Hyperprolactinaemia
Abstract
1. Introduction
2. Causes and Mechanisms of Hyperprolactinaemia
2.1. Physiological Causes
2.1.1. Endogenous Oestrogens
2.1.2. Breastfeeding
2.1.3. Stress
2.1.4. Exercise
2.1.5. Chest Wall Injury
2.2. Pathological Causes
2.2.1. Prolactin-Secreting Pituitary Tumours
2.2.2. “Stalk-Effect”
2.2.3. Renal Failure
2.2.4. Liver Cirrhosis
2.2.5. Primary Hypothyroidism
2.2.6. Polycystic Ovarian Syndrome
2.2.7. Seizures
2.3. Pharmacological
2.3.1. Antipsychotics
2.3.2. Antidepressants
2.3.3. Antiemetics
2.3.4. Opioids
2.3.5. Antihypertensives
3. Clinical Manifestations
3.1. Reproductive Manifestations
3.1.1. Women
3.1.2. Men
3.2. Bone Manifestations
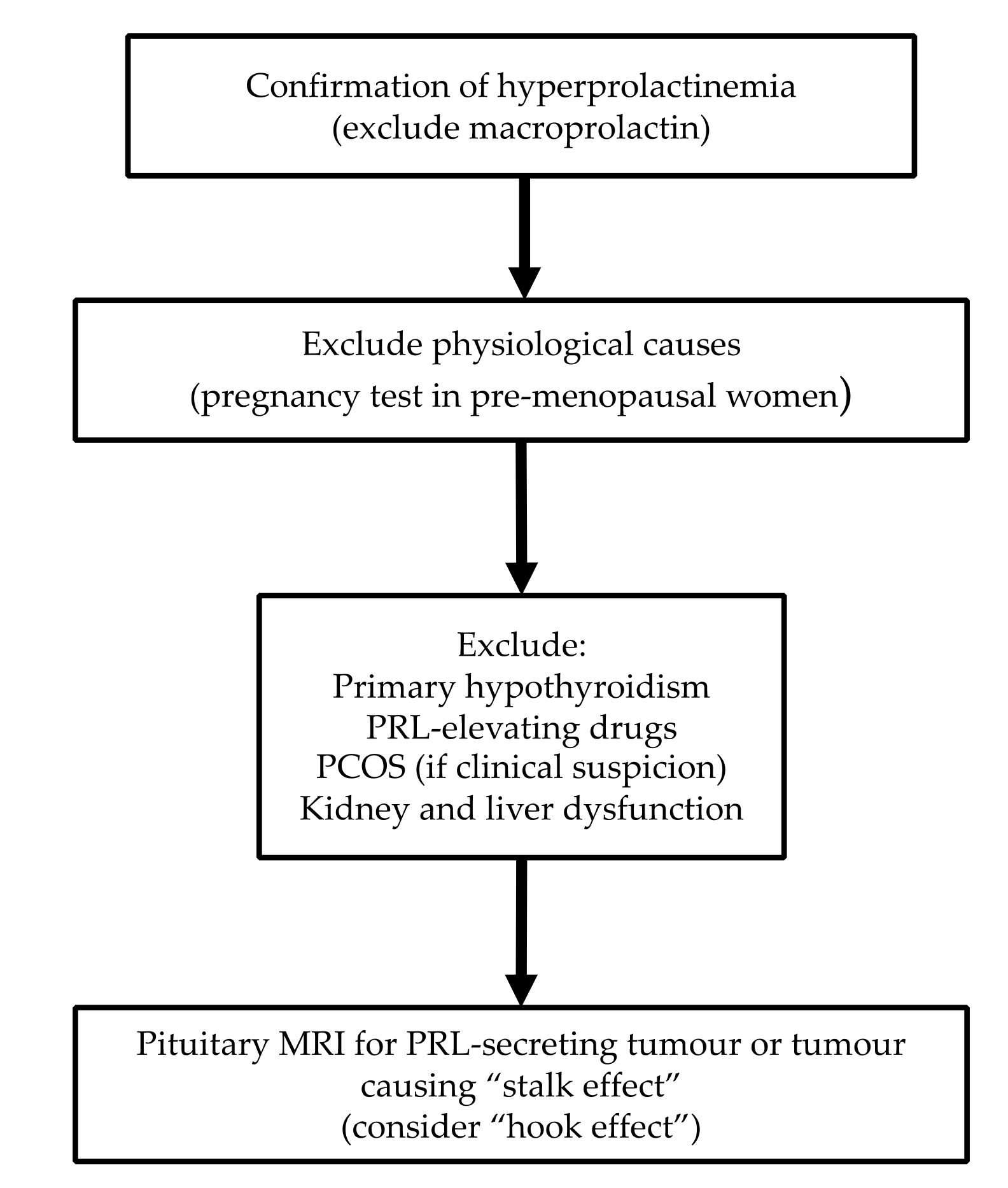
4. Diagnostic Approach
4.1. Blood Sampling for PRL
4.2. Biochemical Interpretation
4.2.1. Macroprolactin
4.2.2. Hook Effect
4.3. Imaging
4.4. Diagnostic Algorithm
5. Management
5.1. Prolactinomas
5.1.1. Conservative Management
5.1.2. Dopamine Agonists
5.1.3. Surgery
5.1.4. Radiotherapy
5.2. “Stalk Effect”
5.3. Other Pathological Causes
5.4. Pharmacological
6. Hyperprolactinaemia and Metabolic/Cardiovascular Risk
6.1. Metabolic Risk
6.2. Cardiovascular Risk
7. Hyperprolactinaemia and Autoimmunity
7.1. Lupus
7.2. Rheumatoid Arthritis
7.3. Multiple Sclerosis
7.4. Other Autoimmune Disorders
8. Cancer
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tucker, H.A. Hormones, mammary growth, and lactation: A 41-year perspective. J. Dairy Sci. 2000, 83, 874–884. [Google Scholar] [CrossRef]
- Trott, J.F.; Vonderhaar, B.K.; Hovey, R.C. Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues—Agog for the future! J. Mammary Gland Biol. Neoplasia 2008, 13, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Riddle, O.; Bates, R.W.; Dykshorn, S.W. The preparation, identification and assay of prolactin—A hormone of the anterior pituitary. Am. J. Physiol. Leg. Content 1933, 105, 191–216. [Google Scholar] [CrossRef]
- Friesen, H.; Guyda, H.; Hardy, J. Biosynthesis of Human Growth Hormone and Prolactin. J. Clin. Endocrinol. Metab. 1970, 31, 611–624. [Google Scholar] [CrossRef]
- Lewis, U.J.; Singh, R.N.; Seavey, B.K. Human prolactin: Isolation and some properties. Biochem. Biophys. Res. Commun. 1971, 44, 1169–1176. [Google Scholar] [CrossRef]
- Prabhakar, V.K.; Davis, J.R. Hyperprolactinaemia. Best Pract. Res. Clin. Obs. Gynaecol. 2008, 22, 341–353. [Google Scholar] [CrossRef]
- Teilum, K.; Hoch, J.C.; Goffin, V.; Kinet, S.; Martial, J.A.; Kragelund, B.B. Solution structure of human prolactin. J. Mol. Biol. 2005, 351, 810–823. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Pontecorvi, A.; Lello, S. Hyperprolactinemia: Pathophysiology and therapeutic approach. Gynecol. Endocrinol. 2015, 31, 506–510. [Google Scholar] [CrossRef]
- Rastrelli, G.; Corona, G.; Maggi, M. The role of prolactin in andrology: What is new? Rev. Endocr. Metab. Disord. 2015, 16, 233–248. [Google Scholar] [CrossRef]
- Grattan, D.R. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef]
- Cabrera-Reyes, E.A.; Limón-Morales, O.; Rivero-Segura, N.A.; Camacho-Arroyo, I.; Cerbón, M. Prolactin function and putative expression in the brain. Endocrine 2017, 57, 199–213. [Google Scholar] [CrossRef]
- Marano, R.J.; Ben-Jonathan, N. Minireview: Extrapituitary Prolactin: An Update on the Distribution, Regulation, and Functions. Mol. Endocrinol. 2014, 28, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Ignacak, A.; Kasztelnik, M.; Sliwa, T.; Korbut, R.A.; Rajda, K.; Guzik, T.J. Prolactin--not only lactotrophin. A “new” view of the “old” hormone. J. Physiol. Pharm. 2012, 63, 435–443. [Google Scholar]
- Levine, S.; Muneyyirci-Delale, O. Stress-Induced Hyperprolactinemia: Pathophysiology and Clinical Approach. Obs. Gynecol. Int. 2018, 2018, 9253083. [Google Scholar] [CrossRef]
- Zaidi, M.; Sun, L.; Liu, P.; Davies, T.F.; New, M.; Zallone, A.; Yuen, T. Pituitary-bone connection in skeletal regulation. Horm. Mol. Biol. Clin. Investig. 2016, 28, 85–94. [Google Scholar] [CrossRef]
- Gregg, C.; Shikar, V.; Larsen, P.; Mak, G.; Chojnacki, A.; Yong, V.W.; Weiss, S. White matter plasticity and enhanced remyelination in the maternal CNS. J. Neurosci. 2007, 27, 1812–1823. [Google Scholar] [CrossRef]
- Tanner, M.J.; Hadlow, N.C.; Wardrop, R. Variation of female prolactin levels with menopausal status and phase of menstrual cycle. Aust. N. Z. J. Obs. Gynaecol. 2011, 51, 321–324. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, Y.; Yang, M.; Xiang, Z. Serum prolactin levels across pregnancy and the establishment of reference intervals. Clin. Chem. Lab. Med. 2018, 56, 803–807. [Google Scholar] [CrossRef]
