Management of Refeeding Syndrome in Medical Inpatients
Abstract
1. Introduction
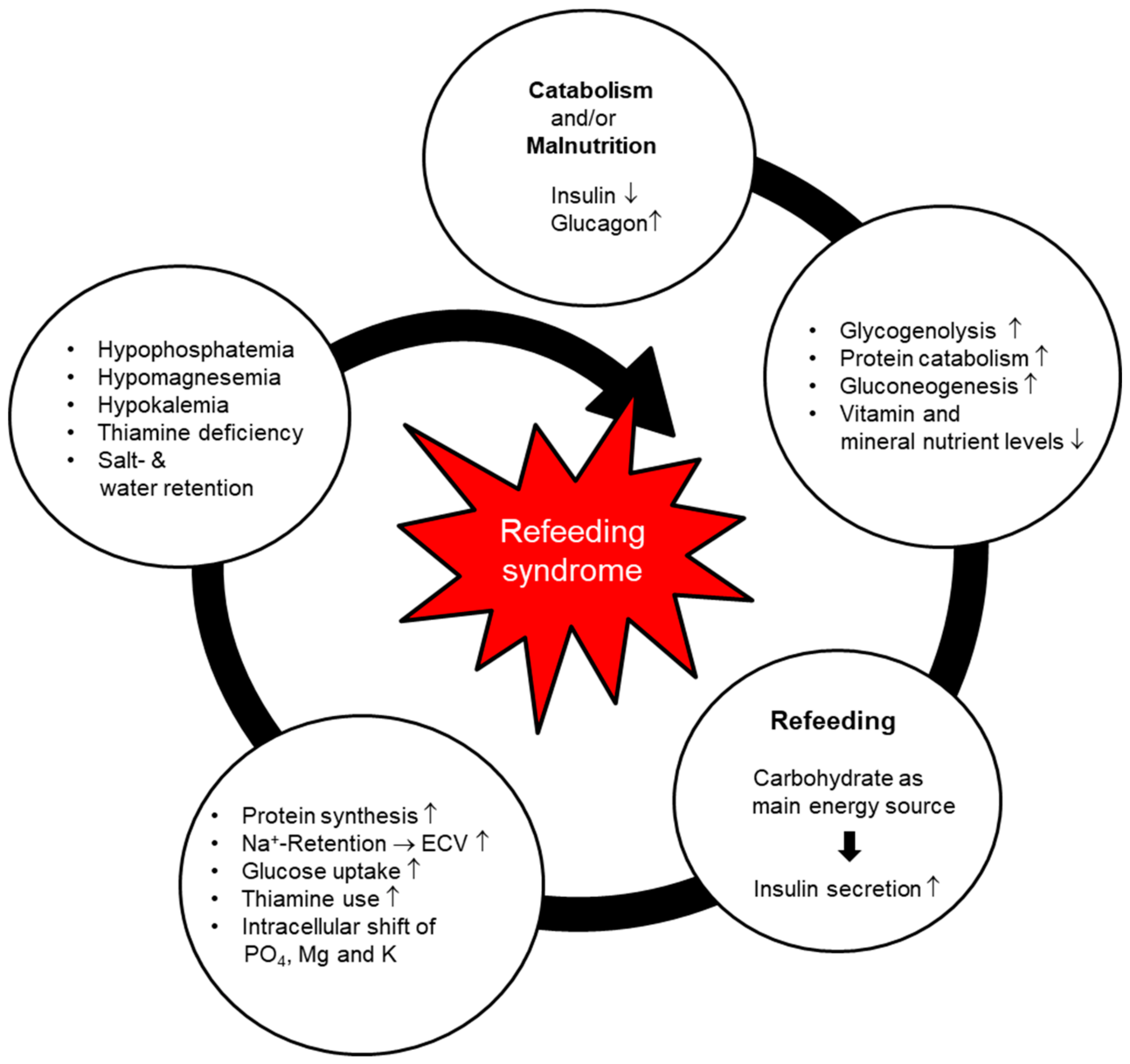
2. Pathophysiology and Clinical Manifestations
- –
- Phosphate is an important electrolyte in the metabolism of macronutrients for both the energy production and transport processes. Phosphate is especially important in the refeeding phase, since glycolysis requires only phosphorylated glucose. Hypophosphatemia may cause several clinical manifestations, such as rhabdomyolysis, hemolysis, respiratory failure, and musculoskeletal disorders. Severe hypophosphatemia (<0.32 mmol/L) is considered a typical hallmark of RFS and in several studies is a central defining criterion [15,18].
- –
- Potassium and magnesium are also important intercellular cations. Severe hypokalemia (<2.5 mmol/L) and/or hypomagnesemia (<0.50 mmol/L) may trigger potentially lethal arrhythmia, neuromuscular dysfunctions such as paresis, rhabdomyolysis, confusion, and respiratory insufficiency [15].
- –
- Thiamine is an essential coenzyme in the metabolism of carbohydrates, allowing the conversion from glucose to adenosine triphosphate (ATP) via the Krebs cycle. When thiamine is lacking (human body stores last for approximately 14 days), glucose is converted to lactate, leading to metabolic acidosis. Thiamine deficiency may also lead to neurologic (Wernicke’s encephalopathy: dry beriberi) or cardiovascular disorders (wet beriberi) [15,16].
- –
- Sodium: The major influence on the serum sodium level during the refeeding phase is the shift of sodium out of the cell as the potassium is pumped back into the cell (sodium-potassium-ATPase pump). In addition, the increased insulin level in the early phase of refeeding leads to sodium retention in the kidneys. Sodium concentration subsequently increases, thus inducing water retention. Noradrenaline and angiotensin II are stimulated and lead to augmented peripheral resistance and vasoconstriction [21]. This may cause peripheral edema and heart failure.
