, X.; Hao, Y.; Zhou, M.; Lu, Z.; Gao, M.; Bao, Y.; Jia, W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc. Diabetol. 2013, 12, 124. [Google Scholar] [CrossRef]
- Chow, W.S.; Xu, A.; Woo, Y.C.; Tso, A.W.K.; Cheung, S.C.W.; Fong, C.H.Y.; Tse, H.F.; Chau, M.T.; Cheung, B.M.Y.; Lam, K.S.L. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Lee, M.S.; Yi, S.A.; Ha, E.S.; Han, S.J.; Kim, H.J.; Kim, D.J.; Lee, K.W. Serum fibroblast growth factor 21 was elevated in subjects with type 2 diabetes mellitus and was associated with the presence of carotid artery plaques. Diabetes Res. Clin. Pract. 2012, 96, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Bartlova, M.; Lacinova, Z.; Michalsky, D.; Kasalicky, M.; Haluzikova, D.; Matoulek, M.; Dostalova, I.; Humenanska, V.; Haluzik, M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009, 71, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.O.; Molina-Carrion, M.; Abdul-Ghani, M.A.; Folli, F.; DeFronzo, R.A.; Tripathy, D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009, 32, 1542–1546. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Gao, F.; Fan, J.; Zhou, J.; Wang, X.; Zhang, H.; Pan, X.; Bao, Y.; Xiang, K.; et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J. Hepatol. 2010, 53, 934–940. [Google Scholar] [CrossRef]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, H. Epidemiology of non-alcoholic fatty liver disease. Digit. Distrib. 2010, 28, 155–161. [Google Scholar] [CrossRef]
- Van der Voort, E.A.M.; Koehler, E.M.; Dowlatshahi, E.A.; Hofman, A.; Stricker, B.H.; Janssen, H.L.A.; Schouten, J.N.L.; Nijsten, T. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: Results from a population based study. J. Am. Acad. Dermatol. 2014, 70, 517–524. [Google Scholar] [CrossRef]
