The Influence of Histologic Inflammation on the Improvement of Liver Stiffness Values Over 1 and 3 Years
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Protocol
2.2. Liver Biopsy and Histology
2.3. Transient Elastography
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
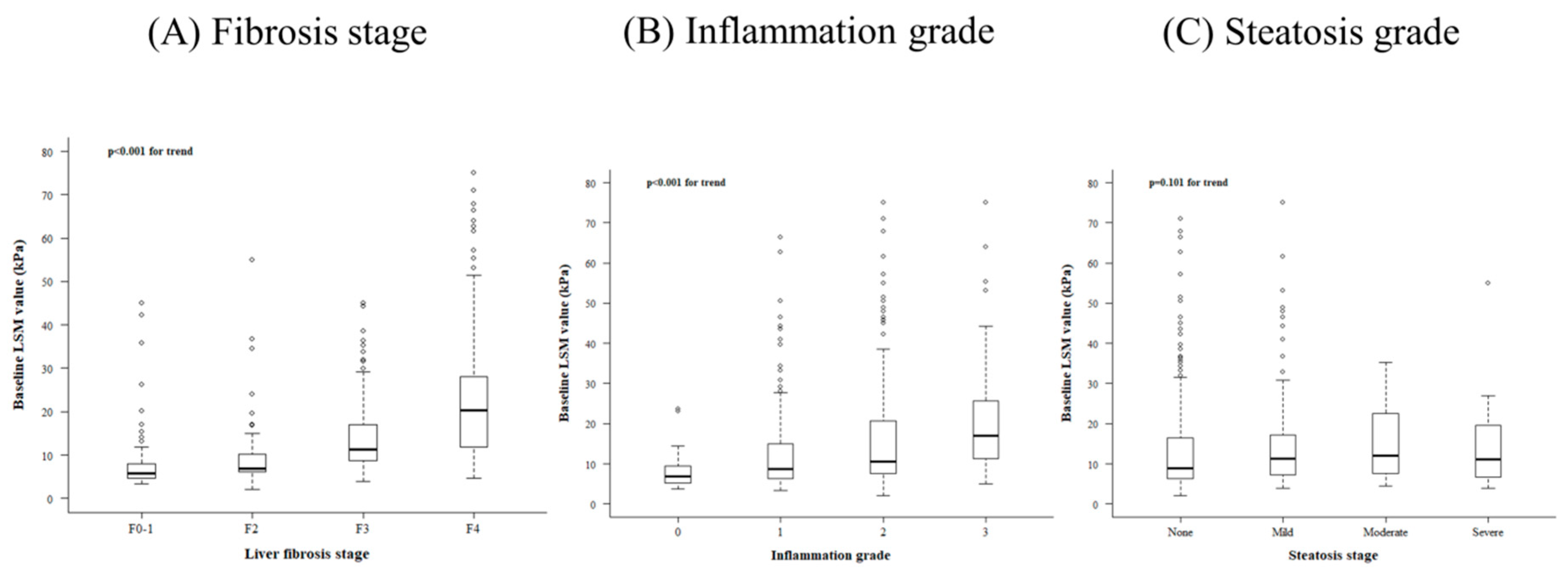
3.2. Related Factors Determining Baseline LS Value
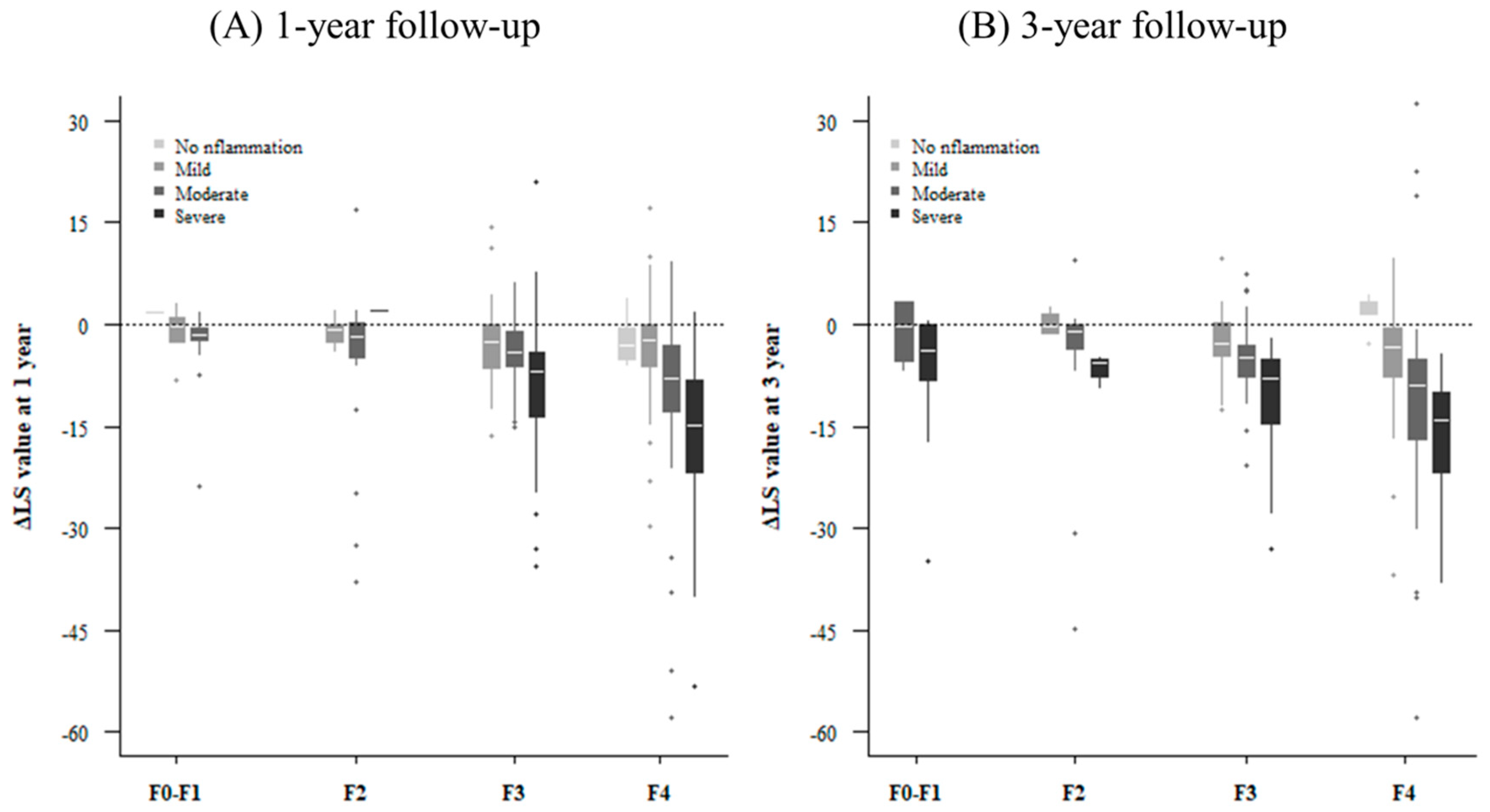
3.3. Factors Associated with Improvement of LS Value
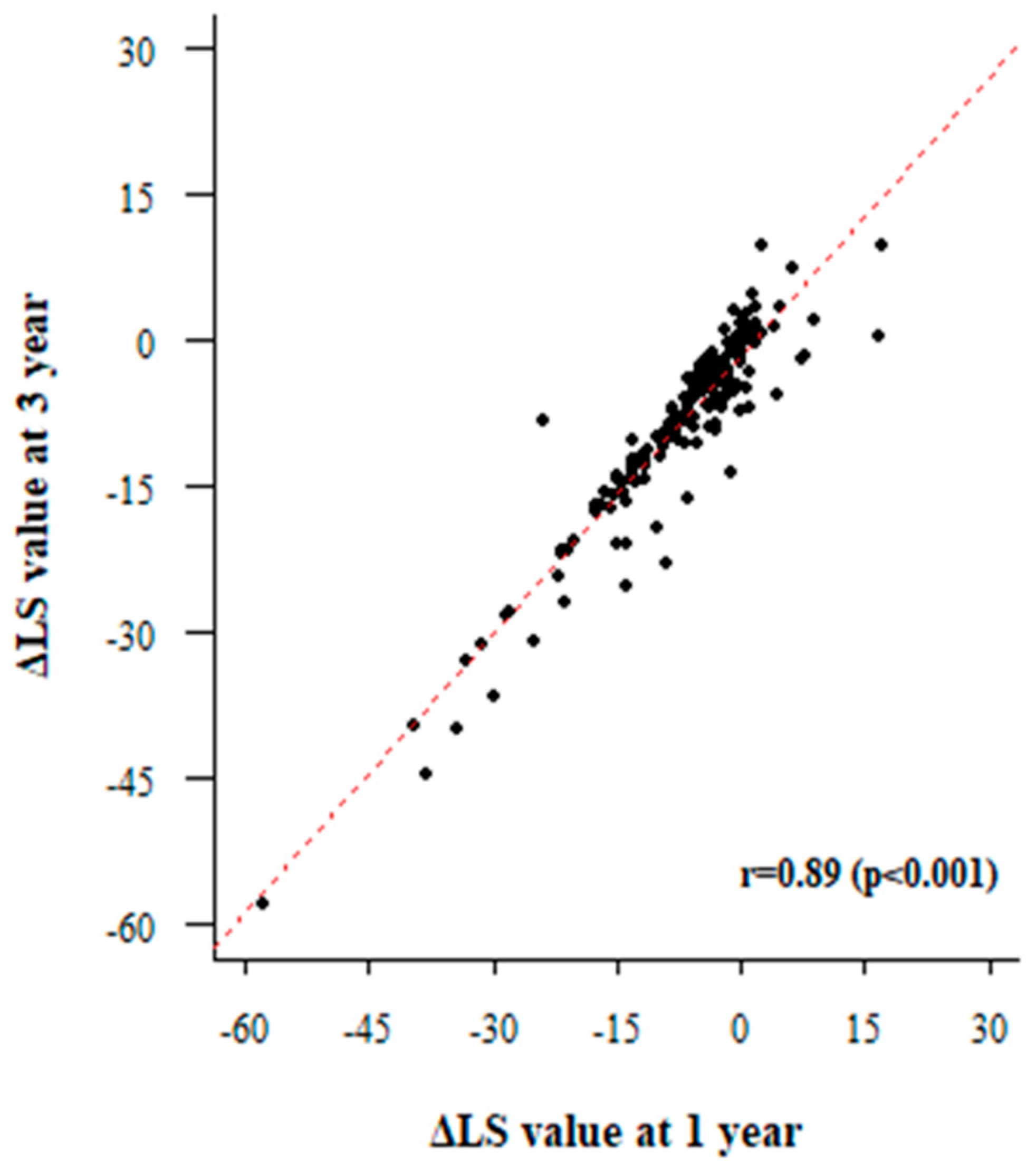
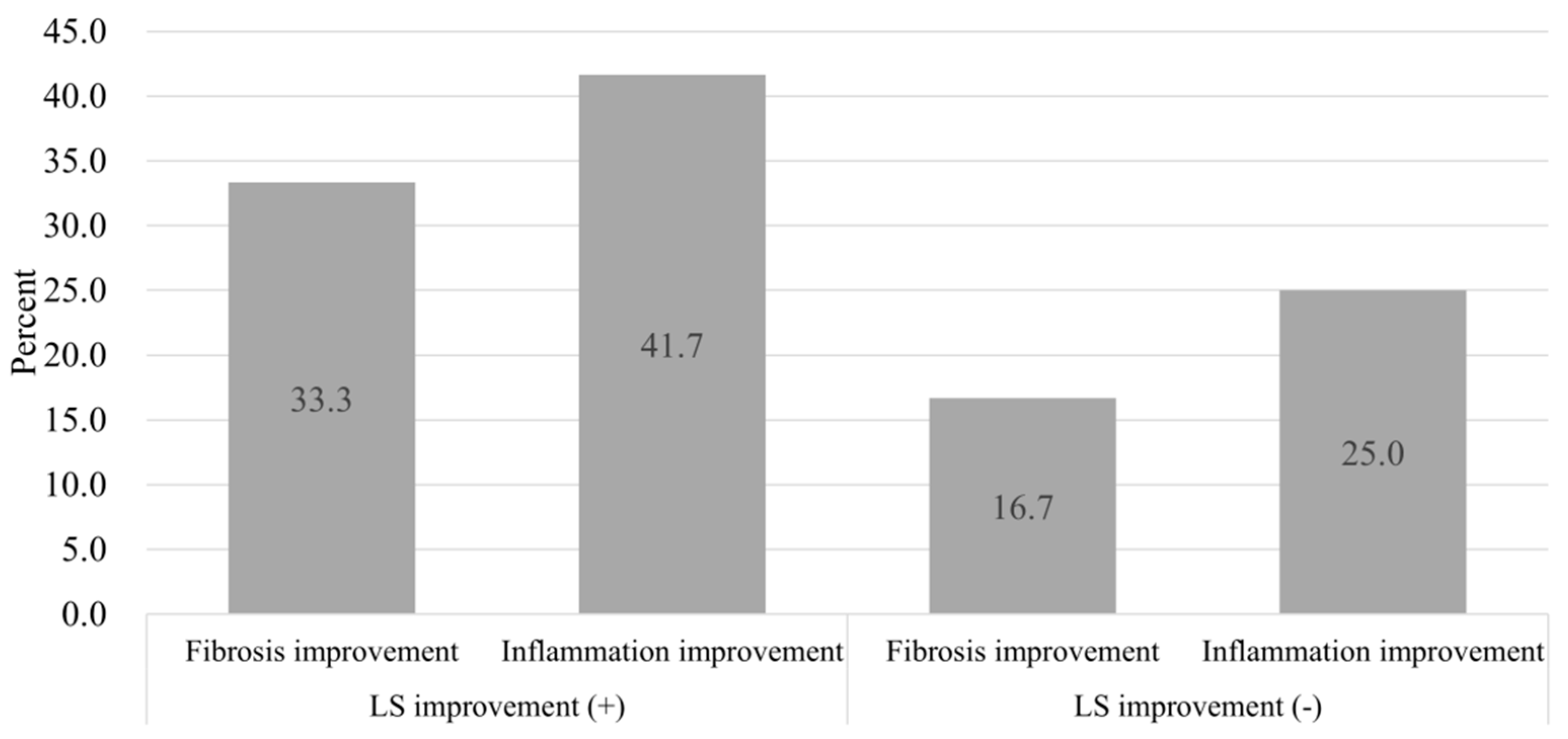
3.4. Paired Liver Biopsy with Paired LSM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mueller, S.; Millonig, G.; Sarovska, L.; Friedrich, S.; Reimann, F.M.; Pritsch, M.; Eisele, S.; Stickel, F.; Longerich, T.; Schirmacher, P.; et al. Increased liver stiffness in alcoholic liver disease: Differentiating fibrosis from steatohepatitis. World J. Gastroenterol. 2010, 16, 966–972. [Google Scholar] [CrossRef]
- Mueller, S.; Sandrin, L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepat. Med. 2010, 2, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Kim, J.K.; Park, Y.N.; Han, K.H. Discordance between liver biopsy and Fibroscan(R) in assessing liver fibrosis in chronic hepatitis b: Risk factors and influence of necroinflammation. PLoS ONE 2012, 7, e32233. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lu, S.N.; Chen, C.H.; Chang, K.C.; Hung, C.H.; Tai, W.C.; Tsai, M.C.; Tseng, P.L.; Hu, T.H.; Wang, J.H. The changes of liver stiffness and its associated factors for chronic hepatitis B patients with entecavir therapy. PLoS ONE 2014, 9, e93160. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Kalafateli, M.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Interventions for hereditary haemochromatosis: An attempted network meta-analysis. Cochrane Database Syst. Rev. 2017, 3. [Google Scholar] [CrossRef]