- Alvarez-Tutor, E.; Forga-LLenas, L.; Rodriguez-Erdozain, R.; Goñi-Iriarte, M.J.; Mendendez-Torre, E.; Alvarez-Tutor, J. Persistent increase of PRL after oral contraceptive treatment. Alterations in dopaminergic regulation as possible etiology. Arch. Gynecol. Obs. 1999, 263, 45–50. [Google Scholar] [CrossRef]
- Banaszewska, B.; Pawelczyk, L.; Spaczynski, R.Z.; Dziura, J.; Duleba, A.J. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: Prospective, randomized, crossover trial. J. Clin. Endocrinol. Metab. 2007, 92, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Crowley, W.R. Neuroendocrine regulation of lactation and milk production. Compr. Physiol. 2015, 5, 255–291. [Google Scholar] [CrossRef] [PubMed]
- Muneyyirci-Delale, O.; Goldstein, D.; Reyes, F.I. Diagnosis of stress-related hyperprolactinemia. Evaluation of the hyperprolactinemia rest test. N. Y. State J. Med. 1989, 89, 205–208. [Google Scholar] [PubMed]
- Hackney, A.C.; Davis, H.C.; Lane, A.R. Growth Hormone-Insulin-Like Growth Factor Axis, Thyroid Axis, Prolactin, and Exercise. Front. Horm. Res. 2016, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.; Society, E. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef]
- Vilar, L.; Fleseriu, M.; Bronstein, M.D. Challenges and pitfalls in the diagnosis of hyperprolactinemia. Arq. Bras. Endocrinol. Metab. 2014, 58, 9–22. [Google Scholar] [CrossRef]
- Saraç, F.; Tütüncüoğlu, P.; Ozgen, A.G.; Saygili, F.; Yilmaz, C.; Bilgen, I.; Memiş, A. Prolactin levels and examination with breast ultrasound or mammography. Adv. Ther. 2008, 25, 59–66. [Google Scholar] [CrossRef]
- Jarrell, J.; Franks, S.; McInnes, R.; Gemayel, K.; Guyda, H.; Arronet, G.H.; Naftolin, F. Breast examination does not elevate serum prolactin. Fertil. Steril. 1980, 33, 49–51. [Google Scholar] [CrossRef]
- Hammond, K.R.; Steinkampf, M.P.; Boots, L.R.; Blackwell, R.E. The effect of routine breast examination on serum prolactin levels. Fertil. Steril. 1996, 65, 869–870. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. Oxf. 2010, 72, 377–382. [Google Scholar] [CrossRef]
- Maiter, D.; Delgrange, E. Therapy of endocrine disease: The challenges in managing giant prolactinomas. Eur. J. Endocrinol. 2014, 170, R213–R227. [Google Scholar] [CrossRef] [PubMed]
- Vilar, L.; Freitas, M.C.; Naves, L.A.; Casulari, L.A.; Azevedo, M.; Montenegro, R.; Barros, A.I.; Faria, M.; Nascimento, G.C.; Lima, J.G.; et al. Diagnosis and management of hyperprolactinemia: Results of a Brazilian multicenter study with 1234 patients. J. Endocrinol. Investig. 2008, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Vilar, L.; Vilar, C.F.; Lyra, R.; Lyra, R.; Naves, L.A. Acromegaly: Clinical features at diagnosis. Pituitary 2017, 20, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A.; Endocrine, S. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef]
- Schernthaner-Reiter, M.H.; Trivellin, G.; Stratakis, C.A. MEN1, MEN4, and Carney Complex: Pathology and Molecular Genetics. Neuroendocrinology 2016, 103, 18–31. [Google Scholar] [CrossRef]
- Thakker, R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell. Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Ozawa, A.; Mateo, C.M.; Marx, S.J. The MEN1 gene and pituitary tumours. Horm. Res. 2009, 71 (Suppl. S2), 131–138. [Google Scholar] [CrossRef]
- Beckers, A.; Aaltonen, L.A.; Daly, A.F.; Karhu, A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr. Rev. 2013, 34, 239–277. [Google Scholar] [CrossRef]
- Kurtkaya-Yapicier, O.; Scheithauer, B.W.; Carney, J.A.; Kovacs, K.; Horvath, E.; Stratakis, C.A.; Vidal, S.; Vella, A.; Young, W.F.; Atkinson, J.L.; et al. Pituitary adenoma in Carney complex: An immunohistochemical, ultrastructural, and immunoelectron microscopic study. Ultrastruct. Pathol. 2002, 26, 345–353. [Google Scholar] [CrossRef]
- Sun, F.; Sun, X.; Du, X.; Xing, H.; Yang, B. Factors related to endocrine changes and hormone substitution treatment during pre- and post-operation stages in craniopharyngioma. Oncol. Lett. 2017, 13, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, N.; Thanabalasingham, G.; Shore, H.C.; Trifanescu, R.; Ansorge, O.; Meston, N.; Turner, H.E.; Wass, J.A. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin. Endocrinol. Oxf. 2006, 65, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Vilar, L.; Abucham, J.; Albuquerque, J.L.; Araujo, L.A.; Azevedo, M.F.; Boguszewski, C.L.; Casulari, L.A.; Cunha Neto, M.B.C.; Czepielewski, M.A.; Duarte, F.H.G.; et al. Controversial issues in the management of hyperprolactinemia and prolactinomas—An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch. Endocrinol. Metab. 2018, 62, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Tartaglione, T.; Capobianco, A.; Anile, C.; De Marinis, L. Diagnosis of endocrine disease: Primary empty sella: A comprehensive review. Eur. J. Endocrinol. 2017, 177, R275–R285. [Google Scholar] [CrossRef]
