Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biochemical Parameters
2.3. Liver Stiffness Measurement
2.4. Statistical Analysis
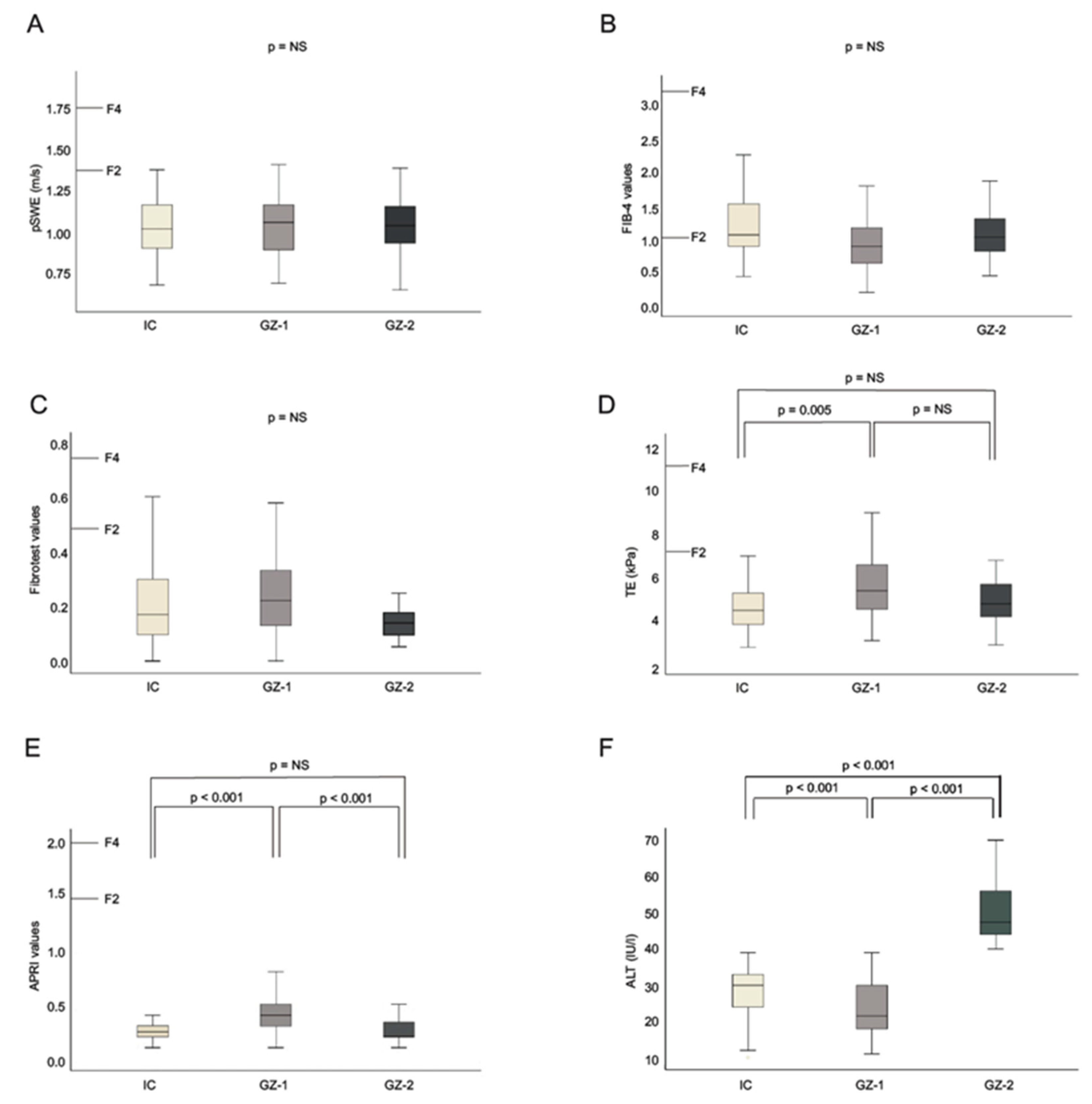
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dienstag, J.L. Hepatitis B Virus Infection. N. Engl. J. Med. 2008, 359, 1486–1500. [Google Scholar] [CrossRef]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of Worldwide Prevalence of Chronic Hepatitis B Virus Infection: A Systematic Review of Data Published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Manesis, E.K. HBeAg-Negative Chronic Hepatitis B: From Obscurity to Prominence. J. Hepatol. 2006, 45, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, F.; Surace, L.; Cavallone, D.; Colombatto, P.; Ricco, G.; Salvati, N.; Coco, B.; Romagnoli, V.; Gattai, R.; Salvati, A.; et al. Long-Term Outcome of Inactive and Active, Low Viraemic HBeAg-Negative-Hepatitis B Virus Infection: Benign Course towards HBsAg Clearance. Liver Int. 2017, 37, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yang, H.I.; Iloeje, U.H.; REVEAL-HBV Study Group. Hepatitis B Virus DNA Levels and Outcomes in Chronic Hepatitis B. Hepatology 2009, 49 (Suppl. 5), S72–S84. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H.; American Association for the Study of Liver Diseases. AASLD Guidelines for Treatment of Chronic Hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef]
- Hadziyannis, S.J. Unrevealing the Natural Course of the So-Called “Inactive HBsAg or HBV Carrier State”. Hepatol. Int. 2007, 1, 281–284. [Google Scholar] [CrossRef][Green Version]
- Management of Hepatitis B Virus Infection. EASL-The Home of Hepatology. European Association for the Study of the Liver. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- WHO | Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Available online: http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/ (accessed on 29 June 2019).
- Lok, A.S.F.; McMahon, B.J. Chronic Hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef]
- Castera, L.; Pinzani, M. Non-Invasive Assessment of Liver Fibrosis: Are We Ready? Lancet 2010, 375, 1419–1420. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Poynard, T.; Castera, L. Critical Comparison of Elastography Methods to Assess Chronic Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Castera, L. Noninvasive Methods to Assess Liver Disease in Patients With Hepatitis B or C. Gastroenterology 2012, 142, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Castéra, L.; Bernard, P.H.; Le Bail, B.; Foucher, J.; Trimoulet, P.