- Yo, I.K.; Kwon, O.S.; Park, J.W.; Lee, J.J.; Lee, J.H.; Won, I.S.; Na, S.Y.; Jang, P.K.; Park, P.H.; Choi, D.J.; et al. The factors associated with longitudinal changes in liver stiffness in patients with chronic hepatitis B. Clin. Mol. Hepatol. 2015, 21, 32–40. [Google Scholar] [CrossRef][Green Version]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008, 47, 380–384. [Google Scholar] [CrossRef]
- Coco, B.; Oliveri, F.; Maina, A.M.; Ciccorossi, P.; Sacco, R.; Colombatto, P.; Bonino, F.; Brunetto, M.R. Transient elastography: A new surrogate marker of liver fibrosis influenced by major changes of transaminases. J. Viral Hepat. 2007, 14, 360–369. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Mirza, S.; Siddiqui, A.R.; Hamid, S.; Umar, M.; Bashir, S. Extent of liver inflammation in predicting response to interferon alpha & Ribavirin in chronic hepatitis C patients: A cohort study. BMC Gastroenterol. 2012, 12, 71. [Google Scholar] [CrossRef]
- Hartl, J.; Denzer, U.; Ehlken, H.; Zenouzi, R.; Peiseler, M.; Sebode, M.; Hubener, S.; Pannicke, N.; Weiler-Normann, C.; Quaas, A.; et al. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J. Hepatol. 2016, 65, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Wong, G.L.; Choi, P.C.; Chan, A.W.; Li, M.K.; Chan, H.Y.; Chim, A.M.; Yu, J.; Sung, J.J.; Chan, H.L. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.Y.; Baik, S.K. Transient elastography versus hepatic venous pressure gradient for diagnosing portal hypertension: A systematic review and meta-analysis. Clin. Mol. Hepatol. 2017, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, C.; He, D.; Zhang, H.; Xia, J.; Shi, D.; Kong, L.; He, X.; Wang, Y. Influence of hepatic inflammation on fibroscan findings in diagnosing fibrosis in patients with chronic hepatitis B. Ultrasound Med. Biol. 2015, 41, 1538–1544. [Google Scholar] [CrossRef]
- Raizner, A.; Shillingford, N.; Mitchell, P.D.; Harney, S.; Raza, R.; Serino, J.; Jonas, M.M.; Lee, C.K. Hepatic inflammation may influence liver stiffness measurements by transient elastography in children and young adults. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 512–517. [Google Scholar] [CrossRef]
- Sagir, A.; Erhardt, A.; Schmitt, M.; Haussinger, D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008, 47, 592–595. [Google Scholar] [CrossRef]