- Lo, J.C.; Beck, G.J.; Kaysen, G.A.; Chan, C.T.; Kliger, A.S.; Rocco, M.V.; Chertow, G.M.; Study, F. Hyperprolactinemia in end-stage renal disease and effects of frequent hemodialysis. Hemodial. Int. 2017, 21, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, C.H.; Rajeev, H. Correlation of Serum Prolactin Level to Child Pugh Scoring System in Cirrhosis of Liver. J. Clin. Diagn. Res. 2017, 11, OC30–OC33. [Google Scholar] [CrossRef]
- Sharma, L.K.; Sharma, N.; Gadpayle, A.K.; Dutta, D. Prevalence and predictors of hyperprolactinemia in subclinical hypothyroidism. Eur. J. Intern. Med. 2016, 35, 106–110. [Google Scholar] [CrossRef]
- Honbo, K.S.; van Herle, A.J.; Kellett, K.A. Serum prolactin levels in untreated primary hypothyroidism. Am. J. Med. 1978, 64, 782–787. [Google Scholar] [CrossRef]
- Shivaprasad, K.S.; Siddardha, K. Pituitary Hyperplasia from Primary Hypothyroidism. N. Engl. J. Med. 2019, 380, e9. [Google Scholar] [CrossRef]
- Kyritsi, E.M.; Dimitriadis, G.K.; Angelousi, A.; Mehta, H.; Shad, A.; Mytilinaiou, M.; Kaltsas, G.; Randeva, H.S. The value of prolactin in predicting prolactinοma in hyperprolactinaemic polycystic ovarian syndrome. Eur. J. Clin. Investig. 2018, 48, e12961. [Google Scholar] [CrossRef]
- Kyritsi, E.M.; Dimitriadis, G.K.; Kyrou, I.; Kaltsas, G.; Randeva, H.S. PCOS remains a diagnosis of exclusion: A concise review of key endocrinopathies to exclude. Clin. Endocrinol. Oxf. 2017, 86, 1–6. [Google Scholar] [CrossRef]
- Filho, R.B.; Domingues, L.; Naves, L.; Ferraz, E.; Alves, A.; Casulari, L.A. Polycystic ovary syndrome and hyperprolactinemia are distinct entities. Gynecol. Endocrinol. 2007, 23, 267–272. [Google Scholar] [CrossRef]
- Nass, R.D.; Sassen, R.; Elger, C.E.; Surges, R. The role of postictal laboratory blood analyses in the diagnosis and prognosis of seizures. Seizure 2017, 47, 51–65. [Google Scholar] [CrossRef]
- Lusić, I.; Pintarić, I.; Hozo, I.; Boić, L.; Capkun, V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999, 8, 218–222. [Google Scholar] [CrossRef]
- Peuskens, J.; Pani, L.; Detraux, J.; De Hert, M. The effects of novel and newly approved antipsychotics on serum prolactin levels: A comprehensive review. CNS Drugs 2014, 28, 421–453. [Google Scholar] [CrossRef]
- Montejo, Á.; Arango, C.; Bernardo, M.; Carrasco, J.L.; Crespo-Facorro, B.; Cruz, J.J.; Del Pino, J.; García Escudero, M.A.; García Rizo, C.; González-Pinto, A.; et al. Spanish consensus on the risks and detection of antipsychotic drug-related hyperprolactinaemia. Rev. Psiquiatr. Salud Ment. 2016, 9, 158–173. [Google Scholar] [CrossRef]
- Cortet-Rudelli, C.; Sapin, R.; Bonneville, J.F.; Brue, T. Etiological diagnosis of hyperprolactinemia. Ann. Endocrinol. Paris 2007, 68, 98–105. [Google Scholar] [CrossRef]
- Molitch, M.E. Medication-induced hyperprolactinemia. Mayo Clin. Proc. 2005, 80, 1050–1057. [Google Scholar] [CrossRef]
- Ajmal, A.; Joffe, H.; Nachtigall, L.B. Psychotropic-induced hyperprolactinemia: A clinical review. Psychosomatics 2014, 55, 29–36. [Google Scholar] [CrossRef]
- Tewksbury, A.; Olander, A. Management of antipsychotic-induced hyperprolactinemia. Ment. Health Clin. 2016, 6, 185–190. [Google Scholar] [CrossRef]
- Reeves, S.; Sugita, E.; Baldeweg, S.E.; Howard, R. Management of antipsychotic related hyperprolactinemia in older people: Can we extrapolate from existing guidance? Int. J. Geriatr. Psychiatry 2018, 33, 1743–1744. [Google Scholar] [CrossRef]
- McMurdo, M.E.; Howie, P.W.; Lewis, M.; Marnie, M.; McEwen, J.; McNeilly, A.S. Prolactin response to low dose sulpiride. Br. J. Clin. Pharm. 1987, 24, 133–137. [Google Scholar] [CrossRef]
- Cookson, J.; Hodgson, R.; Wildgust, H.J. Prolactin, hyperprolactinaemia and antipsychotic treatment: A review and lessons for treatment of early psychosis. J. Psychopharmacol. 2012, 26, 42–51. [Google Scholar] [CrossRef]
- Ingram, J.; Taylor, H.; Churchill, C.; Pike, A.; Greenwood, R. Metoclopramide or domperidone for increasing maternal breast milk output: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F241–F245. [Google Scholar] [CrossRef]
- Fountas, A.; Chai, S.T.; Kourkouti, C.; Karavitaki, N. Mechanisms of endocrinology: Endocrinology of opioids. Eur. J. Endocrinol. 2018, 179, R183–R196. [Google Scholar] [CrossRef]
- Romeo, J.H.; Dombrowski, R.; Kwak, Y.S.; Fuehrer, S.; Aron, D.C. Hyperprolactinaemia and verapamil: Prevalence and potential association with hypogonadism in men. Clin. Endocrinol. Oxf. 1996, 45, 571–575. [Google Scholar] [CrossRef]
- Kelley, S.R.; Kamal, T.J.; Molitch, M.E. Mechanism of verapamil calcium channel blockade-induced hyperprolactinemia. Am. J. Physiol. 1996, 270, E96–E100. [Google Scholar] [CrossRef]
