Retinal Microperimetry: A Useful Tool for Detecting Insulin Resistance-Related Cognitive Impairment in Morbid Obesity
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
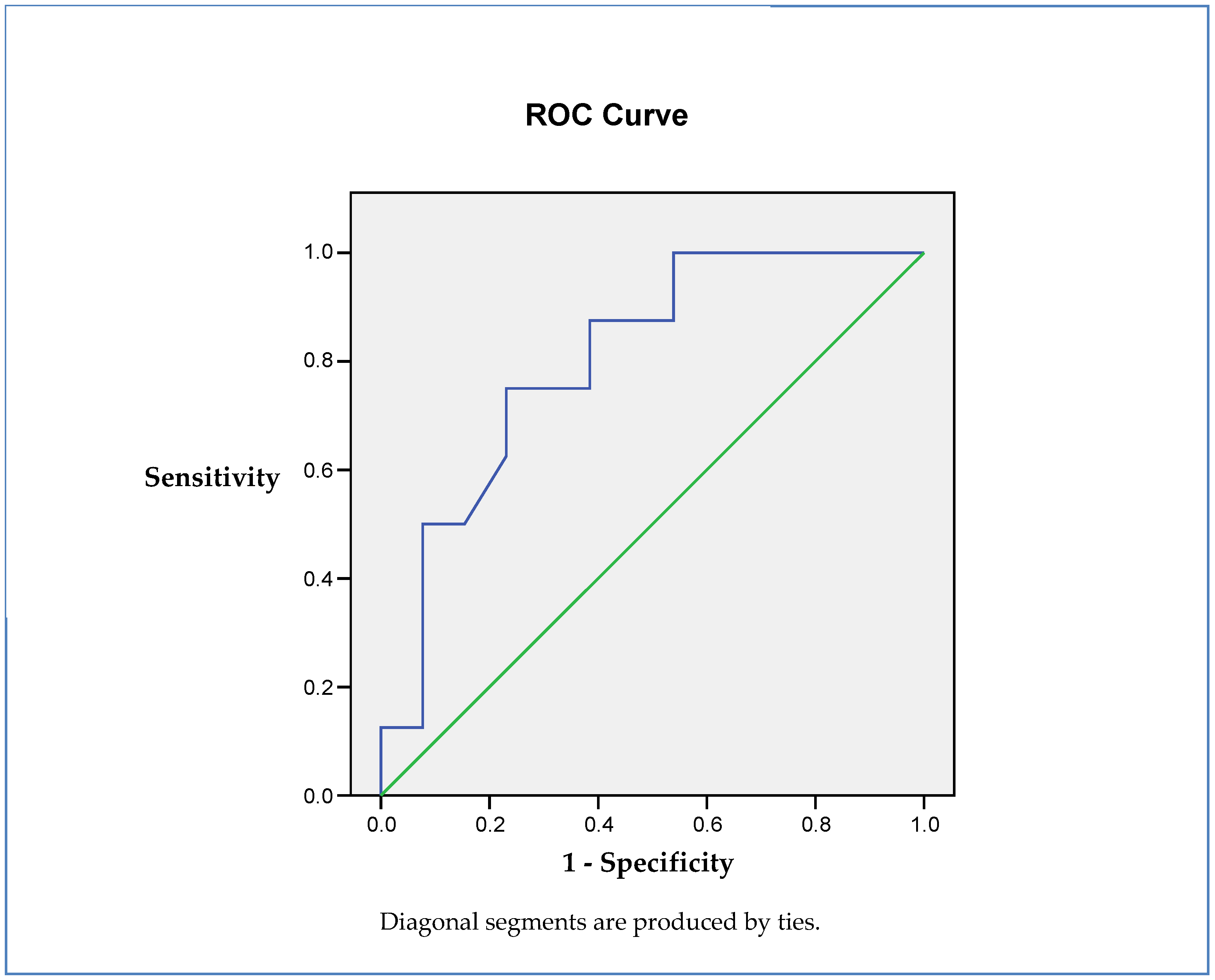
3. Results
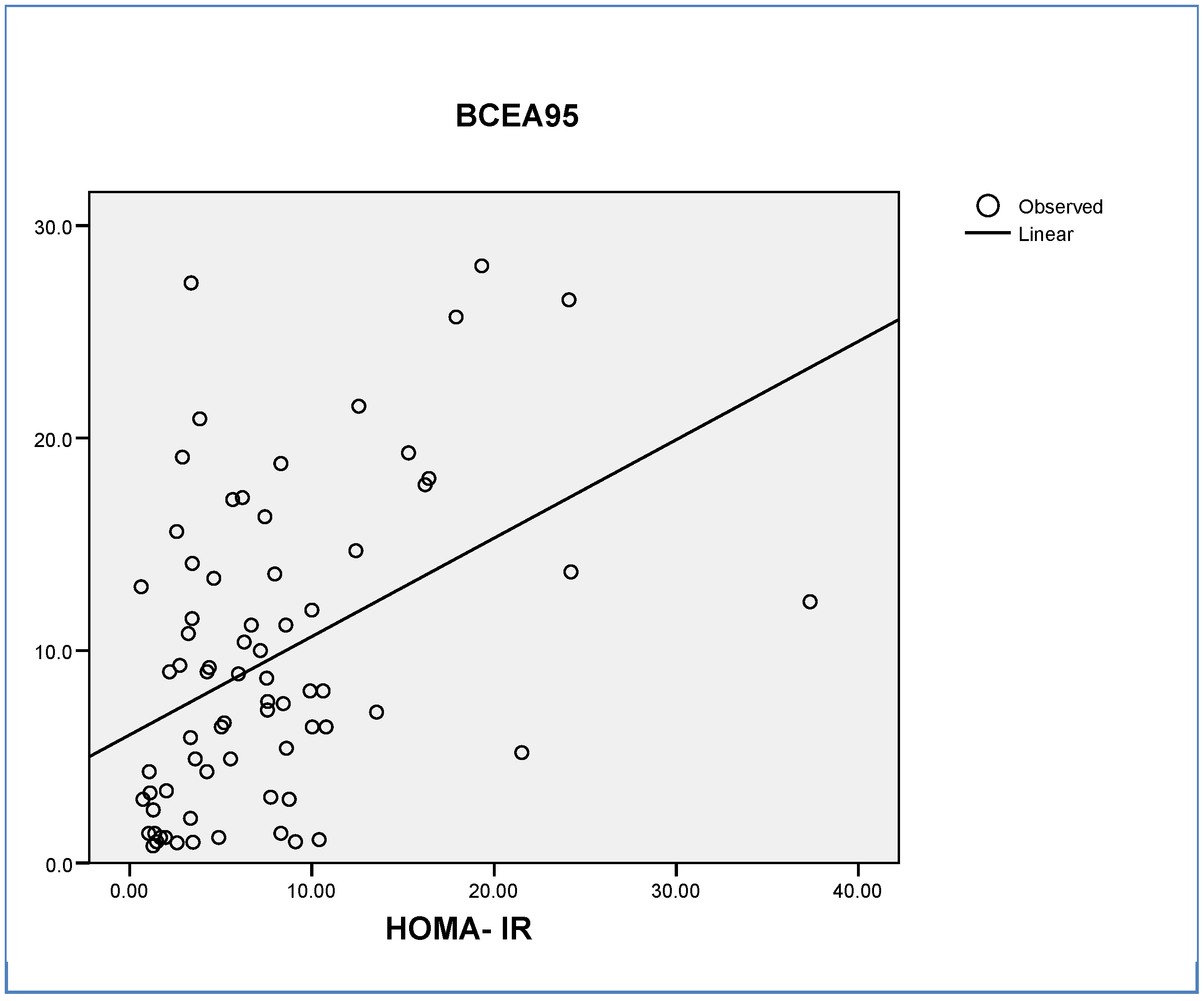
Insulin Resistance (IR) as an Independent Predictor of Cognitive Status
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Biessels, G.J.; Strachan, M.W.J.; Visseren, F.L.J.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24622755 (accessed on 1 October 2019). [CrossRef]
- Exalto, L.G.; Whitmer, R.A.; Kappele, L.J.; Biessels, G.J. An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp. Gerontol. 2012, 47, 858–864. [Google Scholar] [CrossRef]
- Ciudin, A.; Espinosa, A.; Simó-Servat, O.; Ruiz, A.; Alegret, M.; Hernández, C.; Boada, M.; Simo, R. Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. J. Diabetes Complicat. 2017, 31, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Ciudin, A.; Simó-Servat, O.; Hernández, C. Cognitive impairment and dementia: A new emerging complication of type 2 diabetes—The diabetologist’s perspective. Acta Diabetol. 2017. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28210868 (accessed on 1 October 2019).