- Shafaei, S.; Soleimani Amiri, S.; Hajiahmadi, M.; Sadeghi-Haddad-Zavareh, M.; Bayani, M. Histological grading and staging of liver and its relation to viral loads in chronic anti-HBe positive hepatitis. Casp. J. Intern. Med. 2013, 4, 681–685. [Google Scholar]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Unalp, A.; Behling, C.E.; Lavine, J.E.; Neuschwander-Tetri, B.A. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): A histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009, 49, 809–820. [Google Scholar] [CrossRef]
- Sanai, F.M.; Benmousa, A.; Al-Hussaini, H.; Ashraf, S.; Alhafi, O.; Abdo, A.A.; Alameri, H.F.; Akbar, H.O.; Bzeizi, K.I. Is serum alanine transaminase level a reliable marker of histological disease in chronic hepatitis C infection? Liver Int. 2008, 28, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Xun, Y.H.; Hu, C.B.; Zhang, L.; Liu, H.; Lou, G.Q.; Fan, J.G. Clinical and histological features of non-alcoholic fatty liver disease. Chin. J. Hepatol. 2009, 17, 812–816. [Google Scholar]
- Kyrlagkitsis, I.; Portmann, B.; Smith, H.; O’Grady, J.; Cramp, M.E. Liver histology and progression of fibrosis in individuals with chronic hepatitis C and persistently normal ALT. Am. J. Gastroenterol. 2003, 98, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Ehlken, H.; Weiler-Normann, C.; Sebode, M.; Kreuels, B.; Pannicke, N.; Zenouzi, R.; Glaubke, C.; Lohse, A.W.; Schramm, C. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J. Hepatol. 2015, 62, 642–646. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Hoeroldt, B.S.; Dube, A.K.; McFarlane, E.; Underwood, J.C.; Karajeh, M.A.; Gleeson, D. Long-term prognostic significance of persisting histological activity despite biochemical remission in autoimmune hepatitis. Am. J. Gastroenterol. 2015, 110, 993–999. [Google Scholar] [CrossRef]
- Fraquelli, M.; Rigamonti, C.; Casazza, G.; Donato, M.F.; Ronchi, G.; Conte, D.; Rumi, M.; Lampertico, P.; Colombo, M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J. Hepatol. 2011, 54, 621–628. [Google Scholar] [CrossRef]