- Veselinović, T.; Schorn, H.; Vernaleken, I.B.; Schiffl, K.; Klomp, M.; Gründer, G. Impact of different antidopaminergic mechanisms on the dopaminergic control of prolactin secretion. J. Clin. Psychopharmacol. 2011, 31, 214–220. [Google Scholar] [CrossRef]
- Kleinberg, D.L.; Noel, G.L.; Frantz, A.G. Galactorrhea: A study of 235 cases, including 48 with pituitary tumors. N. Engl. J. Med. 1977, 296, 589–600. [Google Scholar] [CrossRef]
- Finken, M.J.; Boersma, B.; Rotteveel, J. Hyperprolactinemia and hyperandrogenism in an adolescent girl presenting with primary amenorrhea. Eur. J. Obs. Gynecol. Reprod. Biol. 2013, 166, 230–231. [Google Scholar] [CrossRef]
- Majumdar, A.; Mangal, N.S. Hyperprolactinemia. J. Hum. Reprod. Sci. 2013, 6, 168–175. [Google Scholar] [CrossRef]
- Moria, Y.; Kortbawi, R.; El-Asmar, N.; Arafah, B.M. Increased androgen secretion in patients with prolactinomas: The impact of altered HPA function. Pituitary 2019, 22, 170–178. [Google Scholar] [CrossRef]
- Carmina, E.; Azziz, R.; Bergfeld, W.; Escobar Morreale, H.F.; Futterweit, W.; Huddleston, H.; Lobo, R.A.; Olsen, E. Female pattern hair loss and androgen excess: A report from the multidisciplinary androgen excess and pcos committee. J. Clin. Endocrinol. Metab. 2019, 104, 2875–2891. [Google Scholar] [CrossRef]
- Serri, O.; Chik, C.L.; Ur, E.; Ezzat, S. Diagnosis and management of hyperprolactinemia. Can. Med. Assoc. J. 2003, 169, 575–581. [Google Scholar]
- De Rosa, M.; Zarrilli, S.; Di Sarno, A.; Milano, N.; Gaccione, M.; Boggia, B.; Lombardi, G.; Colao, A. Hyperprolactinemia in men: Clinical and biochemical features and response to treatment. Endocrine 2003, 20, 75–82. [Google Scholar] [CrossRef]
- Tirosh, A.; Benbassat, C.; Lifshitz, A.; Shimon, I. Hypopituitarism patterns and prevalence among men with macroprolactinomas. Pituitary 2015, 18, 108–115. [Google Scholar] [CrossRef]
- Mazziotti, G.; Frara, S.; Giustina, A. Pituitary Diseases and Bone. Endocr. Rev. 2018, 39, 440–488. [Google Scholar] [CrossRef]
- Ozer, F.F.; Dagdelen, S.; Erbas, T. Relation of RANKL and OPG Levels with Bone Resorption in Patients with Acromegaly and Prolactinoma. Horm. Metab. Res. 2018, 50, 562–567. [Google Scholar] [CrossRef]
- Di Somma, C.; Colao, A.; Di Sarno, A.; Klain, M.; Landi, M.L.; Facciolli, G.; Pivonello, R.; Panza, N.; Salvatore, M.; Lombardi, G. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J. Clin. Endocrinol. Metab. 1998, 83, 807–813. [Google Scholar] [CrossRef]
- Mazziotti, G.; Mancini, T.; Mormando, M.; De Menis, E.; Bianchi, A.; Doga, M.; Porcelli, T.; Vescovi, P.P.; De Marinis, L.; Giustina, A. High prevalence of radiological vertebral fractures in women with prolactin-secreting pituitary adenomas. Pituitary 2011, 14, 299–306. [Google Scholar] [CrossRef]
- D’Sylva, C.; Khan, T.; Van Uum, S.; Fraser, L.A. Osteoporotic fractures in patients with untreated hyperprolactinemia vs. those taking dopamine agonists: A systematic review and meta-analysis. Neuro Endocrinol. Lett. 2015, 36, 745–749. [Google Scholar]
- Casanueva, F.F.; Molitch, M.E.; Schlechte, J.A.; Abs, R.; Bonert, V.; Bronstein, M.D.; Brue, T.; Cappabianca, P.; Colao, A.; Fahlbusch, R.; et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin. Endocrinol. Oxf. 2006, 65, 265–273. [Google Scholar] [CrossRef]
- Saleem, M.; Martin, H.; Coates, P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin. Biochem. Rev. 2018, 39, 3–16. [Google Scholar]
- Lippi, G.; Plebani, M. Macroprolactin: Searching for a needle in a haystack? Clin. Chem. Lab. Med. 2016, 54, 519–522. [Google Scholar] [CrossRef]
- Samson, S.L.; Hamrahian, A.H.; Ezzat, S.; Aace, N.; Pituitary Scientific Committee. American association of clinical endocrinologists, American college of endocrinology disease state clinical review: Clinical relevance of macroprolactin in the absence or presence of true hyperprolactinemia. Endocr. Pract. 2015, 21, 1427–1435. [Google Scholar] [CrossRef]
- Kasum, M.; Orešković, S.; Čehić, E.; Šunj, M.; Lila, A.; Ejubović, E. Laboratory and clinical significance of macroprolactinemia in women with hyperprolactinemia. Taiwan J. Obs. Gynecol. 2017, 56, 719–724. [Google Scholar] [CrossRef]
- Kalsi, A.K.; Halder, A.; Jain, M.; Chaturvedi, P.K.; Sharma, J.B. Prevalence and reproductive manifestations of macroprolactinemia. Endocrine 2019, 63, 332–340. [Google Scholar] [CrossRef]
- Romijn, J.A. Hyperprolactinemia and prolactinoma. Handb. Clin. Neurol. 2014, 124, 185–195. [Google Scholar] [CrossRef]
- Grigg, J.; Worsley, R.; Thew, C.; Gurvich, C.; Thomas, N.; Kulkarni, J. Antipsychotic-induced hyperprolactinemia: Synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology 2017, 234, 3279–3297. [Google Scholar] [CrossRef]