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Transient Elastography and Biomarkers for Liver Fibrosis Assessment and Follow-up of Inactive Hepatitis B Carriers. Aliment. Pharmacol. Ther. 2011, 33, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Bismut, F.; Ratziu, V.; Pieroni, L.; Charlotte, F.; Benhamou, Y.; Poynard, T.; MULTIVIRC Group. Biochemical Markers of Liver Fibrosis in Patients with Hepatitis C Virus Infection: A Prospective Study. Lancet 2001, 357, 1069–1075. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A Simple Noninvasive Index Can Predict Both Significant Fibrosis and Cirrhosis in Patients with Chronic Hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Wunder, K.; Kriener, S.; Sotoudeh, F.; Richter, S.; Bojunga, J.; Herrmann, E.; Poynard, T.; Dietrich, C.F.; Vermehren, J.; et al. Liver Fibrosis in Viral Hepatitis: Noninvasive Assessment with Acoustic Radiation Force Impulse Imaging versus Transient Elastography. Radiology 2009, 252, 595–604. [Google Scholar] [CrossRef]
- Nightingale, K.; Soo, M.S.; Nightingale, R.; Trahey, G. Acoustic Radiation Force Impulse Imaging: In Vivo Demonstration of Clinical Feasibility. Ultrasound Med. Biol. 2002, 28, 227–235. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient Elastography: A New Noninvasive Method for Assessment of Hepatic Fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Marcellin, P.; Ziol, M.; Bedossa, P.; Douvin, C.; Poupon, R.; de Lédinghen, V.; Beaugrand, M. Non-Invasive Assessment of Liver Fibrosis by Stiffness Measurement in Patients with Chronic Hepatitis B. Liver Int. 2009, 29, 242–247. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An Algorithm for the Grading of Activity in Chronic Hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Berg, T.; Asselah, T.; Flisiak, R.; Fung, S.; Gordon, S.C.; Janssen, H.L.A.; Lampertico, P.; Lau, D.; Bornstein, J.D.; et al. Evaluation of APRI and FIB-4 Scoring Systems for Non-Invasive Assessment of Hepatic Fibrosis in Chronic Hepatitis B Patients. J. Hepatol. 2016, 64, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Sporea, I.; Peck-Radosavljevic, M.; Sirli, R.; Tanaka, H.; Iijima, H.; Saito, H.; Ebinuma, H.; Lupsor, M.; Badea, R.; et al. The Influence of Aminotransferase Levels on Liver Stiffness Assessed by Acoustic Radiation Force Impulse Elastography: A Retrospective Multicentre Study. Dig. Liver Dis. 2013, 45, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Lupsor, M.; de Knegt, R.; Dries, V.; Buggisch, P.; Gebel, M.; Maier, B.; Herrmann, E.; Sagir, A.; Zachoval, R.; et al. Point Shear Wave Elastography by Acoustic Radiation Force Impulse Quantification in Comparison to Transient Elastography for the Noninvasive Assessment of Liver Fibrosis in Chronic Hepatitis C: A Prospective International Multicenter Study. Ultraschall Med. Eur. J. Ultrasound 2015, 36, 239–247. [Google Scholar] [CrossRef]