- Kim, S.U.; Seo, Y.S.; Cheong, J.Y.; Kim, M.Y.; Kim, J.K.; Um, S.H.; Cho, S.W.; Paik, S.K.; Lee, K.S.; Han, K.H.; et al. Factors that affect the diagnostic accuracy of liver fibrosis measurement by Fibroscan in patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2010, 32, 498–505. [Google Scholar] [CrossRef]
- Myers, R.P.; Crotty, P.; Pomier-Layrargues, G.; Ma, M.; Urbanski, S.J.; Elkashab, M. Prevalence, risk factors and causes of discordance in fibrosis staging by transient elastography and liver biopsy. Liver Int. 2010, 30, 1471–1480. [Google Scholar] [CrossRef]
- Verveer, C.; Zondervan, P.E.; ten Kate, F.J.; Hansen, B.E.; Janssen, H.L.; de Knegt, R.J. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012, 32, 622–628. [Google Scholar] [CrossRef]
- Liang, X.; Xie, Q.; Tan, D.; Ning, Q.; Niu, J.; Bai, X.; Chen, S.; Cheng, J.; Yu, Y.; Wang, H.; et al. Interpretation of liver stiffness measurement-based approach for the monitoring of hepatitis B patients with antiviral therapy: A 2-year prospective study. J. Viral Hepat. 2018, 25, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Cohen, E.B.; Patel, K.; Bacon, B.; Gordon, S.; Lawitz, E.; Nelson, D.; Nasser, I.A.; Challies, T.; Afdhal, N. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 2012, 10, 932–937.e1. [Google Scholar] [CrossRef] [PubMed]
- Chekuri, S.; Nickerson, J.; Bichoupan, K.; Sefcik, R.; Doobay, K.; Chang, S.; DelBello, D.; Harty, A.; Dieterich, D.T.; Perumalswami, P.V.; et al. Liver Stiffness Decreases Rapidly in Response to Successful Hepatitis C Treatment and Then Plateaus. PLoS ONE 2016, 11, e0159413. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Maida, M.; Macaluso, F.S.; Di Marco, V.; Camma, C.; Cabibi, D.; Craxi, A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 2015, 62, 1101–1110. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Maida, M.; Camma, C.; Cabibbo, G.; Cabibi, D.; Alduino, R.; Di Marco, V.; Craxi, A.; Petta, S. Steatosis affects the performance of liver stiffness measurement for fibrosis assessment in patients with genotype 1 chronic hepatitis C. J. Hepatol. 2014, 61, 523–529. [Google Scholar] [CrossRef]