- Miele, L.; Vallone, S.; Cefalo, C.; La Torre, G.; Di Stasi, C.; Vecchio, F.M.; D’Agostino, M.; Gabrieli, M.L.; Vero, V.; Biolato, M.; et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J. Hepatol. 2009, 51, 778–786. [Google Scholar] [CrossRef]
- Madanagobalane, S.; Anandan, S. The increased prevalence of non-alcoholic fatty liver disease in psoriatic patients: A study from South India. Australas. J. Dermatol. 2012, 53, 190–197. [Google Scholar] [CrossRef]
- Reinehr, T.; Woelfle, J.; Roth, C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2143–2150. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar]
- Madjid, M.; Willerson, J.T. Inflammatory markers in coronary heart disease. Br. Med. Bull. 2011, 100, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines Circulation. J. Am. Coll. Cardiol. 2010, 122, e584–e636. [Google Scholar]
- Rocha-Pereira, P.; Santos-Silva, A.; Rebelo, I.; FigneiRedo, A.; Quintanilha, A.; Teixeira, F. Erythrocyte damage in mild and severe psoriasis. Br. J. Dermatol. 2004, 150, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Okan, G.; Baki, A.M.; Yorulmaz, E.; Doğru-Abbasoğlu, S.; Vural, P. Fibroblast Growth Factor 23 and Placental Growth Factor in Patients with Psoriasis and their Relation to Disease Severity. Ann. Clin. Lab. Sci. 2016, 46, 174–179. [Google Scholar] [PubMed]
- Prié, D.; Forand, A.; Francoz, C.; Elie, C.; Cohen, I.; Courbebaisse, M.; Eladari, D.; Lebrec, D.; Durand, F.; Friedlander, G. Plasma fibroblast growth factor 23 concentration is increased and predicts mortality in patients on the liver-transplant waiting list. PLoS ONE 2013, 8, e66182. [Google Scholar] [CrossRef] [PubMed]
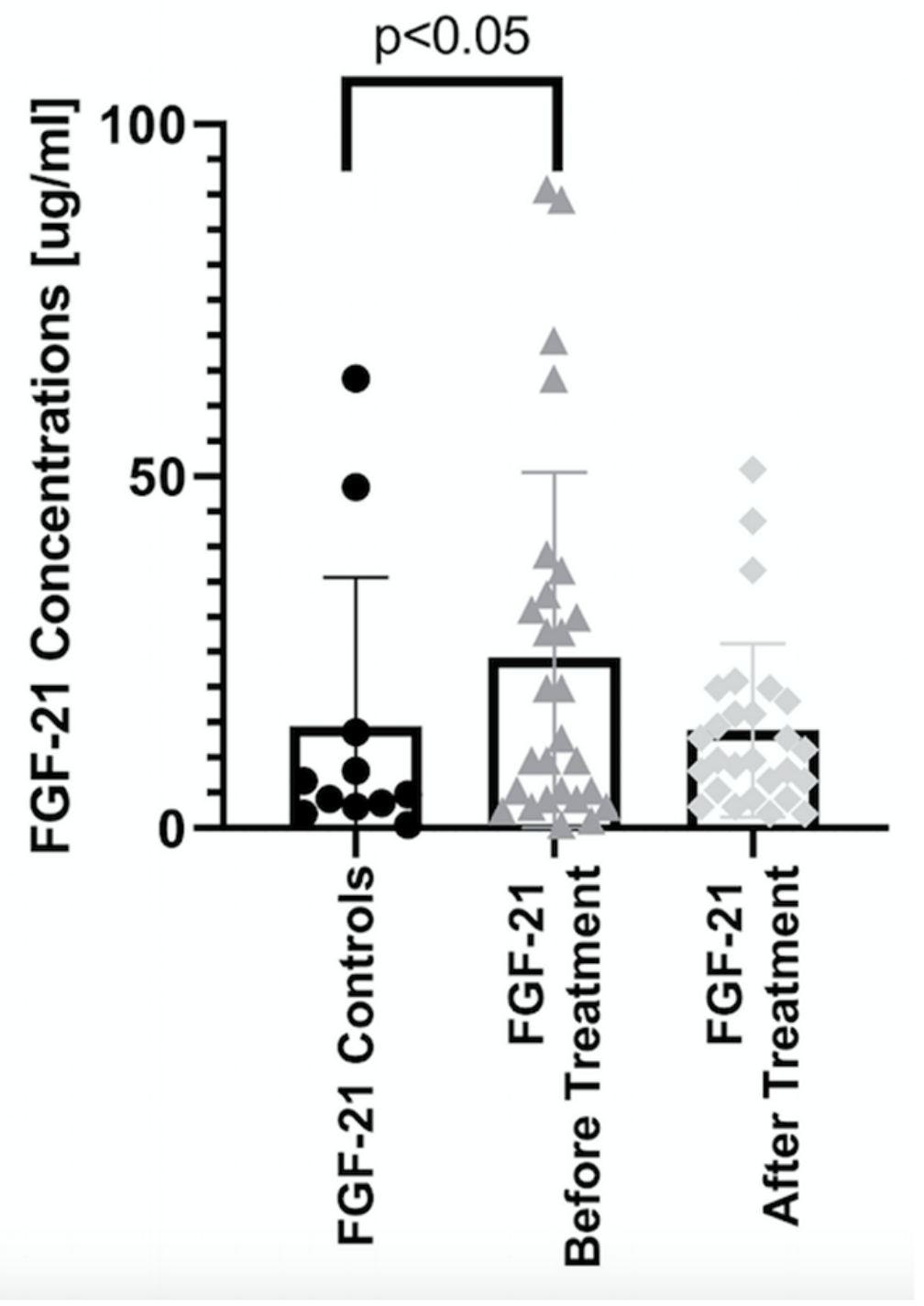
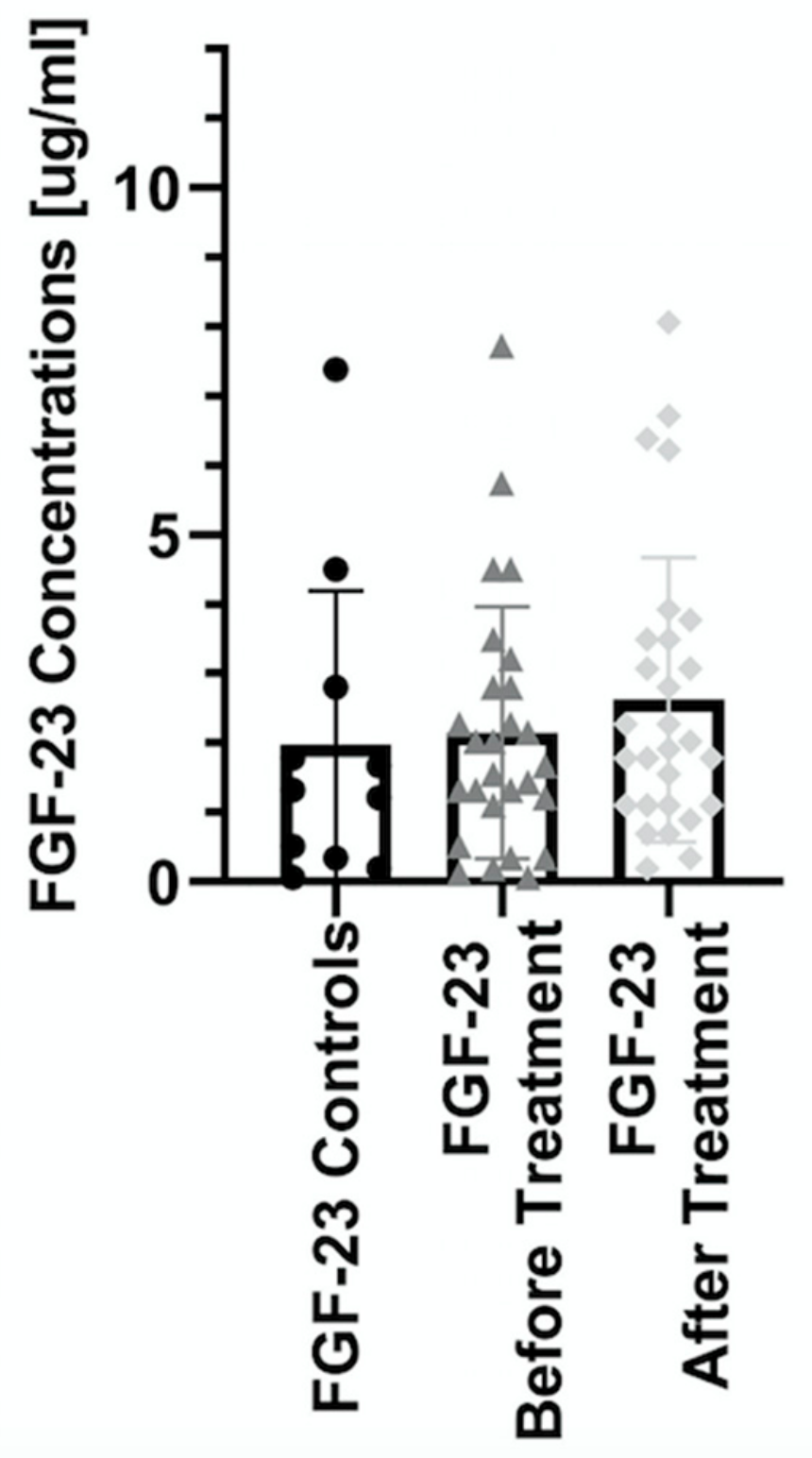
| Parameter | Controls (n = 11) | Patients (n = 33) |
|---|---|---|
| Age (years) | 54.4 ± 9.11 | 54.2 ± 16.8 |
