Serum Spexin is Correlated with Lipoprotein(a) and Androgens in Female Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Bone Mineral Density (BMD) and Body Fat Assessment
2.4. Blood Sampling
2.5. Measurements of Metabolic Parameters
2.6. Measurements of Hormonal Parameters
2.7. Insulin Sensitivity/Resistance Assessment
2.8. Ovarian Measurements
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mirabeau, O.; Perlas, E.; Severini, C.; Audero, E.; Gascuel, O.; Possenti, R.; Birney, E.; Rosenthal, N.; Gross, C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res 2007, 17, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Rucinski, M.; Macchi, V.; Stecco, C.; Malendowicz, L.K.; De Caro, R. Spexin expression in normal rat tissues. J. Histochem. Cytochem. 2010, 58, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Walewski, J.L.; Ge, F.; Gagner, M.; Inabnet, W.B.; Pomp, A.; Branch, A.D.; Berk, P.D. Adipocyte accumulation of long-chain fatty acids in obesity is multifactorial, resulting from increased fatty acid uptake and decreased activity of genes involved in fat utilization. Obes. Surg. 2010, 20, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Walewski, J.L.; Ge, F.; Lobdell, H., 4th; Levin, N.; Schwartz, G.J.; Vasselli, J.; Pomp, A.; Dakin, G.; Berk, P.D. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity 2014, 22, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Walewski, J.L.; Anglade, D.; Berk, P.D. Regulation of hepatocellular fatty acid uptake in mouse models of fatty liver disease with and without functional leptin signaling: Roles of NfKB and SREBP-1C and the effects of spexin. Semin. Liver. Dis. 2016, 36, 360–372. [Google Scholar] [CrossRef]
- Wong, M.K.; Sze, K.H.; Chen, T.; Cho, C.K.; Law, H.C.; Chu, I.K.; Wong, A.O. Goldfish spexin: Solution structure and novel function as a satiety factor in feeding control. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 348–366. [Google Scholar] [CrossRef]
- Kumar, S.; Hossain, J.; Nader, N.; Aguirre, R.; Sriram, S.; Balagopal, P.B. Decreased circulating levels of spexin in obese children. J. Clin. Endocrinol. Metab. 2016, 101, 2931–2936. [Google Scholar] [CrossRef]
- Chen, T.; Wang, F.; Chu, Z.; Sun, L.; Lv, H.; Zhou, W.; Shen, J.; Chen, L.; Hou, M. Circulating spexin decreased and negatively correlated with systemic insulin sensitivity and pancreatic β cell function in obese children. Ann. Nutr. Metab. 2019, 74, 125–131. [Google Scholar] [CrossRef]