- Santharam, S.; Fountas, A.; Tampourlou, M.; Arlt, W.; Ayuk, J.; Gittoes, N.; Toogood, A.; Karavitaki, N. Impact of menopause on outcomes in prolactinomas after dopamine agonist treatment withdrawal. Clin. Endocrinol. Oxf. 2018, 89, 346–353. [Google Scholar] [CrossRef]
- Iacovazzo, D.; De Marinis, L. Treatment of hyperprolactinemia in post-menopausal women: Pros. Endocrine 2015, 48, 76–78. [Google Scholar] [CrossRef]
- Liu, X.; Tang, C.; Wen, G.; Zhong, C.; Yang, J.; Zhu, J.; Ma, C. The Mechanism and Pathways of Dopamine and Dopamine Agonists in Prolactinomas. Front. Endocrinol. Lausanne 2018, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Barlier, A.; Jaquet, P. Quinagolide—A valuable treatment option for hyperprolactinaemia. Eur. J. Endocrinol. 2006, 154, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Di Sarno, A.; Guerra, E.; De Leo, M.; Mentone, A.; Lombardi, G. Drug insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Schade, R.; Andersohn, F.; Suissa, S.; Haverkamp, W.; Garbe, E. Dopamine agonists and the risk of cardiac-valve regurgitation. N. Engl. J. Med. 2007, 356, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Stiles, C.E.; Tetteh-Wayoe, E.T.; Bestwick, J.; Steeds, R.P.; Drake, W.M. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with Cabergoline. J. Clin. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Pivonello, R.; Ferreri, L.; Priscitelli, P.; Colao, A. Cabergoline use for pituitary tumors and valvular disorders. Endocrinol. Metab. Clin. N. Am. 2015, 44, 89–97. [Google Scholar] [CrossRef]
- Vroonen, L.; Lancellotti, P.; Garcia, M.T.; Dulgheru, R.; Rubio-Almanza, M.; Maiga, I.; Magne, J.; Petrossians, P.; Auriemma, R.; Daly, A.F.; et al. Prospective, long-term study of the effect of cabergoline on valvular status in patients with prolactinoma and idiopathic hyperprolactinemia. Endocrine 2017, 55, 239–245. [Google Scholar] [CrossRef]
- Gamble, D.; Fairley, R.; Harvey, R.; Farman, C.; Cantley, N.; Leslie, S.J. Screening for valve disease in patients with hyperprolactinaemia disorders prescribed cabergoline: A service evaluation and literature review. Adv. Drug Saf. 2017, 8, 215–229. [Google Scholar] [CrossRef]
- Steeds, R.P.; Stiles, C.E.; Sharma, V.; Chambers, J.B.; Lloyd, G.; Drake, W. Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: A joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Echo Res. Pract. 2019, 6, G1–G8. [Google Scholar] [CrossRef]
- Gillam, M.P.; Molitch, M.E.; Lombardi, G.; Colao, A. Advances in the treatment of prolactinomas. Endocr. Rev. 2006, 27, 485–534. [Google Scholar] [CrossRef]
- Noronha, S.; Stokes, V.; Karavitaki, N.; Grossman, A. Treating prolactinomas with dopamine agonists: Always worth the gamble? Endocrine 2016, 51, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chng, E.; Dalan, R. Pituitary apoplexy associated with cabergoline therapy. J. Clin. Neurosci. 2013, 20, 1637–1643. [Google Scholar] [CrossRef]
- Ghadirian, H.; Shirani, M.; Ghazi-Mirsaeed, S.; Mohebi, S.; Alimohamadi, M. Pituitary Apoplexy during Treatment of Prolactinoma with Cabergoline. Asian J. Neurosurg. 2018, 13, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arch. Endocrinol. Metab. 2015, 59, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Molitch, M.E. Pituitary Tumors in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 569–581. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Perone, Y.; Di Sarno, A.; Grasso, L.F.; Guerra, E.; Gasperi, M.; Pivonello, R.; Colao, A. Results of a single-center observational 10-year survey study on recurrence of hyperprolactinemia after pregnancy and lactation. J. Clin. Endocrinol. Metab. 2013, 98, 372–379. [Google Scholar] [CrossRef]
- Domingue, M.E.; Devuyst, F.; Alexopoulou, O.; Corvilain, B.; Maiter, D. Outcome of prolactinoma after pregnancy and lactation: A study on 73 patients. Clin. Endocrinol. Oxf. 2014, 80, 642–648. [Google Scholar] [CrossRef]
- Souteiro, P.; Karavitaki, N. Dopamine agonist resistant prolactinomas: Any alternative medical treatment? Pituitary 2019, 1–11. [Google Scholar] [CrossRef]
- Maiter, D. Management of Dopamine Agonist-Resistant Prolactinoma. Neuroendocrinology 2019, 109, 42–50. [Google Scholar] [CrossRef]
- Molitch, M.E. Management of medically refractory prolactinoma. J. Neurooncol. 2014, 117, 421–428. [Google Scholar] [CrossRef]