- Kim, S.U.; Kim, D.Y.; Ahn, S.H.; Kim, H.M.; Lee, J.M.; Chon, C.Y.; Park, Y.N.; Han, K.H.; Park, J.Y. The impact of steatosis on liver stiffness measurement in patients with chronic hepatitis B. Hepato-Gastroenterol. 2010, 57, 832–838. [Google Scholar]
| Variable | Baseline (n = 678) | 1-year (n = 358) | 3-year (n = 244) |
|---|---|---|---|
| Age, year, mean (SD) | 47.12 (12.25) | 48.54 (11.84) | 49.57 (10.42) |
| Sex, n (%) | |||
| Male | 329 (48.5) | 181 (50.6) | 120 (49.2) |
| Female | 349 (51.5) | 177 (49.4) | 124 (50.8) |
| Etiology, n (%) | |||
| HBV | 419 (61.8) | 242 (67.6) | 173 (70.9) |
| HCV | 183 (27.0) | 70 (19.6) | 49 (20.1) |
| Alcoholic | 12 (1.8) | 7 (2.0) | 5 (2.0) |
| Autoimmune | 32 (4.7) | 21 (5.9) | 8 (3.3) |
| NAFLD | 21 (3.1) | 12 (3.4) | 7 (2.9) |
| Others | 11 (1.6) | 6 (1.7) | 2 (0.8) |
| BMI, kg/m2, mean (SD) | 23.97 (3.44) | NA | NA |
| Laboratory findings | |||
| AST, U/L, mean (IQR) | 44.0 (30.0–77.0) | 37.0 (22.0–38.0) | 28.0 (17.0–30.0) |
| ALT, U/L, mean (IQR) | 46.0 (29.0–83.0) | 39.0 (26.0–39.0) | 30.0 (17.0–30.0) |
| Total bilirubin, mg/dL, mean (SD) | 1.11 (1.41) | 0.82 (0.46) | 1.02 (0.92) |
| Albumin, mg/dL, mean (SD) | 4.15 (0.53) | 4.35 (0.34) | 4.34 (0.34) |
| Prothrombin time, INR, mean (SD) | 1.01 (0.46) | 0.97 (0.14) | 0.89 (0.27) |
| LSM value, kPa, mean (IQR) | 10.5 (7.3–19.6) | 8.4 (5.3–12.0) | 7.0 (1.8–10.0) |
| Liver biopsy, n (%) | |||
| Fibrosis | |||
| F0 | 13 (1.9) | ||
| F1 | 96 (14.2) | ||
| F2 | 132 (19.5) | ||
| F3 | 186 (27.4) | ||
| F4 | 251 (37.0) | ||
| Steatosis | |||
| No steatosis | 370 (64.2) | ||
| Mild | 156 (27.1) | ||
| Moderate | 36 (6.3) | ||
| Severe | 14 (2.4) | ||
| Inflammation | |||
| No inflammation | 28 (4.1) | ||
| Mild | 278 (41.0) | ||
| Moderate | 279 (41.2) | ||
| Severe | 93 (13.7) |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Age, year | 0.175 (0.091 to 0.258) | <0.001 | ||
| Sex, male | 0.158 (−1.857 to 2.174) | 0.877 | ||
