Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Design
2.3. Quantification of Paracetamol Plasma Concentrations
2.4. Pharmacokinetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
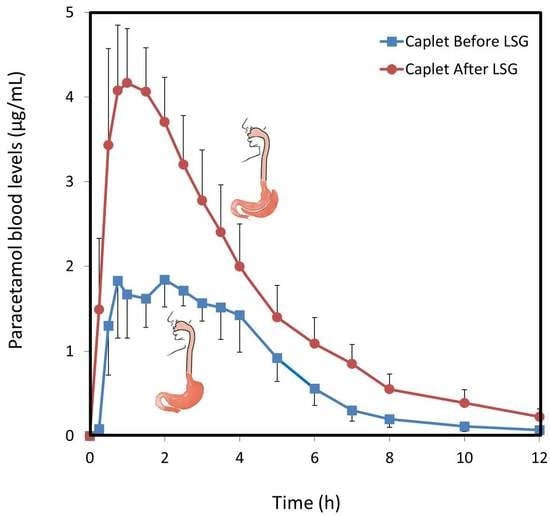
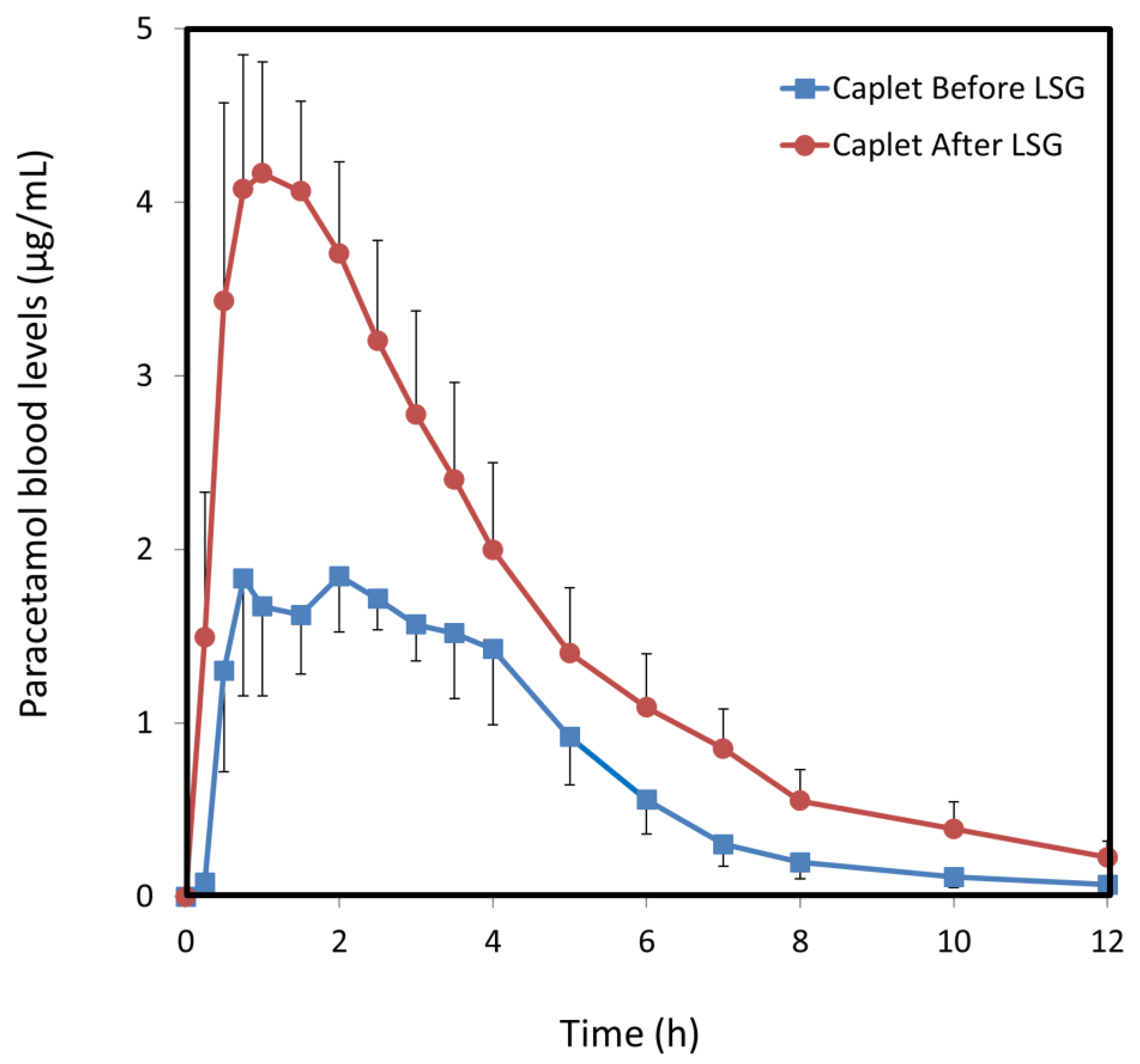
3.2. Paracetamol Caplets
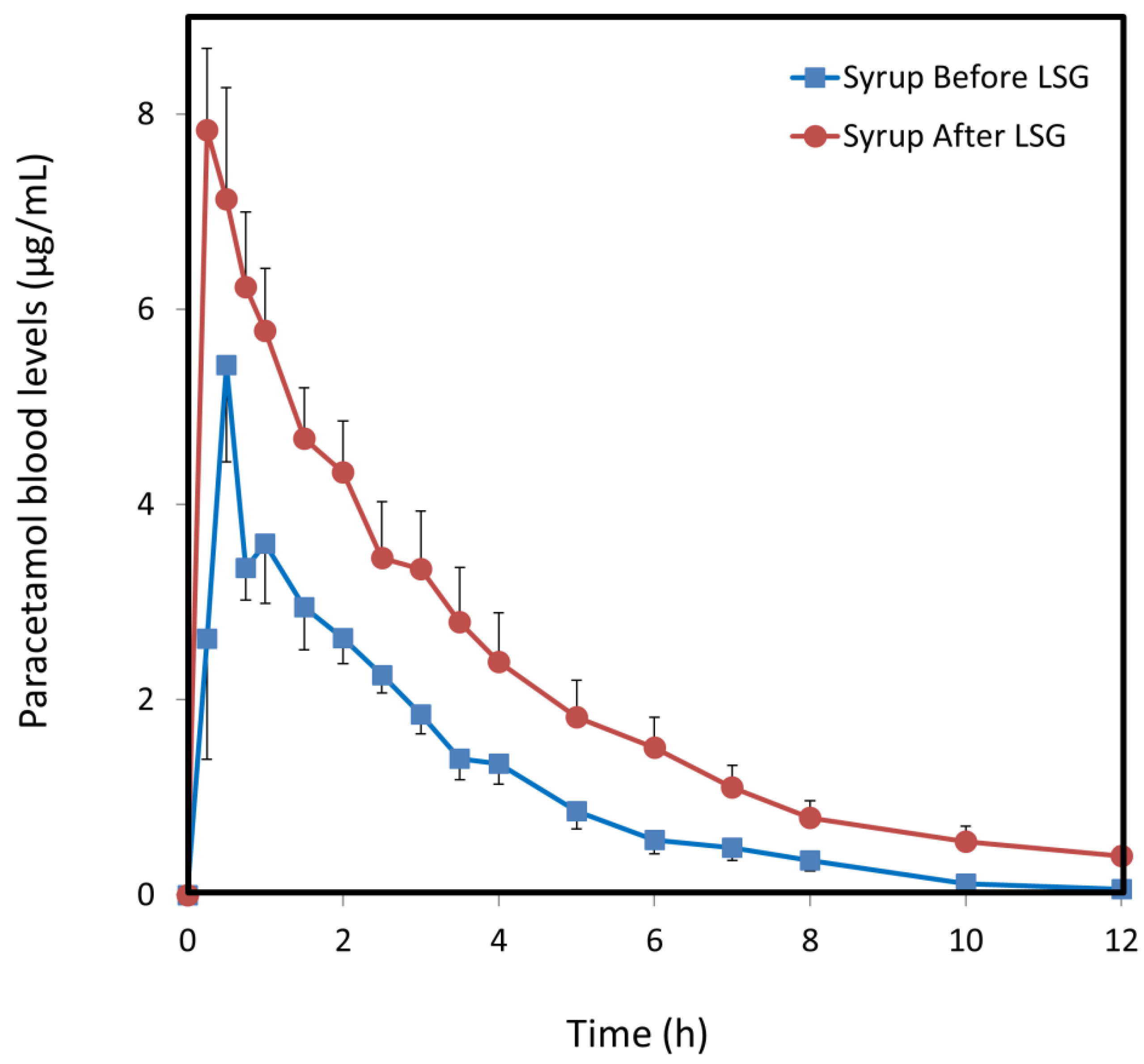
3.3. Paracetamol Syrup
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohula, E.A.; Wiviott, S.D.; McGuire, D.K.; Inzucchi, S.E.; Kuder, J.; Im, K.; Fanola, C.L.; Qamar, A.; Brown, C.; Budaj, A.; et al. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N. Engl. J. Med. 2018, 379, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmio, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients with Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Clarke, M.G.; Evennett, N.J.; John Robinson, S.; Lee Humphreys, M.; Hammodat, H.; Jones, B.; Kim, D.D.; Cutfield, R.; Johnson, M.H.; et al. Laparoscopic Sleeve Gastrectomy Versus Banded Roux-en-Y Gastric Bypass for Diabetes and Obesity: A Prospective Randomised Double-Blind Trial. Obes. Surg. 2018, 28, 293–302. [Google Scholar] [CrossRef] [PubMed]
- English, W.J.; DeMaria, E.J.; Brethauer, S.A.; Mattar, S.G.; Rosenthal, R.J.; Morton, J.M. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg. Obes. Relat. Dis. 2018, 14, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Seki, Y.; Wong, S.K.; Wang, C.; Huang, C.K.; Aly, A.; Baijal, M.; Al-Sabah, S.; Udomsawaengsup, S.; Heo, Y.S.; et al. Bariatric/Metabolic Surgery in the Asia-Pacific Region: APMBSS 2018 Survey. Obes. Surg. 2018, 29, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Azran, C.; Wolk, O.; Zur, M.; Fine-Shamir, N.; Shaked, G.; Czeiger, D.; Sebbag, G.; Kister, O.; Langguth, P.; Dahan, A. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes. Rev. 2016, 17, 1050–1066. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Stier, C.; Raab, H.; Weiner, R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment. Pharmacol. Ther. 2014, 40, 582–609. [Google Scholar] [CrossRef] [PubMed]