- Raverot, G.; Burman, P.; McCormack, A.I.; Heaney, A.P.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O. European Society of Endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2017, 178, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, A.; Shehadeh, N.; Hochberg, I.; Zloczower, M.; Zolotov, S.; Taher, R.; Daoud Naccache, D. Management of cystic prolactinomas: A review. Pituitary 2018, 21, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Tampourlou, M.; Trifanescu, R.; Paluzzi, A.; Ahmed, S.K.; Karavitaki, N. Therapy of endocrine disease: Surgery in microprolactinomas: Effectiveness and risks based on contemporary literature. Eur. J. Endocrinol. 2016, 175, R89–R96. [Google Scholar] [CrossRef] [PubMed]
- Colao, A. Pituitary tumours: The prolactinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Chen, D.M.; Zhang, C.; Pan, M.; Yang, X.P.; Wu, Y.G. Retrospective analysis of 52 patients with prolactinomas following endoscopic endonasal transsphenoidal surgery. Med. Baltim. 2018, 97, e13198. [Google Scholar] [CrossRef]
- Ntali, G.; Karavitaki, N. Efficacy and complications of pituitary irradiation. Endocrinol. Metab. Clin. N. Am. 2015, 44, 117–126. [Google Scholar] [CrossRef]
- Pereira, A.M.; Romijn, J.A.; Dekkers, O.M. Treatment and Follow-Up of Clinically Nonfunctioning Pituitary Macroadenomas. J. Clin. Endocrinol. Metab. 2008, 93, 3717–3726. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Cote, D.J.; Castlen, J.P.; Burke, W.T.; Liu, Y.H.; Smith, T.R.; Laws, E.R. Time Course of Resolution of Hyperprolactinemia After Transsphenoidal Surgery Among Patients Presenting with Pituitary Stalk Compression. World Neurosurg. 2017, 97, 2–7. [Google Scholar] [CrossRef]
- Huang, W.; Molitch, M.E. Evaluation and management of galactorrhea. Am. Fam. Physician 2012, 85, 1073–1080. [Google Scholar]
- Greenman, Y.; Cooper, O.; Yaish, I.; Robenshtok, E.; Sagiv, N.; Jonas-Kimchi, T.; Yuan, X.; Gertych, A.; Shimon, I.; Ram, Z.; et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur. J. Endocrinol. 2016, 175, 63–72. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hugo, E. Prolactin (PRL) in adipose tissue: Regulation and functions. Adv. Exp. Med. Biol. 2015, 846, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Carré, N.; Binart, N. Prolactin and adipose tissue. Biochimie 2014, 97, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, J.; Xu, Y.; Li, M.; Sun, J.; Zhang, J.; Xu, B.; Xu, M.; Chen, Y.; Bi, Y.; et al. Circulating prolactin associates with diabetes and impaired glucose regulation: A population-based study. Diabetes Care 2013, 36, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rice, M.S.; Huang, T.; Hankinson, S.E.; Clevenger, C.V.; Hu, F.B.; Tworoger, S.S. Circulating prolactin concentrations and risk of type 2 diabetes in US women. Diabetologia 2018, 61, 2549–2560. [Google Scholar] [CrossRef]
- Atmaca, A.; Bilgici, B.; Ecemis, G.C.; Tuncel, O.K. Evaluation of body weight, insulin resistance, leptin and adiponectin levels in premenopausal women with hyperprolactinemia. Endocrine 2013, 44, 756–761. [Google Scholar] [CrossRef]
- Dos Santos Silva, C.M.; Barbosa, F.R.; Lima, G.A.; Warszawski, L.; Fontes, R.; Domingues, R.C.; Gadelha, M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obes. Silver Spring 2011, 19, 800–805. [Google Scholar] [CrossRef]
- Schwetz, V.; Librizzi, R.; Trummer, C.; Theiler, G.; Stiegler, C.; Pieber, T.R.; Obermayer-Pietsch, B.; Pilz, S. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab. Brain Dis. 2017, 32, 155–161. [Google Scholar] [CrossRef]
- Medic-Stojanoska, M.; Icin, T.; Pletikosic, I.; Bajkin, I.; Novakovic-Paro, J.; Stokic, E.; Spasic, D.T.; Kovacev-Zavisic, B.; Abenavoli, L. Risk factors for accelerated atherosclerosis in young women with hyperprolactinemia. Med. Hypotheses 2015, 84, 321–326. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Granieri, L.; Galdiero, M.; Simeoli, C.; Perone, Y.; Vitale, P.; Pivonello, C.; Negri, M.; Mannarino, T.; Giordano, C.; et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013, 98, 299–310. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Galdiero, M.; Vitale, P.; Granieri, L.; Lo Calzo, F.; Salzano, C.; Ferreri, L.; Pivonello, C.; Cariati, F.; Coppola, G.; et al. Effect of chronic cabergoline treatment and testosterone replacement on metabolism in male patients with prolactinomas. Neuroendocrinology 2015, 101, 66–81. [Google Scholar] [CrossRef]
- Ozdemir, E.D.; Caglar, G.S.; Akgul, E.; Cengiz, S.D.; Tombak, G. The association between prolactin, high-sensitivity C-reactive protein and Framingham risk score in menopause. Gynecol. Obs. Investig. 2014, 78, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Curhan, G.C.; Forman, J.P. Plasma prolactin level and risk of incident hypertension in postmenopausal women. J. Hypertens. 2010, 28, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, K.E.; Abraham, T.M.; Pedley, A.; Massaro, J.M.; Sutherland, P.; Hoffmann, U.; Fox, C.S. Association Between Prolactin and Incidence of Cardiovascular Risk Factors in the Framingham Heart Study. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Erem, C.; Kocak, M.; Nuhoglu, I.; Yılmaz, M.; Ucuncu, O. Blood coagulation, fibrinolysis and lipid profile in patients with prolactinoma. Clin. Endocrinol. Oxf. 2010, 73, 502–507. [Google Scholar] [CrossRef]