3. Current Level of Evidence
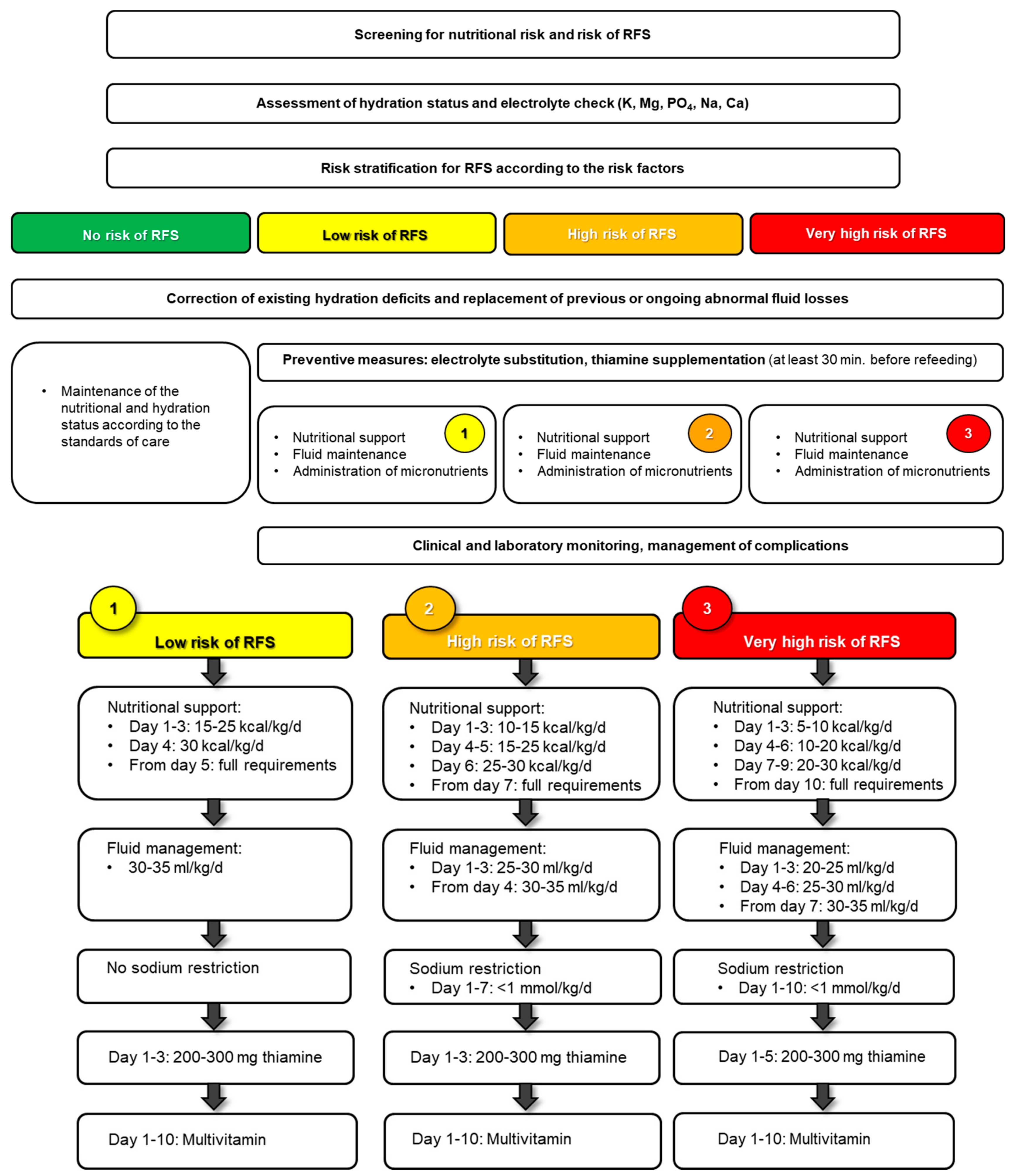
4. Prevention
4.1. Nutritional Support Teams
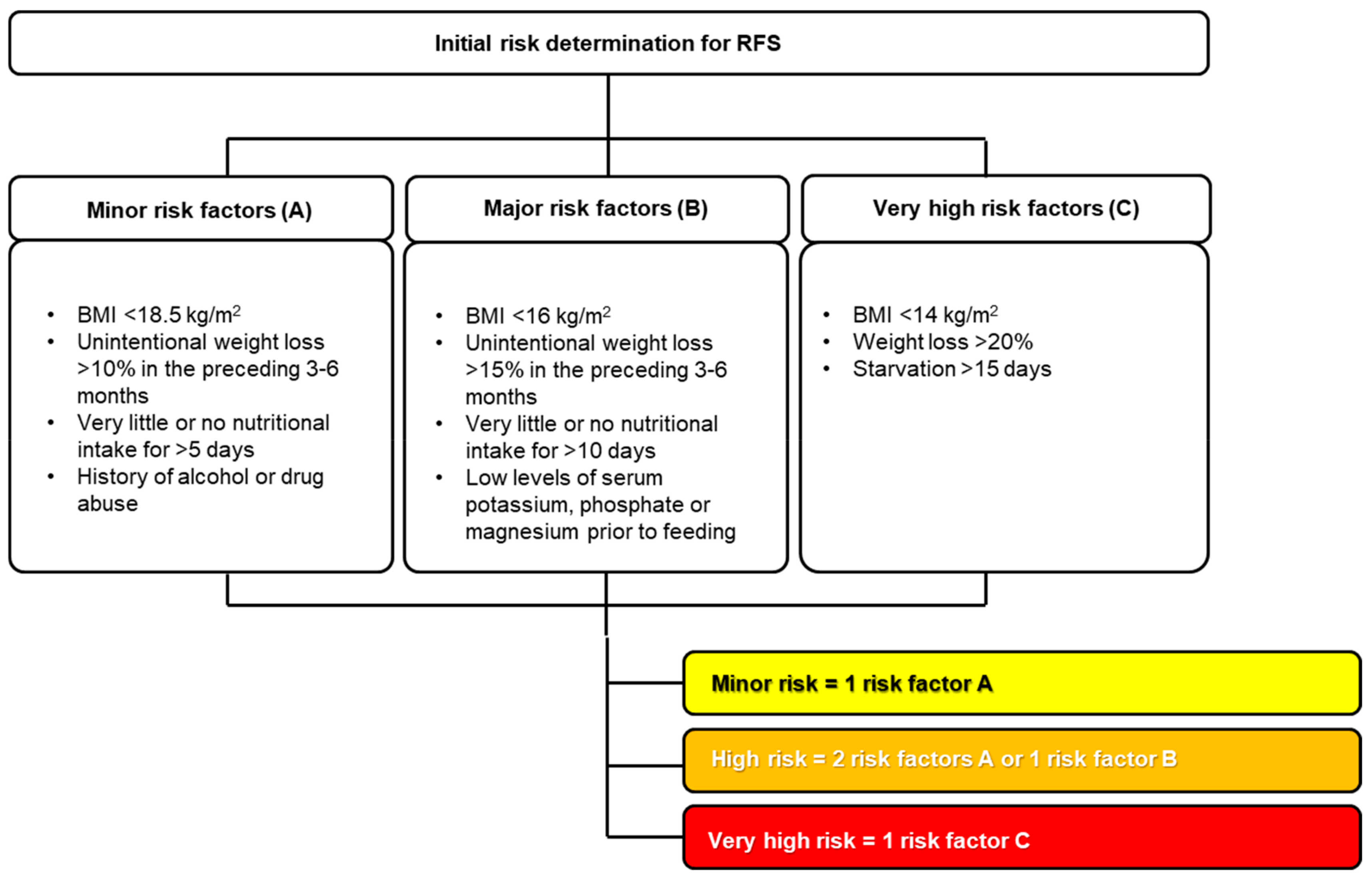
4.2. Individual Risk Assessment
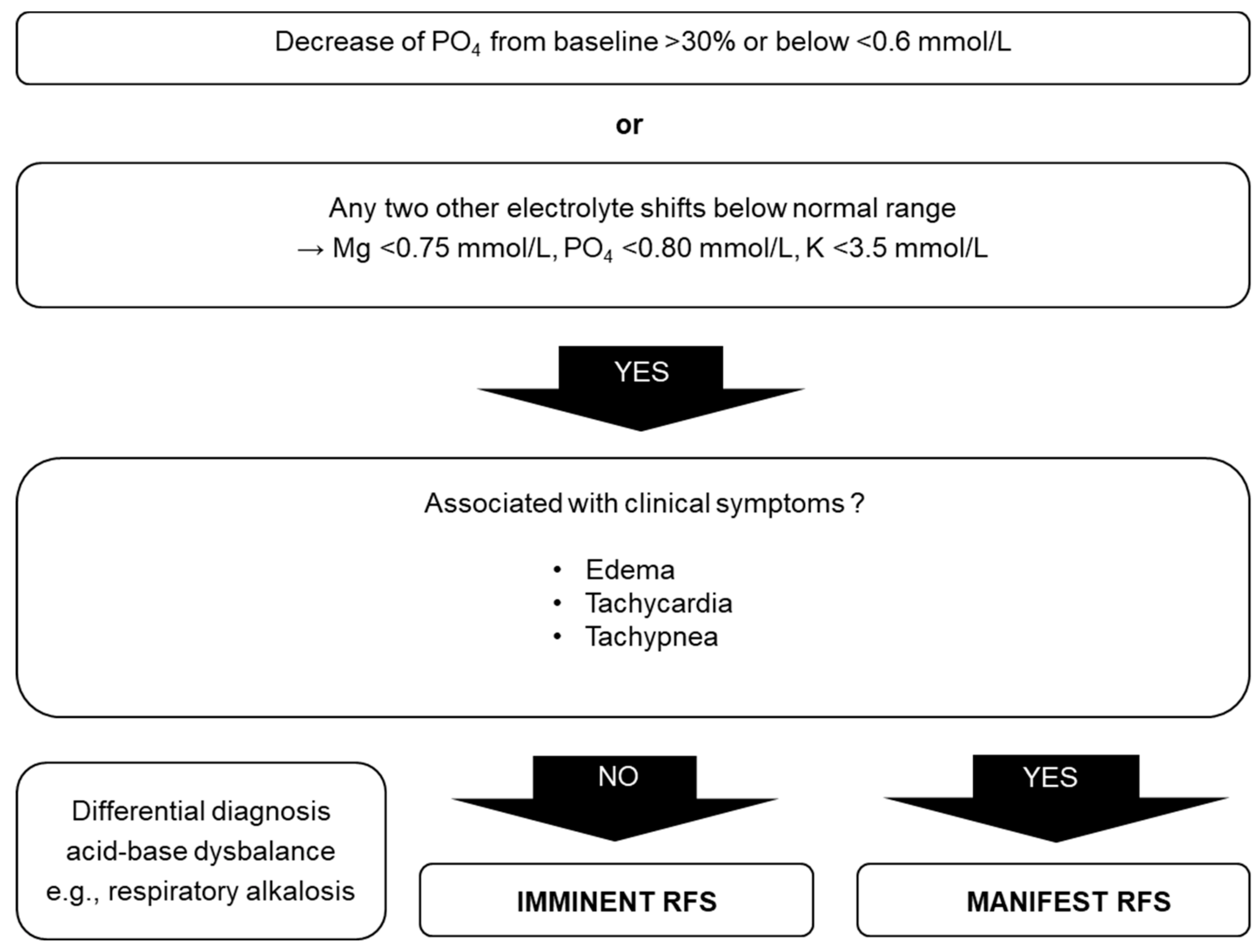
5. Diagnostic Procedure
6. Clinical Management
6.1. Macronutrients
6.2. Fluids
6.3. Micronutrients
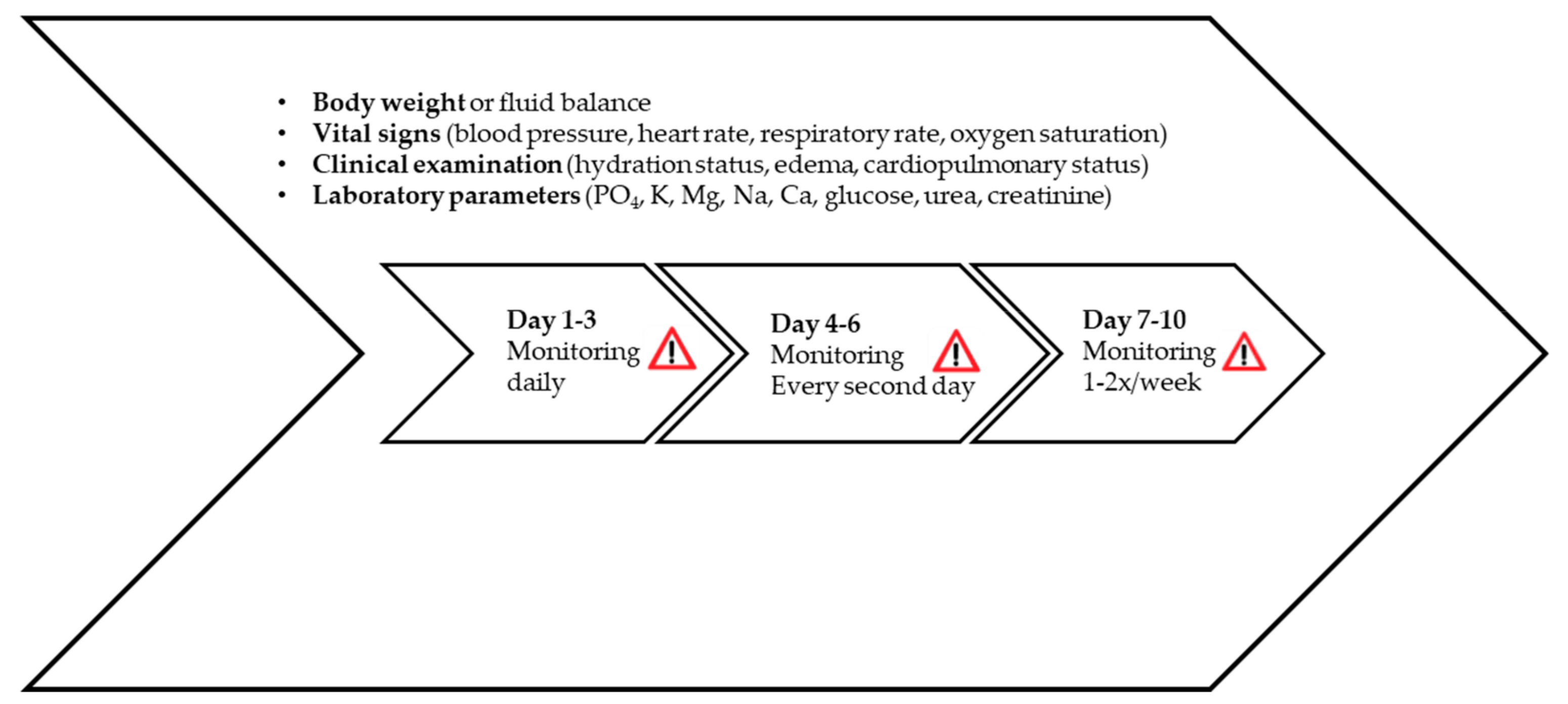
7. Monitoring
8. Important Clinical Sequelae of Refeeding Syndrome and Management of Complications
9. Outlook
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keys, A.; Brožek, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L. The Biology of Human Starvation. (2 Vols); University of Minnesota Press: Minneapolis, MN, USA, 1950. [Google Scholar]
- Schnitker, M.A.; Mattman, P.E.; Bliss, T.L. A clinical study of malnutrition in Japanese prisoners of war. Ann. Intern. Med. 1951, 35, 69–96. [Google Scholar] [PubMed]
- Burger, G.; Drummond, J.; Sandstead, H. Appendices to Malnutrition and Starvation in Western Netherlands, September 1944–July 1945 (Part II); The Hague General State Printing Office: The Hague, The Netherlands, 1948. [Google Scholar]
- Hernandez-Aranda, J.C.; Gallo-Chico, B.; Luna-Cruz, M.L.; Rayon-Gonzalez, M.I.; Flores-Ramirez, L.A.; Ramos Munoz, R.; Ramirez-Barba, E.J. Malnutrition and total parenteral nutrition: A cohort study to determine the incidence of refeeding syndrome. Rev. Gastroenterol. M. 1997, 62, 260–265. [Google Scholar]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Hoess, C.; Pavlicek, V.; et al. Individualized nutritional support in medical inpatients at nutritional risk: A randomized clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Friedli, N.; Baumann, J.; Hummel, R.; Kloter, M.; Odermatt, J.; Fehr, R.; Felder, S.; Baechli, V.; Geiser, M.; Deiss, M.; et al. Refeeding Syndrome is associated with increased mortality in malnourished medical inpatients: Secondary Analysis of a Randomized Trial. Medicine 2019, in press. [Google Scholar]
- Fierz, Y.C.; Kenmeni, R.; Gonthier, A.; Lier, F.; Pralong, F.; Coti Bertrand, P. Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur. J. Clin. Nutr. 2014, 68, 531–533. [Google Scholar] [CrossRef]
- Crook, M.; Hally, V.; Panteli, J. The Importance of the refeeding syndrome. Nutrition 2001, 17, 632–637. [Google Scholar] [CrossRef]
- Felder, S.; Braun, N.; Stanga, Z.; Kulkarni, P.; Faessler, L.; Kutz, A.; Steiner, D.; Laukemann, S.; Haubitz, S.; Huber, A.; et al. Unraveling the Link between Malnutrition and Adverse Clinical Outcomes: Association of Acute and Chronic Malnutrition Measures with Blood Biomarkers from Different Pathophysiological States. Ann. Nutr. Metab. 2016, 68, 164–172. [Google Scholar] [CrossRef]
- Preiser, J.C.; van Zanten, A.R.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and nutritional support of critically ill patients: Consensus and controversies. Crit. Care 2015, 19, 35. [Google Scholar] [CrossRef]
- Solomon, S.M.; Kirby, D.F. The refeeding syndrome: A review. J. Parenter. Enter. Nutr. 1990, 14, 90–97. [Google Scholar] [CrossRef]
- Stanga, Z.; Brunner, A.; Leuenberger, M.; Grimble, R.F.; Shenkin, A.; Allison, S.P.; Lobo, D.N. Nutrition in clinical practice-the refeeding syndrome: Illustrative cases and guidelines for prevention and treatment. Eur. J. Clin. Nutr. 2008, 62, 687–694. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- McCray, S.; Walker, S.; Parrish, C.R. Much ado about refeeding. Pract. Gastroenterol. 2005, 29, 26–44. [Google Scholar]