- Guzmán-Aroca, F.; Reus, M.; Berná-Serna, J.D.; Serrano, L.; Serrano, C.; Gilabert, A.; Cepero, A. Reproducibility of Shear Wave Velocity Measurements by Acoustic Radiation Force Impulse Imaging of the Liver: A Study in Healthy Volunteers. J. Ultrasound Med. 2011, 30, 975–979. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Lissandrin, R.; Zicchetti, M.; Dal Bello, B.; Filice, G.; Filice, C. Point Shear Wave Elastography Method for Assessing Liver Stiffness. World J. Gastroenterol. 2014, 20, 4787–4796. [Google Scholar] [CrossRef]
- Stasi, C.; Milani, S. Non-Invasive Assessment of Liver Fibrosis: Between Prediction/Prevention of Outcomes and Cost-Effectiveness. World J. Gastroenterol. 2016, 22, 1711–1720. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, J.; Zhu, J.; Hu, C.; Gu, Y.; Lai, J.; Zheng, Y.; Gao, Z. Diverse Correlations between Fibrosis-Related Factors and Liver Stiffness Measurement by Transient Elastography in Chronic Hepatitis B. Eur. J. Gastroenterol. Hepatol. 2018, 30, 217–225. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Wong, G.L.H.; Choi, P.C.L.; Chan, A.W.H.; Chim, A.M.L.; Yiu, K.K.L.; Chan, F.K.L.; Sung, J.J.Y.; Wong, V.W.S. Alanine Aminotransferase-Based Algorithms of Liver Stiffness Measurement by Transient Elastography (Fibroscan) for Liver Fibrosis in Chronic Hepatitis B. J. Viral Hepat. 2009, 16, 36–44. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, Y.F.; Lai, H.C.; Kao, J.T.; Peng, C.Y.; Chuang, P.H.; Su, W.P.; Chiang, I.P. Effects of Patient Factors on Noninvasive Liver Stiffness Measurement Using Acoustic Radiation Force Impulse Elastography in Patients with Chronic Hepatitis C. BMC Gastroenterol. 2012, 12, 105. [Google Scholar] [CrossRef]
- Yoon, K.T.; Lim, S.M.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Chon, C.Y.; Cho, M.; Lee, J.W.; Kim, S.U. Liver Stiffness Measurement Using Acoustic Radiation Force Impulse (ARFI) Elastography and Effect of Necroinflammation. Dig. Dis. Sci. 2012, 57, 1682–1691. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | HBeAg-Negative Total (n = 407) | IC (n = 308) | GZ-1 (n = 54) | GZ-2 (n = 45) |
|---|---|---|---|---|
| Patient age (years) Mean±SD | 42 ± 12 | 42 ± 12 | 42 ± 14 | 38 ± 9 |
| Male gender, n (%) | 157 (38%) | 104 (32.5%) | 29 (48%) | 19 (41.3%) |
| Caucasian Ethnicity, n (%) | 212 (52.1%) | 164 (53.2%) | 25 (46%) | 24 (52.2%) |
| BMI (kg/m2), mean±SD | 25.9 ± 5.0 | 25.8 ± 4.9 | 27.5 ± 5.1 | 25 ± 5.1 |
| AST (IU/L) mean±SD | 24.7 ± 8.0 | 23.2 ± 5.9 | 35 ± 12 | 22.9 ± 5.8 |