- Amouyal, C.; Buyse, M.; Lucas-Martini, L.; Hirt, D.; Genser, L.; Torcivia, A.; Bouillot, J.L.; Oppert, J.M.; Aron-Wisnewsky, J. Sleeve Gastrectomy in Morbidly Obese HIV Patients: Focus on anti-retroviral treatment absorption after surgery. Obes. Surg. 2018, 28, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Porat, D.; Azran, C.; Mualem, Y.; Sakran, N.; Abu-Abeid, S. Lithium Toxicity with Severe Bradycardia Post Sleeve Gastrectomy: A Case Report and Review of the Literature. Obes. Surg. 2018, 29, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.T.; Sharma, G.; Nor Hanipah, Z.; Tu, C.; Brethauer, S.A.; Schauer, P.R.; Cetin, D.; Aminian, A. Adjustments to warfarin dosing after gastric bypass and sleeve gastrectomy. Surg. Obes. Relat. Dis. 2018, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Henderson, K.; Burgin, A.; Ward, N.; Whittam, J.; Ammori, B.J.; Ashcroft, D.M.; Rostami-Hodjegan, A. Trends in oral drug bioavailability following bariatric surgery: Examining the variable extent of impact on exposure of different drug classes. Br. J. Clin. Pharmacol. 2012, 74, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Camastra, S.; Politti, U.; Ruffilli, I.; Vita, R.; Navarra, G.; Benvenga, S.; Antonelli, A. TSH Normalization in Bariatric Surgery Patients After the Switch from L-Thyroxine in Tablet to an Oral Liquid Formulation. Obes. Surg. 2017, 27, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ameer, B.; Divoll, M.; Abernethy, D.R.; Greenblatt, D.J.; Shargel, L. Absolute and relative bioavailability of oral acetaminophen preparations. J. Pharm. Sci. 1983, 72, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.A.; Critchley, J.A.; Prescott, L.F. The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br. J. Clin. Pharmacol. 1984, 18, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pawasauskas, J.; Pergolizzi, J.V., Jr.; Lu, L.; Chen, Y.; Wu, S.; Jarrett, B.; Fain, R.; Hill, L.; Devarakonda, K. Pharmacokinetics of Oral and Intravenous Paracetamol (Acetaminophen) When Co-Administered with Intravenous Morphine in Healthy Adult Subjects. Clin. Drug Investig. 2018, 38, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Beig, A.; Dahan, A. Quantification of carbamazepine and its 10, 11-epoxide metabolite in rat plasma by UPLC-UV and application to pharmacokinetic study. Biomed. Chromatogr. 2014, 28, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.K.; Parulan, C.; Samson, R.; Hutchinson, J.; Bushnell, R.; Beja, E.G.; Ang, R.; Royal, M.A. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.; Feinle, C.; Schwizer, W.; Fried, M.; Boesiger, P. Assessment of gastric motor function during the emptying of solid and liquid meals in humans by MRI. J. Magn. Reson Imaging 1999, 9, 75–80. [Google Scholar] [CrossRef]