- Boateng, A.A.; Sriram, K.; Meguid, M.M.; Crook, M. Refeeding syndrome: Treatment considerations based on collective analysis of literature case reports. Nutrition 2010, 26, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.J.; Melnik, G. The refeeding syndrome: An approach to understanding its complications and preventing its occurrence. Pharmacotherapy 1995, 15, 713–726. [Google Scholar]
- Mehler, P.S.; Krantz, M. Anorexia nervosa medical issues. J. Women’s Health 2003, 12, 331–340. [Google Scholar] [CrossRef]
- Friedli, N.; Stanga, Z.; Culkin, A.; Crook, M.; Laviano, A.; Sobotka, L.; Kressig, R.W.; Kondrup, J.; Mueller, B.; Schuetz, P. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018, 47, 13–20. [Google Scholar] [CrossRef]
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition 2017, 35, 151–160. [Google Scholar] [CrossRef]
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981, 21, 165–171. [Google Scholar] [CrossRef]
- Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Inselspital, Bern University Hospital, and University of Bern. Available online: http://www.udem.insel.ch/de/lehre-und-forschung/forschung/wichtige-abbildungen/ (accessed on 12 December 2019).
- National Institute for Health and Clinical Excellence. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition (Clinical Guidance 32). Available online: https://www.nice.org.uk/Guidance/CG32 (accessed on 12 December 2019).