- Hodges, S.K.; Teague, A.M.; Dasari, P.S.; Short, K.R. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatric Diabetes 2018, 19, 212–216. [Google Scholar] [CrossRef]
- Kolodziejski, P.A.; Pruszynska-Oszmalek, E.; Korek, E.; Sassek, M.; Szczepankiewicz, D.; Kaczmarek, P.; Nogowski, L.; Mackowiak, P.; Nowak, K.W.; Krauss, H.; et al. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol. Res. 2018, 67, 45–56. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, T.; Zhao, L.; Zhong, L.L.D.; Lam, W.C.; Fan, B.M.; Bian, Z.X. Circulating spexin levels negatively correlate with age, BMI, fasting glucose, and triglycerides in healthy adult women. J. Endocr. Soc. 2018, 2, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ma, Y.; Gu, M.; Zhang, Y.; Yan, S.; Li, N.; Wang, Y.; Ding, X.; Yin, J.; Fan, N.; et al. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 2015, 71, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alenad, A.; Al-Hazmi, H.; Amer, O.E.; Hussain, S.D.; Alokail, M.S. Spexin levels are associated with metabolic syndrome components. Dis. Markers 2018, 2018, 1679690. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Wani, K.; Yakout, S.M.; Al-Hazmi, H.; Amer, O.E.; Hussain, S.D.; Sabico, S.; Ansari, M.G.A.; Al-Musharaf, S.; Alenad, A.M.; et al. Favorable changes in fasting glucose in a 6-month self-monitored lifestyle modification programme inversely affects spexin levels in females with prediabetes. Sci. Rep. 2019, 9, 9454. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Boucher, J.; Tran, T.T.; Kahn, C.R. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 2009, 58, 803–812. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj Fuleihan, G.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M.; International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Misra, M.; Klibanski, A. Anorexia nervosa, obesity and bone metabolism. Pediatric Endocrinol. Rev. 2013, 11, 21–33. [Google Scholar]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Kumar, S.; Hossain, M.J.; Javed, A.; Kullo, I.J.; Balagopal, P.B. Relationship of circulating spexin with markers of cardiovascular disease: A pilot study in adolescents with obesity. Pediatric Obes. 2018, 13, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Jacobs, D.R.; Steinberger, J.; Hong, C.-P.; Prineas, R.; Luepker, R.; Sinaiko, A.R. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 1999, 48, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejski, P.A.; Pruszynska-Oszmalek, E.; Micker, M.; Skrzypski, M.; Wojciechowicz, T.; Szwarckopf, P.; Skieresz-Szewczyk, K.; Nowak, K.W.; Strowski, M.Z. Spexin: A novel regulator of adipogenesis and fat tissue metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Takamura, M.; Kawashiri, M.A. Lipoprotein(a) as an old and new causal risk factor of atherosclerotic cardiovascular disease. J. Atheroscler. Thromb. 2019, 26, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Utermann, G. Lipoprotein(a): Resurrected by genetics. J. Intern. Med. 2013, 273, 6–30. [Google Scholar] [CrossRef]
- Mora, S.; Kamstrup, P.R.; Rifai, N.; Nordestgaard, B.G.; Buring, J.E.; Ridker, P.M. Lipoprotein(a) and risk of type 2 diabetes. Clin. Chem. 2010, 56, 1252–1260. [Google Scholar] [CrossRef]
- Rainwater, D.L.; Haffner, S.M. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1335–1341. [Google Scholar] [CrossRef]
- Neele, D.M.; de Wit, E.C.; Princen, H.M. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia 1999, 42, 41–44. [Google Scholar] [CrossRef]
- Gencer, B.; Kronenberg, F.; Stroes, E.S.; Mach, F. Lipoprotein(a): The revenant. Eur. Heart J. 2017, 38, 1553–1560. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Garaulet, M.; Perex-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.C.; Matsumoto, A.M.; Fujimoto, W.Y.; Boyko, E.J. Association of bioavailable, free, total testosterone with insulin resistance: Influence of sex hormone-binding globulin and body fat. Diabetes Care 2004, 27, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Yun, S.; Son, G.H.; Hwang, J.I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology 2014, 155, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Qi, X.; Zhou, W.; Liu, X.; Lin, H.; Zhang, Y.; Cheng, C.H. A novel neuropeptide in suppressing luteinizing hormone release in goldfish, Carassius auratus. Mol. Cell Endocrinol. 2013, 374, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Rucinski, M.; Porzionato, A.; Ziolkowska, A.; Szyszka, M.; Macchi, V.; De Caro, R.; Malendowicz, L.K. Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation. Peptides 2010, 31, 676–682. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (n = 80) | NW Group (n = 55) | OB/OW Group (n = 25) | p | |
|---|---|---|---|---|
| BMI (kg/m2) | 22.72 ± 5.40 | 19.72 ± 2.52 | 29.35 ± 3.89 | <0.001 |
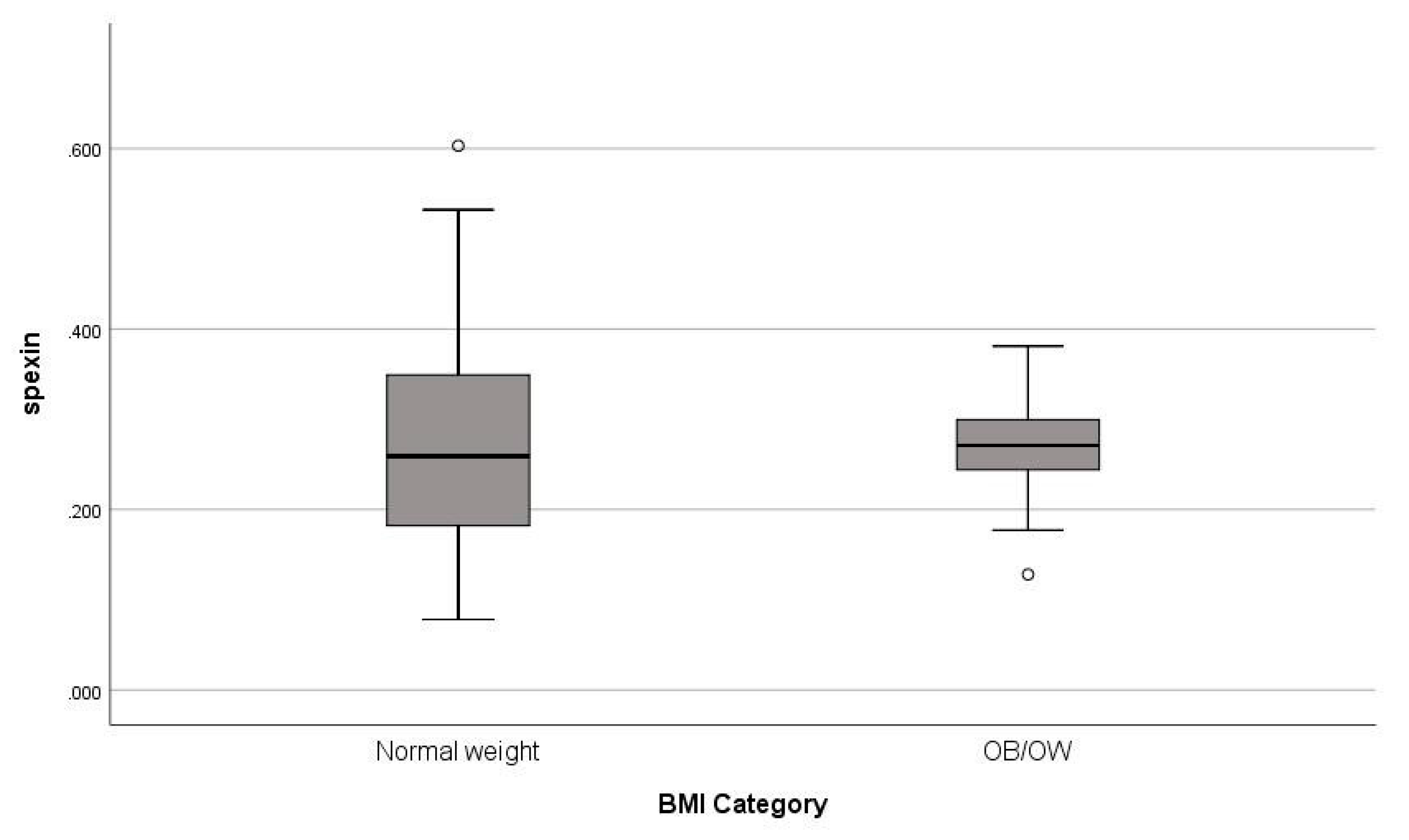
