Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use
Abstract
1. The Rise of Benzodiazepines
2. Costs of Benzodiazepine Use
3. Prescription Monitoring Programs: Impacts and Implications
PMPs and Unintened Patient Harm
4. The Prescriber’s Dilemma
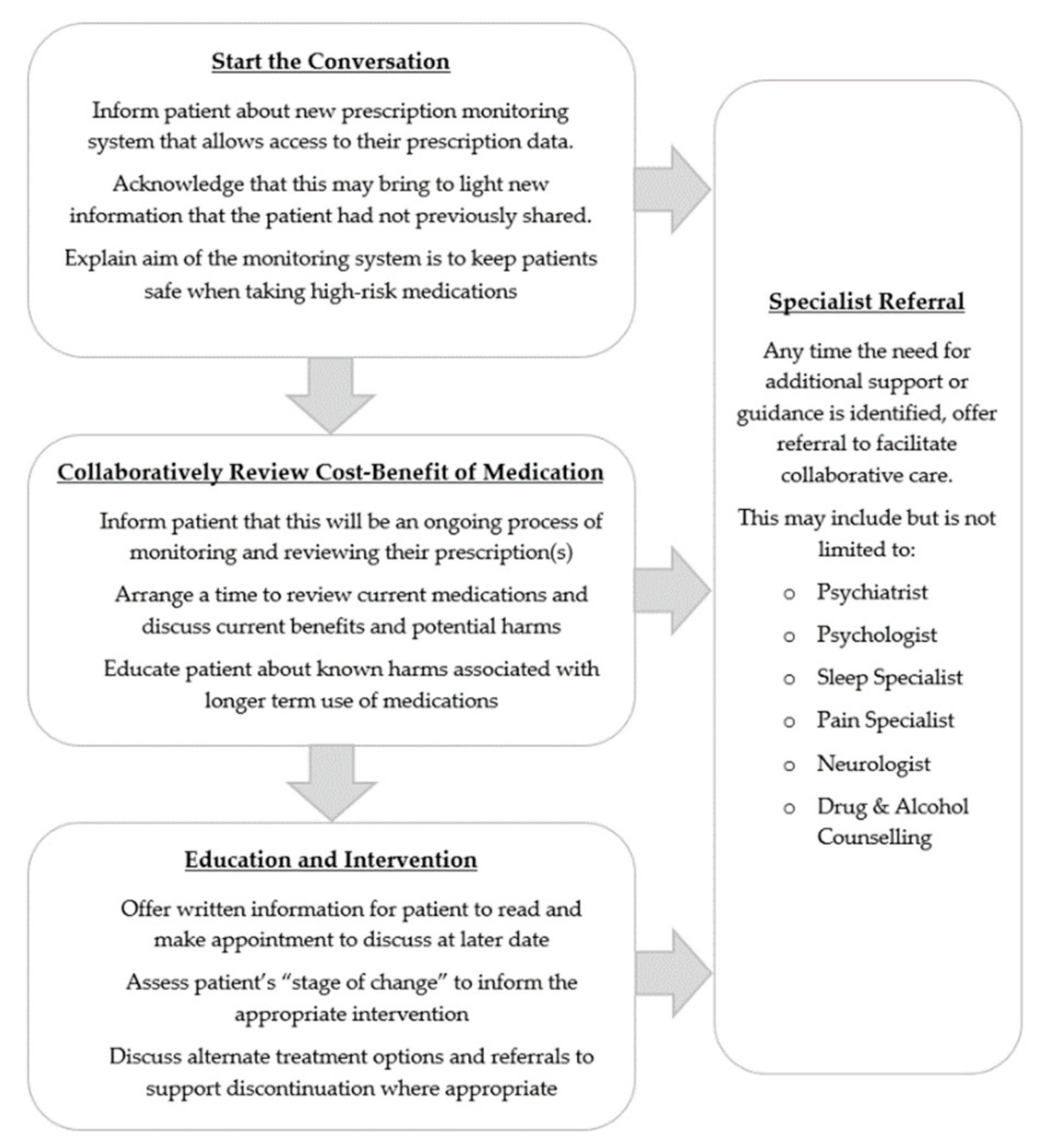
4.1. Steps towards Patient-Centred Care
4.2. Having the Conversation
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blackwell, B. Psychotropic drugs in use today: The role of Diazepam in medical practice. JAMA 1973, 225, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Waldron, I. Increased prescribing of Valium, Librium, and other drugs: An example of the influence of economic and social factors on the practice of medicine. Int. J. Health Serv. 1977, 7, 37–62. [Google Scholar] [CrossRef] [PubMed]
- Koumjian, K. The use of Valium as a form of social control. Soc. Sci. Med. 1981, 15, 245–249. [Google Scholar] [CrossRef]
- Parry, H.J.; Balter, M.B.; Mellinger, G.D.; Cisiin, I.H.; Manheimer, D.I. National patterns of psychotherapeutic drug use. Arch. Gen. Psych. 1973, 28, 769–783. [Google Scholar] [CrossRef]
- Lader, M.H. Benzodiazepines—Pancea or poison? Aust. N. Z. J. Psychiatry 1981, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ashton, H. Benzodiazepines: How They Work & How to Withdraw; University of Newcastle: Newcastle, UK, 2002. [Google Scholar]
- Committee on Safety of Medicines. Benzodiazepines, dependence and withdrawal symptoms. Curr. Probl. 1988, 21, 1–2. [Google Scholar]
- Barker, M.; Greenwood, K.; Jackson, M.; Crowe, S. Cognitive effects of long-term benzodiazepine use: A meta-analysis. CNS Drugs 2004, 18, 37–48. [Google Scholar] [CrossRef]
- Federico, A.; Tamburin, S.; Maier, A.; Faccini, M.; Casari, R.; Morbioli, L.; Lugoboni, F. Multifocal cognitive dysfunction in high-dose benzodiazepine users: A cross-sectional study. Neurol. Sci. 2017, 38, 137–142. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Paquette, A.; Hilmer, S.N.; Holroyd-Leduc, J.; Carnahan, R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging 2012, 29, 639–658. [Google Scholar]
- Lugoboni, F.; Mirijello, A.; Faccini, M.; Casari, R.; Cossari, A.; Musi, G.; Bissoli, G.; Quaglio, G.; Addolorato, G. Quality of life in a cohort of high-dose benzodiazepine dependent patients. Drug Alcohol Depend. 2014, 142, 105–109. [Google Scholar] [CrossRef]
- Paltiel, O.; Marzec-Boguslawska, A.; Soskolne, V.; Massalha, S.; Avitozour, M.; Pfeffer, R.; Cherny, N.; Peretz, T. Use of tranquilizers and sleeping pills among cancer patients is associated with a poorer quality of life. Qual. Life Res. 2004, 13, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Tamburin, S.; Federico, A.; Faccini, M.; Casari, R.; Morbioli, L.; Sartore, V.; Mirijello, A.; Addolorato, G.; Lugoboni, F. Determinants of quality of life in high-dose benzodiazepine misusers. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Graham, R.K. Determinants of treatment-resistant depression: The salience of benzodiazepines. J. Nerv. Ment. Dis. 2015, 203, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Lader, M.H. Anxiety or depression during withdrawal of hypnotic treatments. J. Psychosom. Res. 1994, 38, 113–123. [Google Scholar] [CrossRef]