- Schuetz, P.; Zurfluh, S.; Stanga, Z. Mortality due to refeeding syndrome? You only find what you look for, and you only look for what you know. Eur. J. Clin. Nutr. 2018, 72, 307–308. [Google Scholar] [CrossRef]
- Hofer, M.; Pozzi, A.; Joray, M.; Ott, R.; Hahni, F.; Leuenberger, M.; von Kanel, R.; Stanga, Z. Safe refeeding management of anorexia nervosa inpatients: An evidence-based protocol. Nutrition 2014, 30, 524–530. [Google Scholar] [CrossRef]
- Nightingale, J. Nutrition support teams: How they work, are set up and maintained. Frontline Gastroenterol. 2010, 1, 171–177. [Google Scholar] [CrossRef]
- Ten Dam, S.; Droop, A.; Arjaans, W.; de Groot, S.; van Bokhorst-de van der Schueren, M. Module 11.1 Organisation of a nutritional support team. In Organisation of Nutritional Care. Ethical and Legal Aspects; ESPEN: Luxembourg, 2012. [Google Scholar]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e002173. [Google Scholar] [CrossRef]
- Zeki, S.; Culkin, A.; Gabe, S.M.; Nightingale, J.M. Refeeding hypophosphataemia is more common in enteral than parenteral feeding in adult in patients. Clin. Nutr. 2011, 30, 365–368. [Google Scholar] [CrossRef]
- Goyale, A.; Ashley, S.L.; Taylor, D.R.; Elnenaei, M.O.; Alaghband-Zadeh, J.; Sherwood, R.A.; le Roux, C.W.; Vincent, R.P. Predicting refeeding hypophosphataemia: Insulin growth factor 1 (IGF-1) as a diagnostic biochemical marker for clinical practice. Ann. Clin. Biochem. 2015, 52, 82–87. [Google Scholar] [CrossRef]
- Terlevich, A.; Hearing, S.D.; Woltersdorf, W.W.; Smyth, C.; Reid, D.; McCullagh, E.; Day, A.; Probert, C.S. Refeeding syndrome: Effective and safe treatment with Phosphates Polyfusor. Aliment. Pharmacol. Ther. 2003, 17, 1325–1329. [Google Scholar] [CrossRef]
- Brown, C.A.; Sabel, A.L.; Gaudiani, J.L.; Mehler, P.S. Predictors of hypophosphatemia during refeeding of patients with severe anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 898–904. [Google Scholar] [CrossRef]
- Fan, C.G.; Ren, J.A.; Wang, X.B.; Li, J.S. Refeeding syndrome in patients with gastrointestinal fistula. Nutrition 2004, 20, 346–350. [Google Scholar] [CrossRef]
- Gaudiani, J.L.; Sabel, A.L.; Mehler, P.S. Low prealbumin is a significant predictor of medical complications in severe anorexia nervosa. Int. J. Eat. Disord. 2014, 47, 148–156. [Google Scholar] [CrossRef]
- Kagansky, N.; Levy, S.; Koren-Morag, N.; Berger, D.; Knobler, H. Hypophosphataemia in old patients is associated with the refeeding syndrome and reduced survival. J. Intern. Med. 2005, 257, 461–468. [Google Scholar] [CrossRef]
- Kraaijenbrink, B.; Lambers, W.; Mathus-Vliegen, E.; Siegert, C. Incidence of RFS in internal medicine patients. Ned. J. Med. 2016, 74, 116–121. [Google Scholar]
- Pourhassan, M.; Cuvelier, I.; Gehrke, I.; Marburger, C.; Modreker, M.K.; Volkert, D.; Willschrei, H.P.; Wirth, R. Risk factors of refeeding syndrome in malnourished older hospitalized patients. Clin. Nutr. 2018, 37, 1354–1359. [Google Scholar] [CrossRef]
- Vignaud, M.; Constantin, J.M.; Ruivard, M.; Villemeyre-Plane, M.; Futier, E.; Bazin, J.E.; Annane, D.; AZUREA group (AnorexieRea Study Group). Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: An observational study. Crit. Care 2010, 14, R172. [Google Scholar] [CrossRef]
- Tsiompanou, E.; Lucas, C.; Stroud, M. Overfeeding and overhydration in elderly medical patients: Lessons from the Liverpool Care Pathway. Clin. Med. 2013, 13, 248–251. [Google Scholar] [CrossRef]
- Hearing, S.D. Refeeding syndrome. BMJ 2004, 328, 908–909. [Google Scholar] [CrossRef]
- Marik, P.E.; Bedigian, M.K. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch. Surg. 1996, 131, 1043–1047. [Google Scholar] [CrossRef]
- Dewar, H.; Horvath, R. Refeeding Syndrome: Guidelines; Oxford Radcliffe Hospital NHS Trust: Oxford, UK, 1996. [Google Scholar]
- Stroud, M.; Duncan, H.; Nightingale, J. Guidelines for enteral feeding in adult hospital patients. Gut 2003, 52 (Suppl. 7), vii1–vii12. [Google Scholar] [CrossRef]
- Kraft, M.D.; Btaiche, I.F.; Sacks, G.S. Review of the refeeding syndrome. Nutr. Clin. Pract. 2005, 20, 625–633. [Google Scholar] [CrossRef]
- Stanga, Z.; Sobotka, L.; Schuetz, P. Refeeding Syndrome. In Basics in Clinical Nutrition, 5th ed.; Sobotka, L., Ed.; Galen: Prag, Czech Republic, 2019; in press. [Google Scholar]
- Crook, M.A. Refeeding syndrome: Problems with definition and management. Nutrition 2014, 30, 1448–1455. [Google Scholar] [CrossRef]
- Eichelberger, M.; Joray, M.L.; Perrig, M.; Bodmer, M.; Stanga, Z. Management of patients during hunger strike and refeeding phase. Nutrition 2014, 30, 1372–1378. [Google Scholar] [CrossRef]
- Gonzalez Avila, G.; Fajardo Rodriguez, A.; Gonzalez Figueroa, E. The incidence of the refeeding syndrome in cancer patients who receive artificial nutritional treatment. Nutr. Hosp. 1996, 11, 98–101. [Google Scholar] [PubMed]
- Marvin, V.A.; Brown, D.; Portlock, J.; Livingstone, C. Factors contributing to the development of hypophosphataemia when refeeding using parenteral nutrition. Pharm. World Sci. 2008, 30, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.K.; Michihata, N.; Hetnal, K.; Shafer, M.A.; Moscicki, A.B. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J. Adolesc. Health 2012, 50, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Coskun, R.; Gundogan, K.; Baldane, S.; Guven, M.; Sungur, M. Refeeding hypophosphatemia: A potentially fatal danger in the intensive care unit. Turk. J. Med. Sci. 2014, 44, 369–374. [Google Scholar] [CrossRef]
- Doig, G.S.; Simpson, F.; Heighes, P.T.; Bellomo, R.; Chesher, D.; Caterson, I.D.; Reade, M.C.; Harrigan, P.W. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: A randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir. Med. 2015, 3, 943–952. [Google Scholar] [CrossRef]