| Viral etiology | −5.530 (−8.487 to −2.573) | <0.001 | −3.560 (−5.994 to −1.125) | 0.004 |
| BMI, kg/m2 | 0.115 (−0.223 to 0.452) | 0.505 | ||
| Laboratory findings | ||||
| Platelet, 109/L | −0.073 (−0.088 to −0.058) | <0.001 | −0.024 (−0.039 to −0.010) | 0001 |
| AST, U/L | 0.011 (0.005 to 0.016) | 0.001 | ||
| ALT, U/L | 0.005 (0.000 to 0.009) | 0.032 | ||
| Total bilirubin, mg/mL | 2.781 (2.153 to 3.409) | <0.001 | 1.904 (1.408 to 2.400) | <0.001 |
| Albumin, mg/dL | −12.387 (−14.033 to −10.741) | <0.001 | −5.120 (−6.862 to −3.378) | <0.001 |
| Prothrombin time, INR | 8.789 (6.859 to 10.719) | <0.001 | 4.913 (3.411 to 6.415) | <0.001 |
| Creatinine, mg/dL | −0.706 (−1.775 to 0.363) | 0.195 | ||
| Sodium, mEq/L | −1.428 (−1.809 to −1.047) | <0.001 | −0.494 (−0.789 to −0.198) | 0.001 |
| Liver biopsy | ||||
| Fibrosis | ||||
| F0 | 1 (reference) | 1 (reference) | ||
| F1 | −5.570 (−12.045 to 0.906) | 0.092 | −0.341 (−5.928 to 5.246) | 0.905 |
| F2 | −3.765 (−10.153 to 2.622) | 0.247 | −1.240 (−6.819 to 4.340) | 0.663 |
| F3 | 1.072 (−5.176 to 7.319) | 0.736 | 1.048 (−4.544 to 6.641) | 0.713 |
| F4 | 10.277 (4.048 to 16.506) | 0.001 | 8.306 (2.730 to 13.882) | 0.004 |
| Steatosis | ||||
| No steatosis | 1 (reference) | |||
| Mild | 0.868 (−1.562 to 3.299) | 0.483 | ||
| Moderate | 1.247 (−2.960 to 5.455) | 0.561 | ||
| Severe | 1.339 (−5.092 to 7.770) | 0.683 | ||
| Inflammation | ||||
| No inflammation | 1 (reference) | 1 (reference) | ||
| Mild | 3.897 (−1.265 to 9.058) | 0.139 | 2.347 (−1.487 to 6.182) | 0.230 |
| Moderate | 7.414 (2.289 to 12.538) | 0.005 | 3.374 (−0.489 to 7.237) | 0.087 |
| Severe | 11.492 (5.977 to 17.007) | <0.001 | 6.476 (2.236 to 10.716) | 0.003 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Age, year | −0.028 (−0.115, 0.060) | 0.535 | ||
| Sex, male | 0.263 (−1.785, 2.311) | 0.801 | ||
| Viral etiology | 3.527 (0.489, 6.566) | 0.023 | ||
| BMI, kg/m2 | 0.086 (−0.274, 0.446) | 0.638 | ||
| Laboratory findings | ||||
| Platelet, 109/L | 0.025 (0.008, 0.043) | 0.005 | 0.027 (0.011, 0.042) | 0.001 |