- Manthey, L.; van Loenen-Frosch, F.; Giltay, E.J.; van Veen, T.; Glashouwer, K.; Penninx, B.W.; Zitman, F.G. High dose benzodiazepines prolong reaction times in chronic users who have major depressive and/or anxiety disorders. Br. J. Clin. Pharm. 2014, 77, 571–577. [Google Scholar] [CrossRef]
- Dodds, T.J. Prescribed benzodiazepines and suicide risk: A review of the literature. Prim. Care Companion CNS Disord. 2017, 19, e1–e6. [Google Scholar] [CrossRef]
- Elvik, R. Risk of road accident associated with the use of drugs: A systematic review and meta-analysis of evidence from epidemiological studies. Accid. Anal. Prev. 2013, 60, 254–267. [Google Scholar] [CrossRef]
- Hansen, R.N.; Boudreau, D.M.; Ebel, B.E.; Grossman, D.C.; Sullivan, S.D. Sedative hypnotic medication use and the risk of motor vehicle crash. Res. Pract. 2015, 105, 64–69. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, L.L.; Zhou, Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Clin. Risk Manag. 2014, 10, 437–448. [Google Scholar] [CrossRef]
- Ballokova, A.; Peel, N.M.; Fialova, D.; Scott, I.A.; Gray, L.C.; Hubbard, R.E. Use of benzodiazepines and association with falls in older people admitted to hospital: A prospective cohort study. Drugs Aging 2014, 31, 299–310. [Google Scholar] [CrossRef]
- Diaz-Gutierrez, M.J.; Martinez-Cengotitabengoa, M.; Saez de Adana, E.; Cano, A.I.; Martinez-Cengotitabengoa, M.T.; Besga, A.; Segarra, R.; Gonzalez-Pinto, A. Relationship between the use of benzodiazepines and falls in older adults: A systematic review. Maturitas 2017, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Iqbal, U.; Walther, B.; Atique, S.; Dubey, N.K.; Nguyen, P.A.; Poly, T.N.; Masud, J.H.; Li, Y.J.; Shabbir, S.A. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology 2016, 47, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Wang, Y.; Zhang, Y.; Zhao, Y. Association between benzodiazepine use and dementia: A meta-analysis. PLoS ONE 2015, 10, e0127836. [Google Scholar] [CrossRef] [PubMed]
- Billioti de Gage, S.; Begaud, B.; Bazin, F.; Verdoux, H.; Dartigues, J.F.; Peres, K.; Kurth, T.; Pariente, A. Benzodiazepine use and risk of dementia: Prospective population based study. BMJ 2012, 345, e6231. [Google Scholar] [CrossRef] [PubMed]
- Weich, S.; Pearce, H.L.; Croft, P.; Singh, S.; Crome, I.; Bashford, J.; Frisher, M. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: Retrospective cohort study. BMJ 2014, 348, g1996. [Google Scholar] [CrossRef]
- Belleville, G. Mortality hazard associated with anxiolytic and hypnotic drug use in the national population health survey. Can. J. Psychiatry 2010, 55, 558–567. [Google Scholar] [CrossRef]