- Muzard, L.; Alvarez, J.C.; Gbedo, C.; Czernichow, S.; Carette, C. Tenofovir pharmacokinetic after sleeve-gastrectomy in four severely obese patients living with HIV. Obes. Res. Clin. Pract. 2017, 11, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, C.; Egorin, M.J.; Shah, D.D.; Beumer, J.H.; Rogel, S.; Pavlovsky, S. Imatinib mesylate pharmacokinetics before and after sleeve gastrectomy in a morbidly obese patient with chronic myeloid leukemia. Pharmacotherapy 2009, 29, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Azran, C.; Langguth, P.; Dahan, A. Impaired oral absorption of methylphenidate after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2017, 13, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Azran, C.; Porat, D.; Fine-Shamir, N.; Hanhan, N.; Dahan, A. Oral levothyroxine therapy postbariatric surgery: Biopharmaceutical aspects and clinical effects. Surg. Obes. Relat. Dis. 2019, 15, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kalantzi, L.; Reppas, C.; Dressman, J.B.; Amidon, G.L.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.A.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Acetaminophen (paracetamol). J. Pharm. Sci. 2006, 95, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Prot, J.M.; Maciel, L.; Bricks, T.; Merlier, F.; Cotton, J.; Paullier, P.; Bois, F.Y.; Leclerc, E. First pass intestinal and liver metabolism of paracetamol in a microfluidic platform coupled with a mathematical modeling as a means of evaluating ADME processes in humans. Biotechnol. Bioeng. 2014, 111, 2027–2040. [Google Scholar] [CrossRef] [PubMed]
- Neirinckx, E.; Vervaet, C.; De Boever, S.; Remon, J.P.; Gommeren, K.; Daminet, S.; De Backer, P.; Croubels, S. Species comparison of oral bioavailability, first-pass metabolism and pharmacokinetics of acetaminophen. Res. Vet. Sci. 2010, 89, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H.; Duan, S.X.; von Moltke, L.L.; Greenblatt, D.J.; Patten, C.J.; Miners, J.O.; Mackenzie, P.I. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: Identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J. Pharmacol. Exp. Ther. 2001, 299, 998–1006. [Google Scholar] [PubMed]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Michaut, A.; Moreau, C.; Robin, M.A.; Fromenty, B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014, 34, e171–e179. [Google Scholar] [CrossRef] [PubMed]
- Blouin, R.A.; Kolpek, J.H.; Mann, H.J. Influence of obesity on drug disposition. Clin. Pharm. 1987, 6, 706–714. [Google Scholar] [PubMed]
- Sorrow, P.; Maguire, R.; Murphy, S.K.; Belcher, S.M.; Hoyo, C. Elevated metabolites of acetaminophen in cord blood of children with obesity. Pediatr. Obes. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Divoll, M.; Greenblatt, D.J.; Ameer, B. Obesity, sex, and acetaminophen disposition. Clin. Pharmacol. Ther. 1982, 31, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Greenblatt, D.J.; Divoll, M.; Shader, R.I. Enhanced glucuronide conjugation of drugs in obesity: Studies of lorazepam, oxazepam, and acetaminophen. J. Lab. Clin. Med. 1983, 101, 873–880. [Google Scholar] [PubMed]
- Ardila-Hani, A.; Soffer, E.E. Review article: The impact of bariatric surgery on gastrointestinal motility. Aliment. Pharmacol. Ther. 2011, 34, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.J.; Cheng, M.F.; Yang, W.S.; Tsai, M.S.; Lee, P.C.; Chen, C.N.; Lin, M.T.; Tseng, P.H. A Higher Preoperative Glycemic Profile Is Associated with Rapid Gastric Emptying After Sleeve Gastrectomy for Obese Subjects. Obes. Surg. 2019, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Sioka, E.; Tzovaras, G.; Perivoliotis, K.; Bakalis, V.; Zachari, E.; Magouliotis, D.; Tassiopoulou, V.; Potamianos, S.; Kapsoritakis, A.; Poultsidi, A.; et al. Impact of Laparoscopic Sleeve Gastrectomy on Gastrointestinal Motility. Gastroenterol. Res. Pract. 2018, 2018, 4135813. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J.V., Jr.; Taylor, R., Jr.; Decker, J.F.; Patrick, J.T. Acetaminophen (paracetamol) oral absorption and clinical influences. Pain Pract. 2014, 14, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Sanaka, M.; Kuyama, Y.; Shimomura, Y.; Saitoh, M.; Hattori, K. New mathematical model for accurate description of absorption kinetics of paracetamol given orally with a high calorie liquid meal. Int. J. Clin. Pharmacol. Ther. 2002, 40, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Goon, D.; Klaassen, C.D. Dose-dependent intestinal glucuronidation and sulfation of acetaminophen in the rat in situ. J. Pharmacol. Exp. Ther. 1990, 252, 201–207. [Google Scholar] [PubMed]