- Toulis, K.A.; Robbins, T.; Reddy, N.; Balachandran, K.; Gokhale, K.; Wijesinghe, H.; Cheng, K.K.; Karavitaki, N.; Wass, J.; Nirantharakumar, K. Males with prolactinoma are at increased risk of incident cardiovascular disease. Clin. Endocrinol. Oxf. 2018, 88, 71–76. [Google Scholar] [CrossRef]
- Shelly, S.; Boaz, M.; Orbach, H. Prolactin and autoimmunity. Autoimmun. Rev. 2012, 11, A465–A470. [Google Scholar] [CrossRef]
- Orbach, H.; Zandman-Goddard, G.; Boaz, M.; Agmon-Levin, N.; Amital, H.; Szekanecz, Z.; Szucs, G.; Rovensky, J.; Kiss, E.; Doria, A.; et al. Prolactin and autoimmunity: Hyperprolactinemia correlates with serositis and anemia in SLE patients. Clin. Rev. Allergy Immunol. 2012, 42, 189–198. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F.; Ysrraelit, M.C. Role of prolactin in B cell regulation in multiple sclerosis. J. Neuroimmunol. 2014, 269, 76–86. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Jin, Q.; Kang, Z.; Huo, Y.; He, Z.; Feng, X.; Yin, J.; Wu, X.; Wang, H.; et al. Hyperprolactinemia is associated with a high prevalence of serum autoantibodies, high levels of inflammatory cytokines and an abnormal distribution of peripheral B-cell subsets. Endocrine 2019, 64, 648–656. [Google Scholar] [CrossRef]
- Wang, P.; Lv, T.T.; Guan, S.Y.; Li, H.M.; Leng, R.X.; Zou, Y.F.; Pan, H.F. Increased plasma/serum levels of prolactin in systemic lupus erythematosus: A systematic review and meta-analysis. Postgrad. Med. 2017, 129, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, C.; De Zubiría, A.; Granados, C.; Suárez, J.; Cervera, R. Prolactin and Estradiol Profile in a Cohort of Colombian Women with Systemic Lupus Erythematosus. Isr. Med. Assoc. J. 2016, 18, 537–541. [Google Scholar] [PubMed]
- Ledesma-Soto, Y.; Blanco-Favela, F.; Fuentes-Pananá, E.M.; Tesoro-Cruz, E.; Hernández-González, R.; Arriaga-Pizano, L.; Legorreta-Haquet, M.V.; Montoya-Diaz, E.; Chávez-Sánchez, L.; Castro-Mussot, M.E.; et al. Increased levels of prolactin receptor expression correlate with the early onset of lupus symptoms and increased numbers of transitional-1 B cells after prolactin treatment. BMC Immunol. 2012, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Savino, W. Prolactin: An Immunomodulator in Health and Disease. Front. Horm. Res. 2017, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Leaños-Miranda, A.; Cárdenas-Mondragón, G. Serum free prolactin concentrations in patients with systemic lupus erythematosus are associated with lupus activity. Rheumatol. Oxf. 2006, 45, 97–101. [Google Scholar] [CrossRef]
- Tang, M.W.; Garcia, S.; Gerlag, D.M.; Tak, P.P.; Reedquist, K.A. Insight into the Endocrine System and the Immune System: A Review of the Inflammatory Role of Prolactin in Rheumatoid Arthritis and Psoriatic Arthritis. Front. Immunol. 2017, 8, 720. [Google Scholar] [CrossRef]
- Clapp, C.; Adán, N.; Ledesma-Colunga, M.G.; Solís-Gutiérrez, M.; Triebel, J.; Martínez de la Escalera, G. The role of the prolactin/vasoinhibin axis in rheumatoid arthritis: An integrative overview. Cell. Mol. Life Sci. 2016, 73, 2929–2948. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Salesi, M.; Sadeghihaddadzavareh, S.; Nasri, P.; Namdarigharaghani, N.; Farajzadegan, Z.; Hajalikhani, M. The role of bromocriptine in the treatment of patients with active rheumatoid arthritis. Int. J. Rheum. Dis. 2013, 16, 662–666. [Google Scholar] [CrossRef]
- Tang, M.W.; Garcia, S.; Malvar Fernandez, B.; Gerlag, D.M.; Tak, P.P.; Reedquist, K.A. Rheumatoid arthritis and psoriatic arthritis synovial fluids stimulate prolactin production by macrophages. J. Leukoc. Biol. 2017, 102, 897–904. [Google Scholar] [CrossRef]
- Reyes-Castillo, Z.; Pereira-Suárez, A.L.; Palafox-Sanchez, C.A.; Rangel-Villalobos, H.; Estrada-Chávez, C.; Oregón-Romero, E.; Angel-Chávez, L.I.; Muñoz-Barrios, S.; Bueno-Topete, M.R.; Muñoz-Valle, J.F. The extrapituitary prolactin promoter polymorphism is associated with rheumatoid arthritis and anti-CCP antibodies in Mexican population. Gene 2013, 525, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, R.; Giriş, M.; Gencer, M.; Akcan, U.; Örçen, A. Serum Prolactin Levels in Multiple Sclerosis, Neuromyelitis Optica, and Clinically Isolated Syndrome Patients. Noro Psikiyatr. Arsivi 2016, 53, 353–356. [Google Scholar] [CrossRef] [PubMed]