| ALT (IU/L) mean±SD | 27.4 ± 13.4 | 23.4 ± 7.4 | 54 ± 13 | 23.7 ± 7.4 |
| Median HBsAg (IU/mL) | 1261 | 1125 | 940 | 2090 |
| Median HBV DNA (IU/mL) | 210 | 150 | 157 | 6750 |
| Median TE | 4.8 | 4.7 | 5.3 | 4.9 |
| Median ARFI | 1.1 | 1.1 | 1.1 | 1.1 |
| Median APRI score | 0.3 | 0.3 | 0.4 | 0.3 |
| Median FIB-4 score | 0.9 | 0.9 | 0.9 | 0.8 |
| Median FibroTest score | 0.2 | 0.2 | 0.2 | 0.2 |
| Fibrosis stage (METAVIR), n (%) | 28 (6.8%) | 15 (4.9%) | 5 (9.3%) | 8 (17.8%) |
| F0 | 26 | 14 | 4 | 8 |
| F1 | 2 | 1 | 1 | 0 |
| F3 | 0 | 0 | 0 | 0 |
| F4 | 0 | 0 | 0 | 0 |
| Variable | B | SE | Wald | df | 95% CI of Exp (b) | p | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Age | 0.05 | 0.04 | 2.09 | 1 | 0.98 | 1.13 | 0.15 |
| Gender | −0.52 | 0.82 | 0.39 | 1 | 0.12 | 2.99 | 0.53 |
| Ethnicity | 0.63 | 0.34 | 3.50 | 1 | 0.97 | 3.67 | 0.06 |
| HBsAg | 0.00 | 0.00 | 6.41 | 1 | 1.00 | 1.00 | 0.01 |
| HBV DNA | 0.00 | 0.00 | 0.43 | 1 | 1.00 | 1.00 | 0.51 |
| ALT | 0.01 | 0.03 | 0.24 | 1 | 0.96 | 1.07 | 0.62 |
| TE | 0.71 | 0.30 | 5.45 | 1 | 1.12 | 3.69 | 0.02 |
| pSWE | 1.41 | 2.04 | 0.49 | 1 | 0.08 | 230.37 | 0.48 |
| APRI | 1.77 | 2.77 | 0.41 | 1 | 0.03 | 1326.77 | 0.52 |
| FIB-4 | 0.00 | 0.07 | 0.00 | 1 | 0.87 | 1.15 | 0.98 |
| Fibrotest | −6.85 | 4.10 | 2.78 | 1 | 0.00 | 3.31 | 0.10 |
| Constant | −12.35 | 3.58 | 11.94 | 1 | 0.00 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, C.; Mondorf, A.; Knop, V.; Peiffer, K.-H.; Dietz, J.; Friess, J.; Wedemeyer, H.; Buggisch, P.; Mauss, S.; Berg, T.; et al. Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection. J. Clin. Med. 2019, 8, 2101. https://doi.org/10.3390/jcm8122101
Graf C, Mondorf A, Knop V, Peiffer K-H, Dietz J, Friess J, Wedemeyer H, Buggisch P, Mauss S, Berg T, et al. Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection. Journal of Clinical Medicine. 2019; 8(12):2101. https://doi.org/10.3390/jcm8122101
Chicago/Turabian StyleGraf, Christiana, Antonia Mondorf, Viola Knop, Kai-Henrik Peiffer, Julia Dietz, Julia Friess, Heiner Wedemeyer, Peter Buggisch, Stefan Mauss, Thomas Berg, and et al. 2019. "Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection" Journal of Clinical Medicine 8, no. 12: 2101. https://doi.org/10.3390/jcm8122101
APA StyleGraf, C., Mondorf, A., Knop, V., Peiffer, K.-H., Dietz, J., Friess, J., Wedemeyer, H., Buggisch, P., Mauss, S., Berg, T., Rausch, M., Sprinzl, M., Klinker, H., Hinrichsen, H., Bronowicki, J.-P., Haag, S., Hüppe, D., Lutz, T., Poynard, T., ... Vermehren, J. (2019). Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection. Journal of Clinical Medicine, 8(12), 2101. https://doi.org/10.3390/jcm8122101