| Spexin (ng/mL) § | 0.27 (0.15) | 0.26 (0.17) | 0.28 (0.06) | 0.378 |
| BF (%) | 36.43 ± 9.34 | 29.13 ± 4.56 | 44.33 ± 6.05 | <0.001 |
| Ht (cm) | 163.62 ± 6.15 | 164.42 ± 5.48 | 162.75 ± 6.81 | 0.342 |
| Wt (kg) | 66.60 ± 16.34 | 55.50 ± 5.27 | 78.62 ± 15.77 | <0.001 |
| WC (cm) § | 77.00 (14.00) | 69.00 (11.50) | 84.00 (15.00) | <0.001 |
| HC (cm) | 103.39 ± 10.32 | 96.09 ± 5.63 | 107.05 ± 10.26 | 0.003 |
| WC/HC § | 0.76 (0.09) | 0.73 (0.07) | 0.78 (0.09) | 0.019 |
| FG-score | 8.47 ± 5.96 | 6.68 ± 4.85 | 11.29 ± 6.60 | 0.022 |
| TG (mmol/L) § | 0.93 (0.53) | 0.68 (0.44) | 1.05 (0.45) | 0.013 |
| Glucose (mmol/L) | 4.95 ± 0.51 | 4.75 ± 0.54 | 5.18 ± 0.37 | 0.002 |
| AUC glucose (mmol/L) | 793.03 ± 125.44 | 716.60 ± 94.28 | 838.88 ± 121.51 | 0.017 |
| Insulin (pmol/L) § | 71.88 (65.63) | 48.20 (41.04) | 95.15 (87.99) | <0.001 |
| AUC insulin § (pmol/L) | 69,109.70 (56,754.54) | 47,566.31 (12,885.41) | 99,414.20 (57,473.35) | <0.001 |
| AUC glucose/AUC insulin § | 1.40 (0.91) | 1.82 (0.59) | 0.92 (0.57) | 0.002 |
| HOMA-IR § | 2.19 (2.06) | 1.36 (1.12) | 3.26 (3.44) | <0.001 |
| QUICKI | 0.34 ± 0.03 | 0.36 ± 0.03 | 0.32 ± 0.02 | <0.001 |
| LH (IU/L) § | 5.40 (4.51) | 5.68 (4.60) | 4.62 (3.96) | 0.130 |
| FSH (IU/L) | 5.27 ± 1.17 | 5.29 ± 1.13 | 5.25 ± 1.28 | 0.928 |
| E2 (pmol/L) | 148.05 ± 45.30 | 146.51 ± 47.94 | 150.51 ± 42.47 | 0.800 |
| T (nmol/L) | 6.66 ± 3.68 | 7.46 ± 4.13 | 5.03 ± 1.74 | 0.022 |
| Free-T (pmol/L) § | 7.56 (7.08) | 7.22 (8.54) | 7.81 (10.27) | 0.220 |
| DHEA-S (μmol/L) | 6.20 ± 3.16 | 5.54 ± 2.60 | 7.24 ± 3.76 | 0.118 |
| Δ4-A (nmol/L) | 11.10 ± 4.75 | 10.54 ± 5.34 | 11.97 ± 3.63 | 0.383 |
| SHBG (nmol/L) § | 33.75 (22.88) | 40.70 (23.58) | 24.55 (13.15) | 0.011 |
| FAI § | 4.50 (5.20) | 4.50 (6.95) | 6.40 (3.60) | 0.693 |
| 17OHP (nmol/L) § | 3.42 (2.30) | 3.42 (2.51) | 3.24 (1.39) | 0.699 |
| MOV (mL) § | 10.50 (6.38) | 10.50 (8.00) | 10.00 (5.11) | 0.938 |
| WB z-score | 0.45 ± 0.91 | 0.06 ± 0.95 | 0.78 ± 0.77 | 0.065 |
| LS z-score | 0.36 ± 1.12 | -0.19 ± 1.04 | 0.83 ± 0.99 | 0.030 |
| ALP (IU/L) § | 83.00 (29.00) | 86.00 (28.25) | 80.00 (54.00) | 0.892 |
| iPTH (pmol/L) | 5.32 ± 2.22 | 5.39 ± 1.64 | 5.27 ± 2.62 | 0.895 |
| 25(OH)D (nmol/L) | 53.59 ± 19.02 | 57.58 ± 17.20 | 50.67 ± 20.32 | 0.370 |
| Cortisol (nmol/L) § | 455.24 (193.13) | 463.51 (126.09) | 366.95 (466.55) | 0.879 |
| hsCRP (nmol/L) § | 11.62 (11.43) | 8.95 (8.95) | 13.43 (37.14) | 0.190 |