- Van Rongen, A.; Valitalo, P.A.; Peeters, M.Y.; Boerma, D.; Huisman, F.W.; van Ramshorst, B.; van Dongen, E.P.; van den Anker, J.N.; Knibbe, C.A. Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin. Pharmacokinet. 2016, 55, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Kato, Y.; Hatakeyama, M.; Iwamura, A.; Fukami, T.; Kume, T.; Yokoi, T.; Nakajima, M. Evaluation of expression and glycosylation status of UGT1A10 in Supersomes and intestinal epithelial cells with a novel specific UGT1A10 monoclonal antibody. Drug Metab. Dispos. 2017, 45, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Lloret-Linares, C.; Luo, H.; Rouquette, A.; Labat, L.; Poitou, C.; Tordjman, J.; Bouillot, J.L.; Mouly, S.; Scherrmann, J.M.; Bergmann, J.F.; et al. The effect of morbid obesity on morphine glucuronidation. Pharmacol. Res. 2017, 118, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Milosheska, D.; Lorber, B.; Vovk, T.; Kastelic, M.; Dolzan, V.; Grabnar, I. Pharmacokinetics of lamotrigine and its metabolite N-2-glucuronide: Influence of polymorphism of UDP-glucuronosyltransferases and drug transporters. Br. J. Clin. Pharmacol. 2016, 82, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Ridout, K.K.; Zhu, J.; Lazarus, P. Olanzapine metabolism and the significance of UGT1A448V and UGT2B1067Y variants. Pharmacogenet. Genom. 2011, 21, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, T. Intestinal glucuronidation metabolism may have a greater impact on oral bioavailability than hepatic glucuronidation metabolism in humans: A study with raloxifene, substrate for UGT1A1, 1A8, 1A9, and 1A10. Int. J. Pharm. 2009, 378, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Kasichayanula, S.; Liu, X.; Zhang, W.; Pfister, M.; LaCreta, F.P.; Boulton, D.W. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: An open-label, parallel-group, single-dose study. Clin. Ther. 2011, 33, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S.; Konig, J.; Lutjohann, D.; Giessmann, T.; Kroemer, H.K.; Rimmbach, C.; Rosskopf, D.; Fromm, M.F.; Siegmund, W. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet. Genom. 2008, 18, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Pre-LSG | Post-LSG |