- Parsaik, A.K.; Mascarenhas, S.S.; Khosh-Chashm, D.; Hashmi, A.; John, V.; Okusaga, O.; Singh, B. Mortality associated with anxiolytic and hypnotic drugs: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2016, 50, 520–533. [Google Scholar] [CrossRef]
- Albrecht, B.; Staiger, P.K.; Hall, K.; Miller, P.; Best, D.; Lubman, D.I. Benzodiazepine use and aggressive behaviour: A systematic review. Aust. N. Z. J. Psychiatry 2014, 48, 1096–1114. [Google Scholar] [CrossRef]
- Mancuso, C.E.; Tanzi, M.G.; BGabay, M. Paradoxical reactions to benzodiazepines: Literature review and treatment options. Pharmacotherapy 2004, 24, 1177–1185. [Google Scholar] [CrossRef]
- Maric, N.P.; Latas, M.; Andric Petrovic, S.; Soldatovic, I.; Arsova, S.; Crnkovic, D.; Gugleta, D.; Ivezic, A.; Janjic, V.; Karlovic, D.; et al. Prescribing practices in Southeastern Europe: Focus on benzodiazepine prescription at discharge from nine university psychiatric hospitals. Psychiatry Res. 2017, 258, 59–65. [Google Scholar] [CrossRef]
- Takeshima, N.; Ogawa, Y.; Hayasaka, Y.; Furukawa, T.A. Continuation and discontinuation of benzodiazepine prescriptions: A cohort study based on a large claims database in Japan. Psychiatry Res. 2016, 237, 201–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, Y.; Wilson, E.; Goldner, E.M.; Fischer, B. Benzodiazepine use, misuse, and harm at the population level in Canada: A comprehensive narrative review of data and developments since 1995. Clin. Drug Investig. 2016, 36, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Sakshaug, S.; Handal, M.; Hjellvik, V.; Berg, C.; Ripel, A.; Gustavsen, I.; Morland, J.; Skurtveit, S. Long-term use of z-hypnotics and co-medication with benzodiazepines and opioids. Basic Clin. Pharmacol. Toxicol. 2017, 120, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; King, M.; Schoenbaum, M. Benzodiazepine use in the United States. JAMA Psychiatry 2015, 72, 136–142. [Google Scholar] [CrossRef]
- Schallemberger, J.B.; Colet Cde, F. Assessment of dependence and anxiety among benzodiazepine users in a provincial municipality in Rio Grande do Sul, Brazil. Trends Psychiatry Psychother. 2016, 38, 63–70. [Google Scholar] [CrossRef]
- Lader, M.H. Benzodiazepines revisited: Will we ever learn? Addiction 2011, 106, 2086–2109. [Google Scholar] [CrossRef]
- Jann, M.; Kennedy, W.K.; Lopez, G. Benzodiazepines: A major component in unintentional prescription drug overdoses with opioid analgesics. J. Pharm. Pract. 2014, 27, 5–16. [Google Scholar] [CrossRef]
- Liew, D.; Joules, E.; Booth, J.; Garrett, K.; Fauman, A. Evidence to Inform the Inclusion of Schedule 4 Prescription Medications on a Real-Time Prescription Monitoring System; Austin Health: Melbourne, Australia, 2017. [Google Scholar]