- Whitelaw, M.; Gilbertson, H.; Lam, P.Y.; Sawyer, S.M. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J. Adolesc. Health 2010, 46, 577–582. [Google Scholar] [CrossRef]
- Luque, S.; Berenguer, N.; Mateu de Antonio, J.; Grau, S.; Morales-Molina, J.A. Patients at risk of malnutrition: Assessment of 11 cases of severe malnutrition with individualised total parenteral nutrition. Farm. Hosp. 2007, 31, 238–242. [Google Scholar] [CrossRef]
- Manning, S.; Gilmour, M.; Weatherall, M.; Robinson, G.M. Refeeding syndrome is uncommon in alcoholics admitted to a hospital detoxification unit. Intern. Med. J. 2014, 44, 512–514. [Google Scholar] [CrossRef]
- Gentile, M.G.; Pastorelli, P.; Ciceri, R.; Manna, G.M.; Collimedaglia, S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin. Nutr. 2010, 29, 627–632. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, H.L.; Bair, M.J.; Wu, C.H.; Lin, I.T.; Lee, Y.K.; Chu, C.H. Refeeding syndrome in Southeastern Taiwan: Our experience with 11 cases. World J. Gastroenterol. 2014, 20, 10525–10530. [Google Scholar] [CrossRef]
- Golden, N.H.; Keane-Miller, C.; Sainani, K.L.; Kapphahn, C.J. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J. Adolesc. Health 2013, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, A.; Turrini, T.; Sherwood, K.; Katzman, D.K. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J. Adolesc. Health 2013, 53, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Flesher, M.E.; Archer, K.A.; Leslie, B.D.; McCollom, R.A.; Martinka, G.P. Assessing the metabolic and clinical consequences of early enteral feeding in the malnourished patient. J. Parenter. Enter. Nutr. 2005, 29, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.; Boyce, F.; Sumukadas, D.; Witham, M.D. Changes in serum magnesium and phosphate in older hospitalised patients—Correlation with muscle strength and risk factors for refeeding syndrome. J. Nutr. Health Aging 2010, 14, 872–876. [Google Scholar] [CrossRef]
- Winter, T.A.; O’Keefe, S.J.; Callanan, M.; Marks, T. The effect of severe undernutrition and subsequent refeeding on whole-body metabolism and protein synthesis in human subjects. J. Parenter. Enter. Nutr. 2005, 29, 221–228. [Google Scholar] [CrossRef]
- Olthof, L.E.; Koekkoek, W.; van Setten, C.; Kars, J.C.N.; van Blokland, D.; van Zanten, A.R.H. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: A retrospective study. Clin. Nutr. 2018, 37, 1609–1617. [Google Scholar] [CrossRef]
- Knochel, J.P. Hypophosphatemia. Clin. Nephrol. 1977, 7, 131–137. [Google Scholar]
- Pantoja, F.; Fragkos, K.C.; Patel, P.S.; Keane, N.; Samaan, M.A.; Barnova, I.; Di Caro, S.; Mehta, S.J.; Rahman, F. Refeeding syndrome in adults receiving total parenteral nutrition: An audit of practice at a tertiary UK centre. Clin. Nutr. 2019, 38, 1457–1463. [Google Scholar] [CrossRef]
- Walmsley, R.S. Refeeding syndrome: Screening, incidence, and treatment during parenteral nutrition. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 113–117. [Google Scholar] [CrossRef]
- Hoppe, A.; Metler, M.; Berndt, T.J.; Knox, F.G.; Angielski, S. Effect of respiratory alkalosis on renal phosphate excretion. Am. J. Physiol. 1982, 243, F471–F475. [Google Scholar] [CrossRef]
- Mostellar, M.E.; Tuttle, E.P., Jr. Effects of alkalosis on plasma concentration and urinary excretion of inorganic phosphate in man. J. Clin. Investig. 1964, 43, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Lewington, A.; Allison, S. Basic Concepts of Fluid and Electrolyte Therapy; Bibliomed. Medizinische Verlagsgesellschaft mbH: Melsungen, Germany, 2013. [Google Scholar]
- Huang, C.L.; Kuo, E. Mechanism of hypokalemia in magnesium deficiency. J. Am. Soc. Nephrol. 2007, 18, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Md Ralib, A.; Mat Nor, M.B. Refeeding hypophosphataemia after enteral nutrition in a Malaysian intensive care unit: Risk factors and outcome. Asia Pac. J. Clin. Nutr. 2018, 27, 329–335. [Google Scholar] [PubMed]
- Brannan, P.G.; Vergne-Marini, P.; Pak, C.Y.; Hull, A.R.; Fordtran, J.S. Magnesium absorption in the human small intestine. Results in normal subjects, patients with chronic renal disease, and patients with absorptive hypercalciuria. J. Clin. Investig. 1976, 57, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Gennari, F.J. Hypokalemia. N. Engl. J. Med. 1998, 339, 451–458. [Google Scholar] [CrossRef]
- Marinella, M.A. Refeeding syndrome in cancer patients. Int. J. Clin. Pract. 2008, 62, 460–465. [Google Scholar] [CrossRef]
- Thatte, L.; Oster, J.R.; Singer, I.; Bourgoignie, J.J.; Fishman, L.M.; Roos, B.A. Review of the literature: Severe hyperphosphatemia. Am. J. Med. Sc. 1995, 310, 167–174. [Google Scholar] [CrossRef]
- Weisinger, J.R.; Bellorin-Font, E. Magnesium and phosphorus. Lancet 1998, 352, 391–396. [Google Scholar] [CrossRef]
- Btaiche, I.F.; Khalidi, N. Metabolic complications of parenteral nutrition in adults, part 1. Am. J. Health Syst. Pharm. 2004, 61, 1938–1949. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Bethel, R.A.; Ansley, J.D.; Gibbs, D.M.; Felner, J.M.; Nutter, D.O. Cardiac abnormalities in cachectic patients before and during nutritional repletion. Am. Heart J. 1978, 95, 584–594. [Google Scholar] [CrossRef]
- Kohn, M.R.; Golden, N.H.; Shenker, I.R. Cardiac arrest and delirium: Presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J. Adolesc. Health 1998, 22, 239–243. [Google Scholar] [CrossRef]