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26462756 (accessed on 1 October 2019).
- Moran, C.; Beare, R.; Phan, T.; Srikanth, V. Brain, imaging in type 2 diabetes. In Type 2 Diabetes and Dementia; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 823–830. [Google Scholar] [CrossRef]
- Reijmer, Y.D.; Brundel, M.; de Bresser, J.; Kappelle, L.J.; Leemans, A.; Biessels, G.J. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: A diffusion tensor imaging study. Diabetes Care 2013, 36, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Bäckman, L.; Xu, W. Early cognitive deficits in type 2 diabetes: A population-based study. J. Alzheimer’s Dis. 2016, 53, 1069–1078. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25936649 (accessed on 1 October 2019).
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24183659 (accessed on 1 October 2019).
- Cheung, C.Y.; Ikram, M.K.; Chen, C.; Wong, T.Y. Imaging retina to study dementia and stroke. Prog. Retin. Eye Res. 2017, 57, 89–107. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, Z.; Peng, Y. Potential utility of retinal imaging for Alzheimer’s Disease: A review. Front. Aging Neurosci. 2018, 10, 188. [Google Scholar] [CrossRef]
- Acton, J.H.; Greenstein, V.C. Fundus-driven perimetry (microperimetry) compared to conventional static automated perimetry: Similarities, differences, and clinical applications. Can. J. Ophthalmol. 2013, 48, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Rohrschneider, K.; Bültmann, S.; Springer, C. Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog. Retin. Eye Res. 2008, 27, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ayton, L.N.; Guymer, R.H.; Luu, C.D. Comparison between multifocal electroretinography and microperimetry in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6431–6439. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25159206 (accessed on 1 October 2019). [CrossRef] [PubMed]
- Ciudin, A.; Simó-Servat, O.; Hernández, C.; Arcos, G.; Diego, S.; Sanabria, Á.; Sotolongo, Ó.; Hernández, I.; Boada, M.; Simó, R. Retinal microperimetry: A new tool for identifying patients with type 2 diabetes at risk for developing Alzheimer disease. Diabetes 2017, 66, 3098–3104. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Ciudin, A.; Ortiz-Zúñiga, Á.M.; Hernández, C.; Simó, R. Usefulness of eye fixation assessment for identifying type 2 diabetic subjects at risk of dementia. J. Clin. Med. 2019, 8, 59. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Feng, L.; Chong, M.S.; Lim, W.S.; Lee, T.S.; Collinson, S.L.; Yap, P.; Ng, T.P. Metabolic syndrome and amnestic mild cognitive impairment: Singapore Longitudinal Ageing Study-2 findings. J. Alzheimer’s Dis. 2013, 34, 649–657. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23246920 (accessed on 1 October 2019). [CrossRef]
- Tsai, C.L.; Huang, T.H.; Tsai, M.C. Neurocognitive performances of visuospatial attention and the correlations with metabolic and inflammatory biomarkers in adults with obesity. Exp. Physiol. 2017, 102, 1683–1699. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chen, F.C.; Pan, C.Y.; Tseng, Y.T. The neurocognitive performance of visuospatial attention in children with obesity. Front. Psychol. 2016, 7, 1033. [Google Scholar] [CrossRef]
- Ho, A.J.; Raji, C.A.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Lee, S.; Hibar, D.; Dinov, I.D.; Stein, J.L.; et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 2010, 31, 1326–1339. [Google Scholar] [CrossRef]