| IL-6 (ng/mL) § | 0.75 (0.66) | 0.78 (0.78) | 0.68 (0.63) | 0.935 |
| CHOL (nmol/L) | 4.31 ± 0.87 | 3.69 ± 0.48 | 4.72 ± 0.82 | 0.001 |
| HDL (nmol/L) | 1.32 ± 0.25 | 1.39 ± 0.30 | 1.28 ± 0.21 | 0.240 |
| LDL (nmol/L) | 2.44 ± 0.76 | 1.91 ± 0.43 | 2.83 ± 0.73 | 0.001 |
| ApoA1 (g/L) | 1.33 ± 0.16 | 1.375 ± 0.18 | 1.31 ± 0.15 | 0.297 |
| ApoB (g/L) | 0.70 ± 0.20 | 0.58 ± 0.15 | 0.79 ± 0.18 | 0.004 |
| ApoE (g/L) | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.375 |
| Lp(a) (g/L) § | 0.09 (0.19) | 0.09 (0.16) | 0.09 (0.19) | 0.609 |
| PRL (nmol/L) § | 4.79 (0.52) | 0.67 (0.56) | 0.42 (0.28) | 0.097 |
| Total Sample | OB/OW Group | NW Group | ||||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| Age (years) | −0.138 | 0.226 | 0.260 | 0.219 | −0.215 | 0.115 |
| BMI (kg/m2) | −0.090 | 0.438 | −0.160 | 0.454 | −0.253 | 0.067 |
| BF (%) | −0.173 | 0.409 | 0.266 | 0.404 | −0.135 | 0.661 |
| Ht (cm) | 0.077 | 0.596 | 0.190 | 0.374 | −0.017 | 0.935 |
| Wt (kg) | 0.044 | 0.763 | −0.041 | 0.848 | 0.026 | 0.901 |
| WC (cm) | 0.032 | 0.861 | 0.108 | 0.632 | −0.123 | 0.719 |
| HC (cm) | 0.041 | 0.821 | 0.387 | 0.075 | −0.543 | 0.085 |
| WC/HC | 0.027 | 0.879 | −0.114 | 0.615 | 0.259 | 0.441 |
| FG-score | 0.092 | 0.594 | 0.333 | 0.245 | −0.132 | 0.559 |
| TG (mg/dL) | −0.232 | 0.174 | −0.342 | 0.102 | −0.042 | 0.897 |
| Glucose (mg/dL) | 0.039 | 0.789 | −0.396 | 0.056 | 0.217 | 0.287 |
| AUC glucose (mg/dL) | −0.118 | 0.582 | 0.181 | 0.519 | −0.25 | 0.516 |
| Insulin (μIU/mL) | 0.102 | 0.483 | −0.150 | 0.484 | 0.203 | 0.319 |
| AUC insulin (μIU/mL) | 0.213 | 0.317 | 0.309 | 0.262 | 0.017 | 0.966 |
| AUC glucose/AUC insulin | −0.331 | 0.114 | −0.342 | 0.212 | −0.267 | 0.488 |
| HOMA-IR | 0.101 | 0.487 | −0.221 | 0.300 | 0.218 | 0.284 |
| QUICKI | −0.099 | 0.494 | 0.209 | 0.327 | −0.213 | 0.296 |
| LH (mIU/mL) | −0.056 | 0.744 | 0.503 | 0.067 | −0.214 | 0.326 |
| FSH (mIU/mL) | −0.034 | 0.843 | 0.068 | 0.817 | −0.068 | 0.759 |
| E2 (pg/mL) | 0.018 | 0.919 | 0.314 | 0.274 | −0.132 | 0.559 |
| T (ng/mL) | −0.016 | 0.928 | 0.727 * | 0.011 | −0.147 | 0.504 |
| Free-T (ng/dL) | 0.080 | 0.662 | 0.222 | 0.446 | −0.003 | 0.99 |
| DHEA-S (μg/dL) | −0.212 | 0.216 | 0.143 | 0.626 | −0.445 * | 0.038 |
| Δ4-A (ng/mL) | −0.285 | 0.093 | −0.055 | 0.852 | −0.38 | 0.081 |
| SHBG (nmol/L) | −0.270 | 0.112 | −0.471 | 0.089 | −0.149 | 0.51 |
| FAI | 0.081 | 0.653 | 0.755 ** | 0.007 | −0.105 | 0.642 |