- Havala, T.; Shronts, E. Managing the complications associated with refeeding. Nutr. Clin. Pract. 1990, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.; Sriram, K. Acute respiratory failure due to refeeding syndrome and hypophosphatemia induced by hypocaloric enteral nutrition. Nutrition 2009, 25, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L.; Krumdieck, C.L. Death resulting from overzealous total parenteral nutrition: The refeeding syndrome revisited. Am. J. Clin. Nutr. 1981, 34, 393–399. [Google Scholar] [CrossRef]
- De Filippo, E.; Marra, M.; Alfinito, F.; Di Guglielmo, M.L.; Majorano, P.; Cerciello, G.; De Caprio, C.; Contaldo, F.; Pasanisi, F. Hematological complications in anorexia nervosa. Eur. J. Clin. Nutr. 2016, 70, 1305–1308. [Google Scholar] [CrossRef]
- Yawata, Y.; Hebbel, R.P.; Silvis, S.; Howe, R.; Jacob, H. Blood cell abnormalities complicating the hypophosphatemia of hyperalimentation: Erythrocyte and platelet ATP deficiency associated with hemolytic anemia and bleeding in hyperalimented dogs. J. Lab. Clin. Med. 1974, 84, 643–653. [Google Scholar]
- Kheloufi, M.; Boulanger, C.M.; Codogno, P.; Rautou, P.E. Autosis occurs in the liver of patients with severe anorexia nervosa. Hepatology 2015, 62, 657–658. [Google Scholar] [CrossRef]
- Giordano, F.; Arnone, S.; Santeusanio, F.; Pampanelli, S. Brief elevation of hepatic enzymes due to liver ischemia in anorexia nervosa. Eat. Weight Disord. 2010, 15, e294–e297. [Google Scholar] [CrossRef]
- Bally, M.R.; Blaser Yildirim, P.Z.; Bounoure, L.; Gloy, V.L.; Mueller, B.; Briel, M.; Schuetz, P. Nutritional Support and Outcomes in Malnourished Medical Inpatients: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2016, 176, 43–53. [Google Scholar] [CrossRef]
- Elnenaei, M.O.; Alaghband-Zadeh, J.; Sherwood, R.; Awara, M.A.; Moniz, C.; le Roux, C.W. Leptin and insulin growth factor 1: Diagnostic markers of the refeeding syndrome and mortality. Br. J. Nutr. 2011, 106, 906–912. [Google Scholar] [CrossRef]
- Alaei Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. High-dose thiamine supplementation improves glucose tolerance in hyperglycemic individuals: A randomized, double-blind cross-over trial. Eur. J. Nutr. 2013, 52, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Francini-Pesenti, F.; Brocadello, F.; Manara, R.; Santelli, L.; Laroni, A.; Caregaro, L. Wernicke’s syndrome during parenteral feeding: Not an unusual complication. Nutrition 2009, 25, 142–146. [Google Scholar] [CrossRef] [PubMed]
| Clinical Conditions | |
|---|---|
| - Malnourished, catabolic patients - Geriatric patients - Oncologic patients - Trauma patients - Critically ill patients - Hunger strikers or prolonged fasting - Short -bowel syndrome - Bariatric surgery - Anorexia nervosa - Cystic fibrosis | - Chronic wasting disease - Chronic pancreatitis - Chronic infectious disease - Inflammatory bowel syndrome - Liver cirrhosis - Patients with dysphagia - Patients with hemodialysis - Patients with chemotherapy - Patients with chronic alcoholism - Drug dependent patients |
| Reference | Type of Study | Level of Evidence | Initial Energy/day | Proteins/day | Fluids/day | Vitamins (Before/During) |
|---|---|---|---|---|---|---|
| Solomon et al. 1990 [11] | Review | 4 | 20 kcal/kg | 1.2–1.5 g | NR | NR |
| Dewar et al. 2000 [42] | Review, guidelines | 4 | 20 kcal/kg | NR | NR | Thiamine IV or PO for 2 days |
| Crook et al. 2001 [8] | Review | 4 | 10 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 15–25% fat | 20–30% 1.2–1.5 g | 20–30 mL/kg, 0 fluid balance | Thiamine 300 mg IV, than 100 mg daily during refeeding. In addition, Vit B12, Vit B6 and folate |
| Stroud et al. 2003 [43] | Review | 4 | 10–20 kcal/kg | NR | NR | Thiamine and B vitamins IV for 3 days |
| Kraft et al. 2005 [44] | Review, guidelines | 4 | 7.5 kcal/kg | NR | <1000 mL/day | Thiamine 50–100 mg IV or 100 mg PO for 5–7 days and multivitamin |
| NICE 2006 [23] | Review, guidelines | 4 | 10 kcal/kg high risk: 5 kcal/kg | NR | 0 fluid balance | Thiamine 200–300 mg PO for 10 days and multivitamin for 10 days |
| Stanga et al. 2008 [12] | Case series | 4 | 10–15 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 30–40% fat | 15–20% | 20–30 mL/kg, 0 fluid balance | Thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days |
| Mehanna et al. 2008 [16] | Review | 4 | 10 kcal/kg high risk: 5 kcal/kg | NR | carefully fluid repletion | Thiamine 200–300 mg PO for 10 days and multivitamin for 10 days |
| Boateng et al. 2010 [15] | Case series | 4 | 10 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 15–25% fat | 20–30% 1.2–1.5 g | 20–30 mL/kg, 0 fluid balance | Thiamine 300 mg IV, then 100 mg daily during refeeding. In addition, Vit B12, Vit B6 and folate |
| ESPEN 2019 [45] | Review, guidelines | 4 | 10–15 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 30–40% fat | 15–20% | 20–30 mL/kg, 0 fluid balance | Thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days |
| Crook et al. 2014 [46] | Review | 4 | 10 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 15–25% fat | 20–30% 1.2–1.5 g | 20–30 mL/kg, 0 fluid balance | Thiamine 300 mg IV, then 100 mg daily during refeeding. In addition, Vit B12, Vit B6 and folate |
| Friedli et al. 2017 [20] | Systematic review | 3a | 10–15 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 30–40% fat | 15–20% | 20–30 mL/kg, 0 fluid balance | Thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days |
| Friedli et al. 2018 [19] | Systematic review, consensus paper | 3a | 10–15 kcal/kg high risk: 5 kcal/kg 50–60% CHO, 30–40% fat | 15–20% | 20–30 mL/kg, 0 fluid balance | Thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days |
| Reference | Type of Study | Level of Evidence | N | Preventive Medication | Therapeutic Medication | Effectivity |
|---|---|---|---|---|---|---|