| AST, U/L | −0.012 (−0.018, −0.007) | <0.001 | ||
| ALT, U/L | −0.011 (−0.017, −0.005) | <0.001 | ||
| Total bilirubin, mg/mL | −1.830 (−2.606, −1.055) | <0.001 | −1.716 (−2.420, −1.011) | <0.001 |
| Albumin, mg/dL | 5.882 (3.793, 7.971) | <0.001 | ||
| Prothrombin time, INR | −5.399 (−6.994, −3.803) | <0.001 | −4.647 (−6.126, −3.167) | <0.001 |
| Creatinine, mg/dL | 3.825 (−1.620, 9.271) | 0.168 | ||
| Sodium, mEq/L | 0.438 (0.051, 0.825) | 0.027 | ||
| Liver biopsy | ||||
| Fibrosis | ||||
| F0 | 1 (reference) | |||
| F1 | 3.909 (−4.872, 12.691) | 0.382 | ||
| F2 | 1.493 (−6.893, 9.880) | 0.726 | ||
| F3 | −0.692 (−8.670, 7.285) | 0.865 | ||
| F4 | −2.352 (−10.337, 5.633) | 0.563 | ||
| Steatosis | ||||
| No steatosis | 1 (reference) | |||
| Mild | −0.855 (−3.287, 1.576) | 0.489 | ||
| Moderate | 0.123 (−3.929, 4.174) | 0.952 | ||
| Severe | −3.166 (−8.953, 2.620) | 0.282 | ||
| Inflammation | ||||
| No inflammation | 1 (reference) | 1 (reference) | ||
| Mild | −1.312 (−9.011, 6.387) | 0.738 | −1.256 (−8.298, 5.786) | 0.726 |
| Moderate | −3.925 (−11.578, 3.727) | 0.314 | −3.196 (−10.201, 3.808) | 0.370 |
| Severe | −9.875 (−17.673, −2.078) | 0.013 | −8.581 (−15.715, −1.447) | 0.019 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Age, year | −0.013 (−0.137, 0.11) | 0.832 | ||
| Sex, male | −2.451 (−5.004, 0.102) | 0.060 | ||
| Viral etiology | −2.200 (−6.681, 2.281) | 0.334 | ||
| BMI, kg/m2 | 0.042 (−0.372, 0.456) | 0.842 | ||
| Laboratory findings | ||||
| Platelet, 109/L | 0.028 (0.005, 0.052) | 0.020 | ||
| AST, U/L | −0.013 (−0.022, −0.005) | 0.003 | ||
| ALT, U/L | −0.005 (−0.010, 0.001) | 0.091 | ||
| Total bilirubin, mg/mL | −1.638 (−2.745, −0.531) | 0.004 | −1.129 (−2.153, −0.105) | 0.031 |
| Albumin, mg/dL | 7.138 (4.336, 9.940) | <0.001 | 4.135 (1.187, 7.084) | 0.006 |
| Prothrombin time, INR | −7.089 (−10.301, −3.876) | <0.001 | −5.954 (−8.978, −2.931) | <0.001 |
| Creatinine, mg/dL | 1.223 (−6.467, 8.912) | 0.754 | ||
| Sodium, mEq/L | 0.429 (−0.108, 0.967) | 0.117 | ||
| Liver biopsy | ||||
| Fibrosis | ||||
| F0 | 1 (reference) | |||