- NIH: National Institute on Drug Abuse. Overdose Death Rates. Available online: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (accessed on 27 November 2019).
- National Records of Scotland. Drug related deaths in. Scotland in 2018; National Records of Scotland: Edinburgh, UK, 2019. [Google Scholar]
- Office for National Statistics. Statistical Bulletin: Deaths Related to Drug Poisoning in England and Wales: 2018 Registrations; Office for National Statistics: London, UK, 2019. [Google Scholar]
- Statistics Canada. Causes of Death. 2017. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/190530/dq190530c-eng.htm (accessed on 3 October 2019).
- Gravensteen, I.K.; Ekeberg, O.; Thiblin, I.; Helweg-Larsen, K.; Hem, E.; Rogde, S.; Tollefsen, I.M. Psychoactive substances in natural and unnatural deaths in Norway and Sweden: A study on victims of suicide and accidents compared with natural deaths in psychiatric patients. BMC Psychiatry 2019, 19, 33. [Google Scholar] [CrossRef]
- PDMP Center of Excellence at Brandeis University. Briefing on PDMP effectiveness. Available online: https://www.pdmpassist.org/pdf/Resources/Briefing%20on%20PDMP%20Effectiveness%203rd%20revision.pdf (accessed on 2 October 2019).
- Finley, E.P.; Garcia, A.; Rosen, K.; McGeary, D.; Pugh, M.J.; Potter, J.S. Evaluating the impact of prescription drug monitoring program implementation: A scoping review. BMC Health Serv. Res. 2017, 17, 420. [Google Scholar] [CrossRef]
- Fink, D.S.; Schleimer, J.P.; Sarvet, A.; Grover, K.K.; Delcher, C.; Castillo-Carniglia, A.; Kim, J.H.; Rivera-Aguirre, A.E.; Henry, S.G.; Martins, S.S.; et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann. Intern. Med. 2018, 168, 783–790. [Google Scholar] [CrossRef]
- Deyo, R.A.; Hallvik, S.E.; Hildebran, C.; Marino, M.; Springer, R.; Irvine, J.M.; O′Kane, N.; Van Otterloo, J.; Wright, D.A.; Leichtling, G.; et al. Association of prescription drug monitoring program use with opioid prescribing and health outcomes: A comparison of program users and nonusers. J. Pain 2018, 19, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Haegerich, T.M.; Paulozzi, L.J.; Manns, B.J.; Jones, C.M. What we know, and don′t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014, 145, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Grecu, A.M.; Dave, D.M.; Saffer, H. Mandatory access prescription drug monitoring programs and prescription drug abuse. J. Policy Anal. Manag. 2018, 38, 181–209. [Google Scholar] [CrossRef]
- Liang, D.; Shi, Y. Prescription drug monitoring programs and drug overdose deaths involving benzodiazepines and prescription opioids. Drug Alcohol Rev. 2019, 38, 494–502. [Google Scholar] [CrossRef]
- PDMP Center of Excellence at Brandeis University. PDMP Prescriber Use Mandates: Characteristics, Current Status, and Outcomes in Selected States. Available online: http://www.pdmpassist.org/pdf/Resources/Briefing_on_mandates_3rd_revision_A.pdf (accessed on 2 October 2019).