- Yokum, S.; Ng, J.; Stice, E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: A prospective MRI study. Int. J. Obes. 2012, 6, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; Jansen, P.R.; Lamb, H.J. Obesity, brain volume, and white matter microstructure at MRI: A cross-sectional UK biobank study. Radiology 2019, 292, 270. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. The possible factors affecting microglial activation in cases of obesity with cognitive dysfunction. Metab. Brain Dis. 2018, 3, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE 2012, 7, e30378. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Schrijvers, E.M.C.; Witteman, J.C.M.; Sijbrands, E.J.G.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Insulin metabolism and the risk of Alzheimer disease: Rotterdam Study. Neurology 2010, 75, 1982–1987. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Kenna, H.A.; Wroolie, T.E.; Kelley, R.; Silverman, D.; Brooks, J.; Williams, K.E.; Powers, B.N.; Hallmayer, J.; Reiss, A. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol. Aging 2011, 2, 1942–1948. [Google Scholar] [CrossRef]
- Ursache, A.; Wedin, W.; Tirsi, A.; Convit, A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology 2012, 37, 1270–1276. [Google Scholar] [CrossRef]
- Espeland, M.A.; Bryan, R.N.; Goveas, J.S.; Robinson, J.G.; Siddiqui, M.S.; Liu, S.; Hogan, P.E.; Casanova, R.; Coker, L.H.; Yaffe, K.; et al. Influence of type 2 diabetes on brain volumes and changes in brain volumes: Results from the Women’s Health Initiative Magnetic Resonance Imaging studies. Diabetes Care 2013, 36, 90–97. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22933440 (accessed on 1 October 2019). [CrossRef]
- Reijmer, Y.D.; Leemans, A.; Brundel, M.; Kappelle, L.J.; Biessels, G.J.; Utrecht Vascular Cognitive Impairment Study Group. Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes 2013, 62, 2112–2115. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23349494 (accessed on 1 October 2019). [CrossRef] [PubMed]
- Willette, A.A.; Xu, G.; Johnson, S.C.; Birdsill, A.C.; Jonaitis, E.M.; Sager, M.A.; Hermann, B.P.; La Rue, A.; Asthana, S.; Bendlin, B.B. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care 2013, 36, 443–449. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23069842 (accessed on 1 October 2019). [CrossRef] [PubMed]
- Chua, L.-M.; Lim, M.-L.; Chong, P.-R.; Hu, Z.P.; Cheung, N.S.; Wong, B.-S. Impaired neuronal insulin signaling precedes Aβ42 accumulation in female AβPPSW/PS1ΔE9 mice. J. Alzheimer’s Dis. 2012, 29, 783–791. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22337827 (accessed on 1 October 2019). [CrossRef] [PubMed]
- Macklin, L.; Griffith, C.M.; Cai, Y.; Rose, G.M.; Yan, X.-X.; Patrylo, P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp. Gerontol. 2017, 88, 9–18. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28025127 (accessed on 1 October 2019). [CrossRef] [PubMed]
- de Felice, F.G. Alzheimer’s disease and insulin resistance: Translating basic science into clinical applications. J. Clin. Investig. 2013, 123, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J. Clin. Investig. 2012, 22, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Perez, T.; Chang, H.; Mehta, P.; Steffener, J.; Pradabhan, G.; Ichise, M.; Manly, J.; Devanand, D.P.; Bagiella, E. Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J. Alzheimer’s Dis. 2016, 51, 501–514. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Strain, G.; Devlin, M.; Cohen, R.; Paul, R.; Crosby, R.D.; Mitchell, J.E.; Gunstad, J. Improved memory function two years after bariatric surgery. Obesity 2014, 22, 32–38. [Google Scholar] [CrossRef]
- Jones, E.G. A new view of specific and nonspecific thalamocortical connections. Adv. Neurol. 1998, 77, 49–71. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9709817 (accessed on 1 October 2019).