- El Tahlawi, S.M.; El Eishi, N.H.; Kahhal, R.K.; Hegazy, R.A.; El Hanafy, G.M.; Abdel Hay, R.M.; Shaker, O.G. Do Prolactin and its Receptor Play a Role in Alopecia Areata? Indian J. Derm. 2018, 63, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lajevardi, V.; Hallaji, Z.; Daneshpazhooh, M.; Ghandi, N.; Shekari, P.; Khani, S. Evaluation of prolactin levels in patients with newly diagnosed pemphigus vulgaris and its correlation with pemphigus disease area index. Int. J. Womens Derm. 2016, 2, 53–55. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Hankinson, S.E. Prolactin and breast cancer etiology: An epidemiologic perspective. J. Mammary Gland Biol. Neoplasia 2008, 13, 41–53. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Eliassen, A.H.; Zhang, X.; Qian, J.; Sluss, P.M.; Rosner, B.A.; Hankinson, S.E. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013, 73, 4810–4819. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Chanson, P.; Binart, N. New insights in prolactin: Pathological implications. Nat. Rev. Endocrinol. 2015, 11, 265–275. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Arslan, A.A.; Lokshin, A.E.; Liu, M.; Lundin, E.; Koenig, K.L.; Berrino, F.; Hallmans, G.; Idahl, A.; Krogh, V.; et al. Circulating prolactin levels and risk of epithelial ovarian cancer. Cancer Causes Control 2013, 24, 741–748. [Google Scholar] [CrossRef]
- Ilan, Y.; Sibirsky, O.; Livni, N.; Gofrit, O.; Barack, V.; Goldin, E. Plasma and tumor prolactin in colorectal cancer patients. Dig. Dis. Sci. 1995, 40, 2010–2015. [Google Scholar] [CrossRef]
- Bhatavdekar, J.M.; Patel, D.D.; Giri, D.D.; Karelia, N.H.; Vora, H.H.; Ghosh, N.; Shah, N.G.; Trivedi, S.N.; Balar, D.B. Comparison of plasma prolactin and CEA in monitoring patients with adenocarcinoma of colon and rectum. Br. J. Cancer 1992, 66, 977–980. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Lee, K.T.; Tsai, C.J.; Chen, Y.J.; Wang, S.N. Prolactin promotes hepatocellular carcinoma through Janus kinase 2. World J. Surg. 2012, 36, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Chen, K.H.; Ghosh, M.K.; Rivera, L.; Dill, R.; Ma, L.; Villa, P.A.; Kawaminami, M.; Walker, A.M. Anti-metastatic outcome of isoform-specific prolactin receptor targeting in breast cancer. Cancer Lett. 2015, 366, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Goffin, V. Prolactin receptor targeting in breast and prostate cancers: New insights into an old challenge. Pharm. Ther. 2017, 179, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yuan, Y.; Chong, Q.Y.; Yang, Y.; Li, R.; Li, X.; Kong, X.; Qian, P.; Xiong, Z.; Pandey, V.; et al. Autocrine Prolactin Stimulates Endometrial Carcinoma Growth and Metastasis and Reduces Sensitivity to Chemotherapy. Endocrinology 2017, 158, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Neradugomma, N.K.; Subramaniam, D.; Tawfik, O.W.; Goffin, V.; Kumar, T.R.; Jensen, R.A.; Anant, S. Prolactin signaling enhances colon cancer stemness by modulating Notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis 2014, 35, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Romijn, J.A.; de Boer, A.; Vandenbroucke, J.P. The risk for breast cancer is not evidently increased in women with hyperprolactinemia. Pituitary 2010, 13, 195–198. [Google Scholar] [CrossRef] [PubMed]
| Physiological | Pathological | Pharmacological |
|---|---|---|
|
|
|
| Drug Class | No Significant HPRL | HPRL in <25% of Patients | HPRL in 25–50% of Patients | HPRL in >50% of Patients |
|---|---|---|---|---|
| Typical antipsychotics |
|
| ||
| Atypical antipsychotics |
|
|
| |
| Tricyclic antidepressants |
|
|
| |
| Monoamine oxidase inhibitors |
| |||
| Antiemetics |
| |||
| Antihypertensives |
|
|
| Drug | Mild HPRL (<50 µg/L) (% of Patients) | Moderate HPRL (50–100 µg/L) (% of Patients) | Severe HPRL (>100 µg/L) (% of Patients) |
|---|---|---|---|
| Aripiprazole * |
|
| |
| Olanzapine * |
|
|
|
| Quetiapine * |
|
|
|
| Depot Risperidone * |
|
|
|
| Oral Risperidone * |
|
|
|
| Oral Paliperidone * |
|
|
|
| Depot Paliperidone * |
|
|
|
| Phenothiazines ** |
| ||
| Amisulpride *** |
| ||
| Sulpiride ∞ |
| ||
| Haloperidol † |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samperi, I.; Lithgow, K.; Karavitaki, N. Hyperprolactinaemia. J. Clin. Med. 2019, 8, 2203. https://doi.org/10.3390/jcm8122203
Samperi I, Lithgow K, Karavitaki N. Hyperprolactinaemia. Journal of Clinical Medicine. 2019; 8(12):2203. https://doi.org/10.3390/jcm8122203
Chicago/Turabian StyleSamperi, Irene, Kirstie Lithgow, and Niki Karavitaki. 2019. "Hyperprolactinaemia" Journal of Clinical Medicine 8, no. 12: 2203. https://doi.org/10.3390/jcm8122203
APA StyleSamperi, I., Lithgow, K., & Karavitaki, N. (2019). Hyperprolactinaemia. Journal of Clinical Medicine, 8(12), 2203. https://doi.org/10.3390/jcm8122203