- Haffajee, R.L.; Jena, A.B.; Weiner, S.G. Mandatory use of prescription drug monitoring programs. JAMA 2015, 313, 891–892. [Google Scholar] [CrossRef]
- Boyles, P. Real-time prescription monitoring: Lessons from Tasmania. Aust. Prescr. 2019, 42, 48–49. [Google Scholar] [CrossRef]
- Reynolds, A.; Boyles, P. Clinical care and regulation of opioid use: The Tasmanian model. Med. Today 2017, 183, 17–21. [Google Scholar]
- Brown, R.; Morgan, A. The opioid epidemic in North America: Implications for Australia. Trends Issues Crime Crim. Justice 2019, 578, 1–15. [Google Scholar]
- State Government of Victoria. Real-Time Prescription Monitoring. Available online: http://www2.health.vic.gov.au/public-health/drugs-and-poisons/real-time-prescription-monitoring (accessed on 2 October 2019).
- Wastila, L.J.; Bishop, C. The influence of multiple copy prescription programs on analgesic utilization. J. Pharm. Care Pain Symptom Control 1996, 4, 3–19. [Google Scholar] [CrossRef]
- Fishman, S.M.; Papazian, J.S.; Gonzalez, S.; Riches, P.S.; Gilson, A. Regulating opioid prescribing through prescription monitoring programs: Balancing drug diversion and treatment of pain. Pain Med. 2004, 5, 309–324. [Google Scholar] [CrossRef]
- Paulozzi, L.J.; Kilbourne, E.M.; Desai, H.A. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011, 12, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Murphy, A.L.; Turner, J.P.; Gardner, D.M.; Silvius, J.L.; Bouck, Z.; Gordon, D.; Tannenbaum, C. Policies for deprescribing: An international scan of intended and unintended outcomes of limiting sedative-hypnotic use in community-dwelling older adults. Healthc. Policy 2019, 14, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.; Singh, S.; Byrne, L.; Maharaj, K.; Guttmacher, L. Consequences of the 1989 New York state triplicate benzodiazepine prescription regulations. JAMA 1991, 266, 2392–2397. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kreling, D.H. The effect of the Medicare Part D benzodiazepine exclusion on the utilization patterns of benzodiazepines and substitute medications. Res. Soc. Adm. Pharm. 2014, 10, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.L.; Buckley, N.A.; Cairns, R.; Pearson, S. Comparison of prescribing patterns before and after implementation of a national policy to reduce inappropriate Alprazolam prescribing in Australia. JAMA Netw. Open 2019, 2, e1911590. [Google Scholar] [CrossRef] [PubMed]
- Gugelmann, H.; Perrone, J.; Nelson, L. Windmills and pill mills: Can PDMPs tilt the prescription drug epidemic? J. Med. Toxicol. 2012, 8, 378–386. [Google Scholar] [CrossRef][Green Version]
- Perrone, J.; Nelson, L.S. Medication reconciliation for controlled substances: An “ideal” prescription-drug monitoring program. New. Engl. J. Med. 2012, 366, 2341–2343. [Google Scholar] [CrossRef]
- Ross-Degnan, D.; Simoni-Wastila, L.; Brown, J.S.; Gao, X.; Mah, C.; Cosler, L.E.; Fanning, T.; Gallagher, P.; Salzman, C.; Shader, R.I.; et al. A controlled study of the effects of state surveillance on indicators of problematic and non-problematic benzodiazepine use in a medicaid population. Int. J. Psychiatry Med. 2004, 34, 103–123. [Google Scholar] [CrossRef]