| Weight (kg) | 69.4 ± 15.2 | 84.8 ± 20.9 * |
| Height (cm) | 166 ± 8.83 | 171 ± 10 |
| BMI | 25 ± 3.59 | 28.9 ± 6.42 * |
| Parameter | Before Treatment | After Treatment | After Acitretin | After Methotrexate |
|---|---|---|---|---|
| PASI | 15 (5.4–32.7) | 3.4 (0.7–15) ^^^ | 3.4 (0.7–15) | 2.6 (1–7.6) |
| Hemoglobin (mg/dL) | 13.6 ± 1.66 | 13.2 ± 1.45 | 13 ± 1.5 | 13 ± 1.16 |
| Red Blood Cells (× 103/mL) | 4.38 ± 0.56 | 4.28 ± 0.47 | 4.3 ± 0.46 | 4.2 ± 0.32 |
| White Blood Cells (× 103/mL) | 7.04 (4.92–12) | 6.42 (4.2–10.3) ^ | 6.44 (4.2–8.9) | 6.4 (4.3–10.34) |
| Platelets (× 103/mL) | 251 ± 73.2 | 231 ± 59.5 | 238 ± 62.2 | 238 ± 62.4 |
| Glucose (mg/dL) | 84 (53–215) | 87 (55–140) | 86 (55–110) | 90 (65–140) |
| C-Reactive Protein (mg/L) | 5.13 (1–34.7) | 1.87 (0.5–15) ^^ | 2.37 (1–13.2) | 1.6 (0.5–5.8) |
| Alanine Aminotransferase (IU/L) | 24 (9–48) | 19 (9–49) | 19 (9–42) | 23 (12–49) |
| Aspartate Aminotransferase (IU/L) | 21 (14–86) | 19 (12–52) | 20 (12–39) | 20 (13–52) |
| Total Cholesterol (mg/dL) | 170 ± 39.1 | 168 ± 38.6 | 170 ± 39.6 | 165 ± 31.7 |
| Triglycerides (mg/dL) | 137 ± 63.1 | 120 ± 54.6 | 124 ± 58 | 125.3 ± 53.9 |
| Controls | Patients | BMI I | BMI II | BMI III | |
|---|---|---|---|---|---|
| FGF 21 | |||||
| Before | 4.75 (0.42–63.9) | 19.9 (0.42–1993) * | 9.6 (1.12–699) * # | 9.6 (2.49–197) * # | 28.9 (0.42–1993) * # |
| After | 12 (2–11507) * | 12.8 (4.15–11507) * | 9.6 (2–50.9) * | 13.6 (3.02–205) * | |
| FGF 23 | |||||
| Before | 1.32 (0.06–7.38) | 1.85 (0.06–10.4) | 3.21 (1.43–10.4) * # | 1.21 (0.12–2.8) * # | 2.09 (0.06–7.72) * # |
| After | 1.97 (0.19–8.06) | 2.29 (0.19–6.71) | 1.44 (0.34–6.38) | 2.28 (0.89–8.06) |
| Controls | Study Group | PASI I | PASI II | PASI III | |
|---|---|---|---|---|---|
| FGF 21 | |||||