| 17OHP (ng/mL) | −0.176 | 0.329 | −0.007 | 0.983 | −0.271 | 0.235 |
| MOV (mL) | −0.079 | 0.643 | −0.024 | 0.935 | −0.087 | 0.693 |
| WB z-score | −0.262 | 0.240 | −0.319 | 0.313 | −0.061 | 0.867 |
| LS z-score | −0.124 | 0.582 | −0.049 | 0.879 | −0.040 | 0.913 |
| ALP (IU/L) | −0.159 | 0.447 | −0.456 | 0.088 | 0.225 | 0.532 |
| iPTH (pg/mL) | −0.074 | 0.719 | 0.079 | 0.781 | −0.387 | 0.239 |
| 25(OH)D (ng/mL) | −0.007 | 0.972 | 0.004 | 0.990 | 0.117 | 0.732 |
| Cortisol (μg/dL) | −0.270 | 0.213 | −0.308 | 0.306 | −0.467 | 0.174 |
| hsCRP (mg/L) | 0.273 | 0.208 | 0.161 | 0.567 | 0.395 | 0.333 |
| IL-6 (ng/mL) § | −0.085 | 0.687 | 0.307 | 0.265 | −0.486 | 0.154 |
| CHOL (mg/dL) | −0.011 | 0.953 | −0.300 | 0.226 | 0.062 | 0.849 |
| HDL (mg/dL) | −0.062 | 0.736 | 0.011 | 0.965 | −0.161 | 0.616 |
| LDL (mg/dL) | 0.135 | 0.509 | −0.089 | 0.752 | 0.288 | 0.391 |
| ApoA1 (mg/dL) | −0.003 | 0.990 | 0.238 | 0.393 | −0.315 | 0.345 |
| ApoB (mg/dL) | 0.056 | 0.787 | −0.027 | 0.924 | −0.014 | 0.968 |
| ApoE (mg/dL) | 0.104 | 0.619 | −0.194 | 0.507 | 0.189 | 0.577 |
| Lp(a) (mg/dL) | 0.402 * | 0.046 | 0.465 | 0.094 | 0.278 | 0.408 |
| PRL (ng/mL) | −0.057 | 0.781 | 0.077 | 0.785 | −0.182 | 0.592 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacopoulou, F.; Apostolaki, D.; Mantzou, A.; Doulgeraki, A.; Pałasz, A.; Tsimaris, P.; Koniari, E.; Efthymiou, V. Serum Spexin is Correlated with Lipoprotein(a) and Androgens in Female Adolescents. J. Clin. Med. 2019, 8, 2103. https://doi.org/10.3390/jcm8122103
Bacopoulou F, Apostolaki D, Mantzou A, Doulgeraki A, Pałasz A, Tsimaris P, Koniari E, Efthymiou V. Serum Spexin is Correlated with Lipoprotein(a) and Androgens in Female Adolescents. Journal of Clinical Medicine. 2019; 8(12):2103. https://doi.org/10.3390/jcm8122103
Chicago/Turabian StyleBacopoulou, Flora, Despoina Apostolaki, Aimilia Mantzou, Artemis Doulgeraki, Artur Pałasz, Pantelis Tsimaris, Eleni Koniari, and Vasiliki Efthymiou. 2019. "Serum Spexin is Correlated with Lipoprotein(a) and Androgens in Female Adolescents" Journal of Clinical Medicine 8, no. 12: 2103. https://doi.org/10.3390/jcm8122103
APA StyleBacopoulou, F., Apostolaki, D., Mantzou, A., Doulgeraki, A., Pałasz, A., Tsimaris, P., Koniari, E., & Efthymiou, V. (2019). Serum Spexin is Correlated with Lipoprotein(a) and Androgens in Female Adolescents. Journal of Clinical Medicine, 8(12), 2103. https://doi.org/10.3390/jcm8122103