- Ogeil, R.P.; Heilbronn, C.; Lloyd, B.; Lubman, D.I. Benefits and challenges to the implementation of real-time prescription monitoring. Med. Today 2015, 16, 65–68. [Google Scholar]
- Islam, M.M.; McRae, I.S. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: Pros, cons and tensions. BMC Pharm. Toxicol. 2014, 15, 1–7. [Google Scholar] [CrossRef]
- Dasgupta, N.; Creppage, K.; Austin, A.; Ringwalt, C.; Sanford, C.; Proescholdbell, S.K. Observed transition from opioid analgesic deaths toward heroin. Drug Alcohol Depend. 2014, 145, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Unick, G.J.; Rosenblum, D.; Mars, S.; Ciccarone, D. Intertwined epidemics: National demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS ONE 2013, 8, e54496. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Shea, D.G.; Shi, Y.; Moran, J.R. State prescription drug monitoring programs and fatal drug overdoses. Am. J. Manag. Care 2017, 23, 297–303. [Google Scholar] [PubMed]
- Hildebran, C.; Leichtling, G.; Irvine, J.M.; Cohen, D.J.; Hallvik, S.E.; Deyo, R.A. Clinical styles and practice policies: Influence on communication with patients regarding worrisome prescription drug monitoring program data. Pain Med. 2016, 17, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Yuanhong Lai, A.; Smith, K.C.; Vernick, J.S.; Davis, C.S.; Caleb Alexander, G.; Rutkow, L. Perceived Unintended Consequences of Prescription Drug Monitoring Programs. Subst. Use Misuse 2019, 54, 345–349. [Google Scholar] [CrossRef]
- Breier, A.; Charney, D.; Nelson, C. Seizures induced by abrupt discontinuation from Alprazolam. Am. J. Psychiatry 1984, 141, 1606–1607. [Google Scholar]
- American Psychiatric Association. Benzodiazepine Dependence, Toxicity, and Abuse; APA: Washington, DC, USA, 1990. [Google Scholar]
- Rickels, K.; Schweizer, E.; Case, G.; Greenblatt, D. Long term therapeutic use of benzodiazepines- I. Effects of abrupt discontinuation. Arch. Gen. Psych. 1990, 47, 899–907. [Google Scholar] [CrossRef]
- Ashton, H. Protracted withdrawal syndromes from benzodiazepines. J. Subst. Abus. Treat. 1991, 8, 19–28. [Google Scholar] [CrossRef]
- Bachhuber, M.A.; Maughan, B.C.; Mitra, N.; Feingold, J.; Starrels, J.L. Prescription monitoring programs and emergency department visits involving benzodiazepine misuse: Early evidence from 11 United States metropolitan areas. Int. J. Drug Policy 2016, 28, 120–123. [Google Scholar] [CrossRef]
- Okumura, Y.; Shimizu, S.; Matsumoto, T. Prevalence, prescribed quantities, and trajectory of multiple prescriber episodes for benzodiazepines: A 2-year cohort study. Drug Alcohol Depend. 2016, 158, 118–125. [Google Scholar] [CrossRef]
- Fisher, J.; Sanyal, C.; Frail, D.; Sketris, I. The intended and unintended consequences of benzodiazepine monitoring programmes: A review of the literature. J. Clin. Pharm. 2012, 37, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J. New Insights into Victorian Pharmaceutical Drug Overdose Death. Available online: https://www.turningpoint.org.au/education/talking-point/victorian-pharmaceutical-drug-overdose-death (accessed on 5 October 2019).