| Hofer et al. 2014 [25] | Retrospective study | 3b | 86 | Hypocaloric feeding, restricted fluid administration (0 fluid balance), thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days, electrolyte supplementation (unless prefeeding serum levels are high): PO4 0.5–0.8 mmol/kg/day, K 1–2.2 mmol/kg/day, Mg 0.3–0.4 mmol/kg/day | Hypocaloric feeding, restricted fluid administration, electrolytes substitution according to the serum level | Yes |
| Eichelberger et al. 2014 [47] | Retrospective study | 3b | 37 | Hypocaloric feeding, restricted fluid administration (0 fluid balance), thiamine 200–300 mg IV or PO for 3 days and multivitamin for 10 days, electrolyte supplementation (unless prefeeding serum levels are high): PO4 0.5–0.8 mmol/kg/day, K 1–2.2 mmol/kg/day, Mg 0.3–0.4 mmol/kg/day | Hypocaloric feeding, restricted fluid administration, electrolytes substitution according to the serum level | Yes |
| Terlevich et al. 2003 [31] | Prospective study | 4 | 30 | NR | 50 mmol PO4 over 24h | Yes |
| Gonzalez Aviva et al. 1996 [48] | Prospective study | 3b | 106 | PO4 supplementation | NR | Yes |
| Marvin et al. 2008 [49] | Case control study | 3b | 140 | During the first 24 h slow PN regimen providing <70% of protein and calories but >12 mmol PO4 | NR | Yes |
| Garber et al. 2011 [50] | Retrospective study | 4 | 40 | No effective preventive measures found | NR | No |
| Coskun et al. 2014 [51] | Retrospective study | 4 | 117 | Lower energy intake | NR | No |
| Doig et al. 2015 [52] | RCT | 1b | 339 | NR | Lower caloric intake | Yes |
| Whitelaw et al. 2010 [53] | Retrospective study | 4 | 46 | Prophylactic administration of PO4, lower initial energy intake, monitoring of PO4 | Supplementation of PO4 | Yes |
| Luque et al. 2007 [54] | Retrospective study | 4 | 11 | PO4 supplementation, thiamine 3.51 mg/d | NR | Yes |
| Manning et al. 2014 [55] | Prospective study | 2b | 36 | Repeated electrolyte testing | NR | No |
| Fan et al. 2004 [33] | Retrospective study | 4 | 158 | PO4 supplementation | NR | Yes, if PO4 <0.30 |
| Gentile et al. 2010 [56] | Retrospective study | 4 | 33 | Prophylactic administration of PO4 and K, cautious nutritional rehabilitation | NR | Yes |
| Vignaud et al. 2010 [38] | Retrospective study | 4 | 68 | For patients at risk for initial nutritional support 10 kcal/kg/day falling to as low as 5 kcal/kg/day | NR | Yes |
| Chen et al. 2014 [57] | Retrospective study | 4 | 56 | Thiamine and multivitamin supplementation, 15 kcal/kg/day | NR | Yes |
| Golden et al. 2013 [58] | Retrospective study | 4 | 310 | Lower caloric intake | NR | No |
| Leclerc et al. 2013 [59] | Retrospective study | 4 | 29 | Hypocaloric feeding | NR | No |
| Flesher et al. 2005 [60] | Retrospective study | 4 | 51 | Thiamine supplementation, cautious feeding | NR | No |
| Rio et al. 2013 [28] | Prospective | 2b | 243 | Hypocaloric feeding | NR | No |
| Potassium | Magnesium | Phosphate | |
|---|---|---|---|
| Mild deficiency | 3.1–3.5 mmol/L Oral replacement with 20 mmol (as KCl or other salts) OR i.v. replacement with 20 mmol KCl over 4 to 8 h. Check levels the next day. | 0.5–0.7 mmol/L Oral replacement with 10–15 mmol MgCl2 or Mg-citrate or Mg-L-aspartate Oral Mg should be given in divided doses to minimize diarrhea (absorption process is saturated at about 5–10 mmol Mg) | 0.61–0.8 mmol/L Oral replacement with 0.3 mmol/kg/day PO4 (divided doses to minimize diarrhea) OR i.v. replacement with 0.3 mmol/kg/day PO4 (as K3PO4 or Na3PO4) over 8–12 h. Check levels the next day. |
| Moderate deficiency | 2.5–3.0 mmol/L i.v. replacement with 20–40 mmol KCl over 4–8 h. Check levels after 8 h; if not normal, give an additional 20 mmol KCl. | 0.32–0.6 mmol/L i.v. replacement with 0.6 mmol/kg/day PO4 (as K3PO4 or Na3PO4) over 8–12 h. Check levels after 8–12 h and repeat infusion if necessary (max. of 50 mmol PO4 in 24 h). | |
| Severe deficiency | <2.5 mmol/L i.v. replacement with 40 mmol KCl over 4–8 h. Check levels after 8 h; if not normal, give an additional 40 mmol KCl. | <0.5 mmol/L i.v. replacement with 20–24 mmol MgSO4 (4–6 g) over 4–8 h. Reassess every 8 to 12 h. | <0.32 mmol/L Same replacement therapy as for moderate deficiency. |
| System | Symptoms |
|---|---|
| Cardiovascular | Tachycardia Arrhythmias Hypotension Congestive heart failure Shock Edemas Sudden death |
| Gastrointestinal | Maldigestion and malabsorption Vomiting Constipation Abdominal pain |
| Musculoskeletal | Weakness Myalgia Rhabdomyolysis Osteomalacia |
| Respiratory | Tachypnea Dyspnea Respiratory failure Ventilator dependency Diaphragm muscle weakness |
| Neurologic | Anorexia Paresthesia Tremor Wernicke encephalopathy Korsakoff syndrome Ataxia Tetany Delirium Seizures Coma |
| Metabolic | Hyperglycemia Metabolic alkalosis Metabolic acidosis Respiratory alkalosis Insulin resistance |
| Hematologic | Thrombocytopenia Hemolysis Anemia Leukocyte dysfunction Decreased 2,3-DPG |
| Renal | Acute tubular necrosis |
| Hepatological | Acute liver failure |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reber, E.; Friedli, N.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Management of Refeeding Syndrome in Medical Inpatients. J. Clin. Med. 2019, 8, 2202. https://doi.org/10.3390/jcm8122202
Reber E, Friedli N, Vasiloglou MF, Schuetz P, Stanga Z. Management of Refeeding Syndrome in Medical Inpatients. Journal of Clinical Medicine. 2019; 8(12):2202. https://doi.org/10.3390/jcm8122202
Chicago/Turabian StyleReber, Emilie, Natalie Friedli, Maria F. Vasiloglou, Philipp Schuetz, and Zeno Stanga. 2019. "Management of Refeeding Syndrome in Medical Inpatients" Journal of Clinical Medicine 8, no. 12: 2202. https://doi.org/10.3390/jcm8122202
APA StyleReber, E., Friedli, N., Vasiloglou, M. F., Schuetz, P., & Stanga, Z. (2019). Management of Refeeding Syndrome in Medical Inpatients. Journal of Clinical Medicine, 8(12), 2202. https://doi.org/10.3390/jcm8122202