- Dugger, B.N.; Tu, M.; Murray, M.E.; Dickson, D.W. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci. Lett. 2011, 491, 122–126. [Google Scholar] [CrossRef]
- Parvizi, J.; Van Hoesen, G.W.; Damasio, A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann. Neurol. 2001, 49, 53–66. [Google Scholar] [CrossRef]
- Erskine, D.; Taylor, J.P.; Firbank, M.J.; Patterson, L.; Onofrj, M.; O’Brien, J.T.; McKeith, I.G.; Attems, J.; Thomas, A.J.; Morris, C.M.; et al. Changes to the lateral geniculate nucleus in Alzheimer’s disease but not dementia with Lewy bodies. Neuropathol. Appl. Neurobiol. 2016, 42, 366–376. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25967384 (accessed on 1 October 2019). [CrossRef] [PubMed]
- Ellis, E.M.; Gauvain, G.; Sivyer, B.; Murphy, G.J. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. J. Neurophysiol. 2016, 116, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Donner, T.H.; Oostenveld, R.; Fries, P.; Engel, A.K. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 2008, 60, 709–719. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19038226 (accessed on 1 October 2019). [CrossRef]


| Characteristics | Obese Patients | Controls | p |
|---|---|---|---|
| N | 50 | 30 | NA |
| Gender (females %) | 71.12 | 70.45 | n.s. |
| Age (years) | 45.88 ± 10.23 | 41.17 ± 12.33 | n.s. |
| BMI (kg/m2) | 44.54 ± 5.53 | 22.99 ± 3.51 | <0.001 |
| HOMA-IR | 6.43 ± 2.64 | 1.34 ± 0.68 | <0.001 |
| HbA1c (%Hb DCCT) | 5.96 ± 1.15 | 5.24 ± 0.27 | 0.001 |
| T2D duration (month) | 24 ± 9.1 | NA | NA |
| MoCA score | 24.94 ± 2.74 | 28.95 ± 1.05 | <0.001 |
| MMSE | 28.24 ± 1.14 | 29 ± 0.62 | n.s. |
| MoCA Domains | Obese Patients | Controls | p |
|---|---|---|---|
| Visuo Spatial Executive Function | 4.0 ± 0.791 | 4.93 ± 0.26 | 0.001 |
| Naming | 2.94 ± 0.243 | 3.00 ± 0.00 | n.s. |
| Attention | 4.41 ± 1.73 | 5.67 ± 0.62 | 0.01 |
| Language | 2.71 ± 0.588 | 2.91 ± 0.32 | n.s. |
| Abstraction | 1.71 ± 0.772 | 1.96 ± 1.92 | n.s. |
| Delayed Recall | 3.14 ± 1.581 | 4.11 ± 1.31 | 0.015 |
| Orientation | 6.00 ± 0.00 | 6.00 ± 0.00 | n.s. |
| Total | 24.94 ± 2.74 | 28.95 ± 1.05 | <0.001 |
| Microperimetry Parameters | Obese Patients | Controls | p |
|---|---|---|---|
| N | 50 | 30 | NA |
| Sensitivity (dB) | 27.6 ± 3.81 | 29.28 ± 1.39 | n.s. |
| Fixation P1 (%) | 78.48 ± 26.16 | 97.18 ± 3.2 | 0.001 |
| Fixation P2 (%) | 90.31 ± 17.84 | 98.40 ± 4.39 | 0.001 |
| BCEA63 | 1.50 ± 1.32 | 0.40 ± 0.34 | 0.001 |
| BCEA95 | 8.33 ± 4.66 | 3.01 ± 2.01 | 0.021 |
| Reliability index | 93.52 ± 11.75 | 95.45 ± 15.07 | n.s. |
| Characteristics | Obese Patients with T2D | Obese Patients Without T2D | p |
|---|---|---|---|
| N | 24 | 26 | NA |
| Gender (female %) | 70.37 | 71.42 | n.s. |
| Age (years) | 47.63 ± 8.62 | 44.33 ± 11.41 | n.s. |
| BMI (kg/m2) | 44.36 ± 5.4 | 44.70 ± 5.66 | n.s. |
| HOMA-IR | 8.34 ± 2.08 | 4.71 ± 2.047 | 0.001 |