|---|---|---|
| Age (years) | 38.9 (13.6) | |
| Females | 6 | |
| Males | 3 | |
| Smokers | 4 | |
| Height (cm) | 167 (9) | |
| Weight (kg) | 125 (17) | 99 (19) * |
| BMI (kg/m2) | 43.8 (4.0) | 34.6 (4.6) * |
| SBP (mmHg) | 135 (24) | 133 (20) |
| DBP (mmHg) | 74 (17) | 78 (15) |
| HR (bpm) | 85 (18) | 81 (19) |
| Caplet | Syrup | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| N | 7 | 7 | 5 | 4 |
| t½ (h) | 2.1 (0.7) | 2.4 (0.4) | 1.9 (0.3) | 2.7 (0.3) |
| Tmax (h) | 0.75 | 1 | 0.5 | 0.25 |
| Cmax (µg/mL) | 1.8 (0.7) | 4.2 (0.6) * | 5.4 (1.0) | 7.8 (0.9) |
| AUC0–t (µg·h/mL) | 9.1 (1.3) | 18.6 (3.2) ** | 13.4 (1.5) | 25.6 (5.0) * |
| F (%) | 36 (5.3) | 74 (13.0) ** | 54 (6.3) | 102 (20.3) * |
| CL/F (L/h) | 57 (10.9) | 32 (7.4) ** | 37 (3.2) | 22 (3.4) ** |
| Vd/F (L) | 53 (15.5) | 75 (12.0) | 148 (43) | 101 (16.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porat, D.; Markovic, M.; Zur, M.; Fine-Shamir, N.; Azran, C.; Shaked, G.; Czeiger, D.; Vaynshtein, J.; Replyanski, I.; Sebbag, G.; et al. Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial. J. Clin. Med. 2019, 8, 1949. https://doi.org/10.3390/jcm8111949
Porat D, Markovic M, Zur M, Fine-Shamir N, Azran C, Shaked G, Czeiger D, Vaynshtein J, Replyanski I, Sebbag G, et al. Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial. Journal of Clinical Medicine. 2019; 8(11):1949. https://doi.org/10.3390/jcm8111949
Chicago/Turabian StylePorat, Daniel, Milica Markovic, Moran Zur, Noa Fine-Shamir, Carmil Azran, Gad Shaked, David Czeiger, Julie Vaynshtein, Ilya Replyanski, Gilbert Sebbag, and et al. 2019. "Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial" Journal of Clinical Medicine 8, no. 11: 1949. https://doi.org/10.3390/jcm8111949
APA StylePorat, D., Markovic, M., Zur, M., Fine-Shamir, N., Azran, C., Shaked, G., Czeiger, D., Vaynshtein, J., Replyanski, I., Sebbag, G., & Dahan, A. (2019). Increased Paracetamol Bioavailability after Sleeve Gastrectomy: A Crossover Pre- vs. Post-Operative Clinical Trial. Journal of Clinical Medicine, 8(11), 1949. https://doi.org/10.3390/jcm8111949