| Before | 4.75 (0.42–63.9) | 19.9 (0.42–1993) * | 14.7 (3.02–90.8) * # | 7.49 (0.42–63.9) * # | 34.9 (3.02–1993) * # |
| After | 12 (2–11507) * | 14.5 (2–11507) * | 8.49 (2–43.6) * | 13.6 (3–2493) * | |
| FGF 23 | |||||
| After | 1.32 (0.06–7.38) | 1.85 (0.06–10.4) | 2.03 (0.12–7.72) | 1.43 (0.19–10.4) | 1.85 (0.06–5.74) |
| Before | 1.97 (0.19–8.06) | 3.49 (0.19–8.06) | 1.78 (0.34–6.38) | 1.85 (0.89–6.71) |
| Controls | Study Group | Acitretin | Methotrexat | |
|---|---|---|---|---|
| FGF 21 | ||||
| Before Treatment | 4.75 (0.42–63.9) | 19.9 (0.42–1993) * | 37.7 (1.12–1993) * | 11.2 (0.42–89.3) # |
| After Treatment | - | 12 (2–11507) | 12 (3.02–2493) * | 11.2 (2–11507) |
| FGF 23 | ||||
| Before Treatment | 1.32 (0.06–7.38) | 1.85 (0.06–10.4) | 2.03 (0.34–10.4) | 1.67 (0.06–7.72) |
| After Treatment | - | 1.97 (0.19–8.06) | 1.91 (0.69–6.71) | 2.28 (0.19–8.06) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiluk, P.; Baran, A.; Kaminski, T.W.; Maciaszek, M.; Flisiak, I. The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients. J. Clin. Med. 2019, 8, 2206. https://doi.org/10.3390/jcm8122206
Kiluk P, Baran A, Kaminski TW, Maciaszek M, Flisiak I. The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients. Journal of Clinical Medicine. 2019; 8(12):2206. https://doi.org/10.3390/jcm8122206
Chicago/Turabian StyleKiluk, Paulina, Anna Baran, Tomasz W. Kaminski, Magdalena Maciaszek, and Iwona Flisiak. 2019. "The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients" Journal of Clinical Medicine 8, no. 12: 2206. https://doi.org/10.3390/jcm8122206
APA StyleKiluk, P., Baran, A., Kaminski, T. W., Maciaszek, M., & Flisiak, I. (2019). The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients. Journal of Clinical Medicine, 8(12), 2206. https://doi.org/10.3390/jcm8122206