| HbA1c (%Hb DCCT) | 6.62 ± 1.3 | 5.33 ± 0.28 | 0.001 |
| T2D duration (month) | 24 ± 9.1 | NA | NA |
| MoCA score | 25.5 ± 2.61 | 24.44 ± 2.92 | n.s. |
| MMSE | 28.1 ± 1.53 | 28.09 ± 1.78 | n.s. |
| Retinal sensitivity (dB) | 27.76 ± 2.45 | 27.59 ± 4.58 | n.s. |
| Fixation P1 (%) | 82.48 ± 23.59 | 78.19 ± 27.6 | n.s |
| Fixation P2 (%) | 92.00 ± 17.21 | 89.54 ± 18.04 | n.s. |
| BCEA63 | 1.47 ± 1.44 | 1.51 ± 1.25 | n.s. |
| BCEA95 | 8.14 ± 4.5 | 8.46 ± 4.85 | n.s. |
| Reliability index | 91.52 ± 14.75 | 92.65 ± 12.96 | n.s. |
| Education level | 7.08 ± 0.33 | 7.16 ± 0.28 | n.s. |
| Coefficients(a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | 95% Confidence Interval for B | Collinearity Statistics | ||||
| B | Std. Error | Beta | Lower Bound | Upper BOUND | Tolerance | VIF | ||||
| 1 | (Constant) | 25.200 | 3.734 | 6.749 | 0.000 | 17.684 | 32.715 | |||
| Gender | −0.503 | 0.776 | −0.084 | −0.648 | 0.520 | −2.065 | 1.060 | 0.869 | 1.151 | |
| Age | −0.063 | 0.034 | −0.242 | −1.823 | 0.075 | −0.132 | 0.007 | 0.831 | 1.203 | |
| Type 2 Diabetes | −0.169 | 0.286 | −0.076 | 0.589 | 0.559 | −0.745 | 0.408 | 0.888 | 1.127 | |
| HbA1c levels | 0.233 | 0.375 | 0.081 | 0.620 | 0.538 | −0.523 | 0.989 | 0.869 | 1.151 | |
| BMI | 0.078 | 0.050 | 0.209 | 1.574 | 0.122 | −0.022 | 0.179 | 0.836 | 1.196 | |
| HOMA-IR | −0.149 | 0.053 | −0.375 | −2.836 | 0.007 | −0.255 | −0.043 | 0.842 | 1.188 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciudin, A.; Ortiz, A.M.; Fidilio, E.; Romero, D.; Sánchez, M.; Comas, M.; Gonzalez, O.; Vilallonga, R.; Simó-Servat, O.; Hernández, C.; et al. Retinal Microperimetry: A Useful Tool for Detecting Insulin Resistance-Related Cognitive Impairment in Morbid Obesity. J. Clin. Med. 2019, 8, 2181. https://doi.org/10.3390/jcm8122181
Ciudin A, Ortiz AM, Fidilio E, Romero D, Sánchez M, Comas M, Gonzalez O, Vilallonga R, Simó-Servat O, Hernández C, et al. Retinal Microperimetry: A Useful Tool for Detecting Insulin Resistance-Related Cognitive Impairment in Morbid Obesity. Journal of Clinical Medicine. 2019; 8(12):2181. https://doi.org/10.3390/jcm8122181
Chicago/Turabian StyleCiudin, Andreea, Angel Michael Ortiz, Enzamaria Fidilio, Diana Romero, Marta Sánchez, Marta Comas, Oscar Gonzalez, Ramon Vilallonga, Olga Simó-Servat, Cristina Hernández, and et al. 2019. "Retinal Microperimetry: A Useful Tool for Detecting Insulin Resistance-Related Cognitive Impairment in Morbid Obesity" Journal of Clinical Medicine 8, no. 12: 2181. https://doi.org/10.3390/jcm8122181
APA StyleCiudin, A., Ortiz, A. M., Fidilio, E., Romero, D., Sánchez, M., Comas, M., Gonzalez, O., Vilallonga, R., Simó-Servat, O., Hernández, C., & Simó, R. (2019). Retinal Microperimetry: A Useful Tool for Detecting Insulin Resistance-Related Cognitive Impairment in Morbid Obesity. Journal of Clinical Medicine, 8(12), 2181. https://doi.org/10.3390/jcm8122181