- Allen, B.; Harocopos, A.; Chernick, R. Substance use stigma, primary care, and the New York state prescription drug monitoring program. Behav. Med. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Leichtling, G.J.; Irvine, J.M.; Hildebran, C.; Cohen, D.J.; Hallvik, S.E.; Deyo, R.A. Clinicians′ use of prescription drug monitoring programs in clinical practice and decision-making. Pain Med. 2017, 18, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M. Treatment of Benzodiazepine Dependence. N. Engl. J. Med. 2017, 376, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Perna, G.; Alciati, A.; Riva, A.; Micieli, W.; Caldirola, D. Long-term pharmacological treatments of anxiety disorders: An updated systematic review. Curr. Psychiatry Rep. 2016, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Willems, I.A.; Gorgels, W.J.; Oude Voshaar, R.C.; Mulder, J.; Lucassen, P.L. Tolerance to benzodiazepines among long-term users in primary care. Fam. Pr. 2013, 30, 404–410. [Google Scholar] [CrossRef]
- Votaw, V.R.; Geyer, R.; Rieselbach, M.M.; McHugh, R.K. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol Depend. 2019, 200, 95–114. [Google Scholar] [CrossRef]
- Nicolaidis, C. Police officer, deal-maker, or health care provider? Moving to a patient-centered framework for chronic opioid management. Pain Med. 2011, 12, 890–897. [Google Scholar] [CrossRef]
- Brett, J.; Murnion, B. Management of benzodiazepine misuse and dependence. Aust. Prescr. 2015, 38, 152–155. [Google Scholar] [CrossRef]
- Matthias, M.S.; Parpart, A.L.; Nyland, K.A.; Huffman, M.; Stubbs, D.L.; Sargent, C.; Blair, M.J. The patient–provider relationship in chronic pain care: Providers′ perspectives. Pain Med. 2011, 11, 1688–1697. [Google Scholar] [CrossRef]
- Manchikanti, L. National drug control policy and prescription drug abuse: Facts and fallacies. Pain Physician 2007, 10, 399–424. [Google Scholar] [PubMed]
- Stevenson, F.; Barry, C.; Britten, N.; Barber, N.; Bradley, C.P. Doctor-patient communication about drugs: The evidence for shared decision making. Soc. Sci. Med. 2000, 50, 829–840. [Google Scholar] [CrossRef]
- Elwyn, G.; Edwards, A.; Kinnersley, P.; Grol, R. Shared decision making and the concept of equipoise: The competences of involving patients in healthcare choices. Br. J. Gen. Pract. 2000, 50, 892–899. [Google Scholar] [PubMed]
- Wyse, J.J.; Ganzini, L.; Dobscha, S.K.; Krebs, E.E.; Morasco, B.J. Setting expectations, following orders, safety, and standardization: Clinicians′ strategies to guide difficult conversations about opioid prescribing. J. Gen. Intern. Med. 2019, 34, 1200–1206. [Google Scholar] [CrossRef]
- Nielsen, S.; Bruno, R. Implementing real-time prescription drug monitoring: Are we ready? Drug Alcohol Rev. 2014, 33, 463–465. [Google Scholar] [CrossRef]
- The Royal Australian College of General Practitioners. Prescribing Drugs of Dependence in General Practice, Part B—Benzodiazepines; The Royal Australian College of General Practitioners: Melbourne, Australia, 2015. [Google Scholar]
- Mead, N.; Bower, P. Patient-centredness: A conceptual framework and review of the empirical literature. Soc. Sci. Med. 2000, 51, 1087–1110. [Google Scholar] [CrossRef]
- Ackerman, S.J.; Hilsenroth, M.J. A review of therapist characteristics and techniques positively impacting the therapeutic alliance. Clin. Psychol. Rev. 2003, 23, 1–33. [Google Scholar]
- Oldenhof, E.; Hall, K.; Youssef, G.; Staiger, P.K. Influences on Long-Term Benzodiazepine Users Decision to Continue or Stop Taking Their Medication; Deakin University: Melbourne, Australia, 2019. [Google Scholar]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Panic Disorder; American Psychiatric Association Publishing: Washington, DC, USA, 2009. [Google Scholar]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change: Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef]