| F1 | 2.625 (−18.129, 23.379) | 0.803 | ||
| F2 | 4.321 (−15.918, 24.561) | 0.674 | ||
| F3 | 1.288 (−18.740, 21.317) | 0.899 | ||
| F4 | −0.766 (−20.821, 19.289) | 0.94 | ||
| Steatosis | ||||
| No steatosis | 1 (reference) | |||
| Mild | −1.600 (−4.734, 1.534) | 0.315 | ||
| Moderate | −1.515 (−6.960, 3.930) | 0.584 | ||
| Severe | −6.865 (−13.645, −0.085) | 0.047 | ||
| Inflammation | ||||
| No inflammation | 1 (reference) | 1 (reference) | ||
| Mild | −5.772 (−14.596, 3.051) | 0.199 | −4.631 (−12.982, 3.721) | 0.276 |
| Moderate | −8.888 (−17.625, −0.151) | 0.046 | −6.553 (−14.959, 1.852) | 0.126 |
| Severe | −13.894 (−22.773, −5.015) | 0.002 | −10.725 (−19.299, −2.151) | 0.014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.-J.; Seo, Y.S.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Suh, S.J.; Yim, H.J.; Suk, K.T.; Kim, D.J.; Han, K.-H.; et al. The Influence of Histologic Inflammation on the Improvement of Liver Stiffness Values Over 1 and 3 Years. J. Clin. Med. 2019, 8, 2065. https://doi.org/10.3390/jcm8122065
Yoo J-J, Seo YS, Kim YS, Jeong SW, Jang JY, Suh SJ, Yim HJ, Suk KT, Kim DJ, Han K-H, et al. The Influence of Histologic Inflammation on the Improvement of Liver Stiffness Values Over 1 and 3 Years. Journal of Clinical Medicine. 2019; 8(12):2065. https://doi.org/10.3390/jcm8122065
Chicago/Turabian StyleYoo, Jeong-Ju, Yeon Seok Seo, Young Seok Kim, Soung Won Jeong, Jae Young Jang, Sang Jun Suh, Hyung Joon Yim, Ki Tae Suk, Dong Joon Kim, Kwang-Hyub Han, and et al. 2019. "The Influence of Histologic Inflammation on the Improvement of Liver Stiffness Values Over 1 and 3 Years" Journal of Clinical Medicine 8, no. 12: 2065. https://doi.org/10.3390/jcm8122065
APA StyleYoo, J.-J., Seo, Y. S., Kim, Y. S., Jeong, S. W., Jang, J. Y., Suh, S. J., Yim, H. J., Suk, K. T., Kim, D. J., Han, K.-H., Kim, S. U., Lee, B., & Kim, S. G. (2019). The Influence of Histologic Inflammation on the Improvement of Liver Stiffness Values Over 1 and 3 Years. Journal of Clinical Medicine, 8(12), 2065. https://doi.org/10.3390/jcm8122065