- Gosselin, P.; Ladouceur, R.; Morin, C.M.; Dugas, M.J.; Baillargeon, L. Benzodiazepine discontinuation among adults with GAD: A randomized trial of cognitive-behavioral therapy. J. Consult. Clin. Psychol 2006, 74, 908–919. [Google Scholar] [CrossRef]
- Parr, J.M.; Kavanagh, D.J.; Cahill, L.; Mitchell, G.; Young, R.M. Effectiveness of current treatment approaches for benzodiazepine discontinuation: A meta-analysis. Addiction 2009, 104, 13–24. [Google Scholar] [CrossRef]
- Otto, M.W.; McHugh, R.K.; Simon, N.M.; Farach, F.J.; Worthington, J.J.; Pollack, M.H. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behav. Res. 2010, 48, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belanger, L.; Bastien, C.; Vallieres, A. Long-term outcome after discontinuation of benzodiazepines for insomnia: A survival analysis of relapse. Behav. Res. 2005, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Spiegel, D.A.; Hegel, M.T. Cognitive-behavioral therapy helps prevent relapse and recurrence of panic disorder following Alprazolam discontinuation: A long-term follow-up of the Peoria and Dartmouth studies. J. Consult. Clin. Psychol. 1999, 67, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wurf, G.; O′Neal, P.; Swing, A.; Golin, R. A Mixed Methods Evaluation of the Reconnexion Benzodiazepine Support and Counselling Service; Monash University: Melbourne, Australia, 2019. [Google Scholar]
- Creupelandt, H.; Anthierens, S.; Habraken, H.; Sirdifield, C.; Niroshan Siriwardena, A.; Christiaens, T. A tailored e-learning gives long-term changes in determinants of GPs′ benzodiazepines prescribing: A pretest-posttest study with self-report assessments. Scand. J. Prim. Health Care 2019, 1–8. [Google Scholar] [CrossRef]
- Buchman, D.Z.; Ho, A.; Illes, J. You present like a drug addict: Patient and clinician perspectives on trust and trustworthiness in chronic pain management. Pain Med. 2016, 17, 1394–1406. [Google Scholar] [CrossRef]
- Kennedy-Hendricks, A.; Barry, C.L.; Gollust, S.E.; Ensminger, M.E.; Chisolm, M.S.; McGinty, E.E. Social stigma toward persons with prescription opioid use disorder: Associations with public support for punitive and public health-oriented policies. Psychiatr. Serv. 2017, 68, 462–469. [Google Scholar] [CrossRef]
- Parr, J.M.; Kavanagh, D.J.; Young, R.M.; McCafferty, K. Views of general practitioners and benzodiazepine users on benzodiazepines: A qualitative analysis. Soc. Sci. Med. 2006, 62, 1237–1249. [Google Scholar] [CrossRef]
- Losby, J.L.; Hyatt, J.D.; Kanter, M.H.; Baldwin, G.; Matsuoka, D. Safer and more appropriate opioid prescribing: A large healthcare system′s comprehensive approach. J. Eval. Clin. Pract. 2017, 23, 1173–1179. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldenhof, E.; Anderson-Wurf, J.; Hall, K.; Staiger, P.K. Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use. J. Clin. Med. 2019, 8, 2143. https://doi.org/10.3390/jcm8122143
Oldenhof E, Anderson-Wurf J, Hall K, Staiger PK. Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use. Journal of Clinical Medicine. 2019; 8(12):2143. https://doi.org/10.3390/jcm8122143
Chicago/Turabian StyleOldenhof, Erin, Jane Anderson-Wurf, Kate Hall, and Petra K. Staiger. 2019. "Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use" Journal of Clinical Medicine 8, no. 12: 2143. https://doi.org/10.3390/jcm8122143
APA StyleOldenhof, E., Anderson-Wurf, J., Hall, K., & Staiger, P. K. (2019). Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use. Journal of Clinical Medicine, 8(12), 2143. https://doi.org/10.3390/jcm8122143




