Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Legal
2.2. Sample Size Calculation
2.3. Devices and Mode of Action
2.4. Patient and Sample Selection, Randomization
2.5. Inclusion Criteria
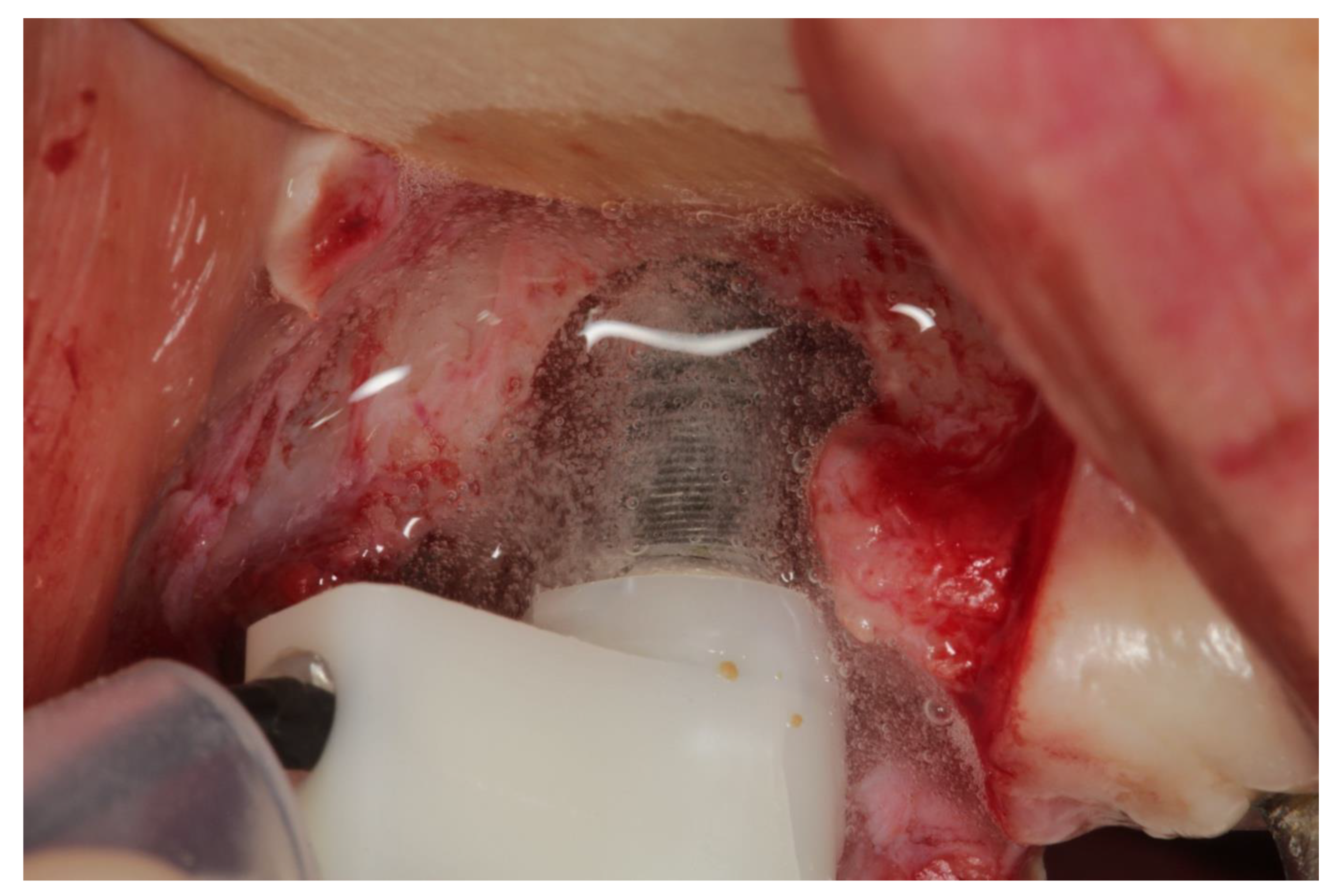
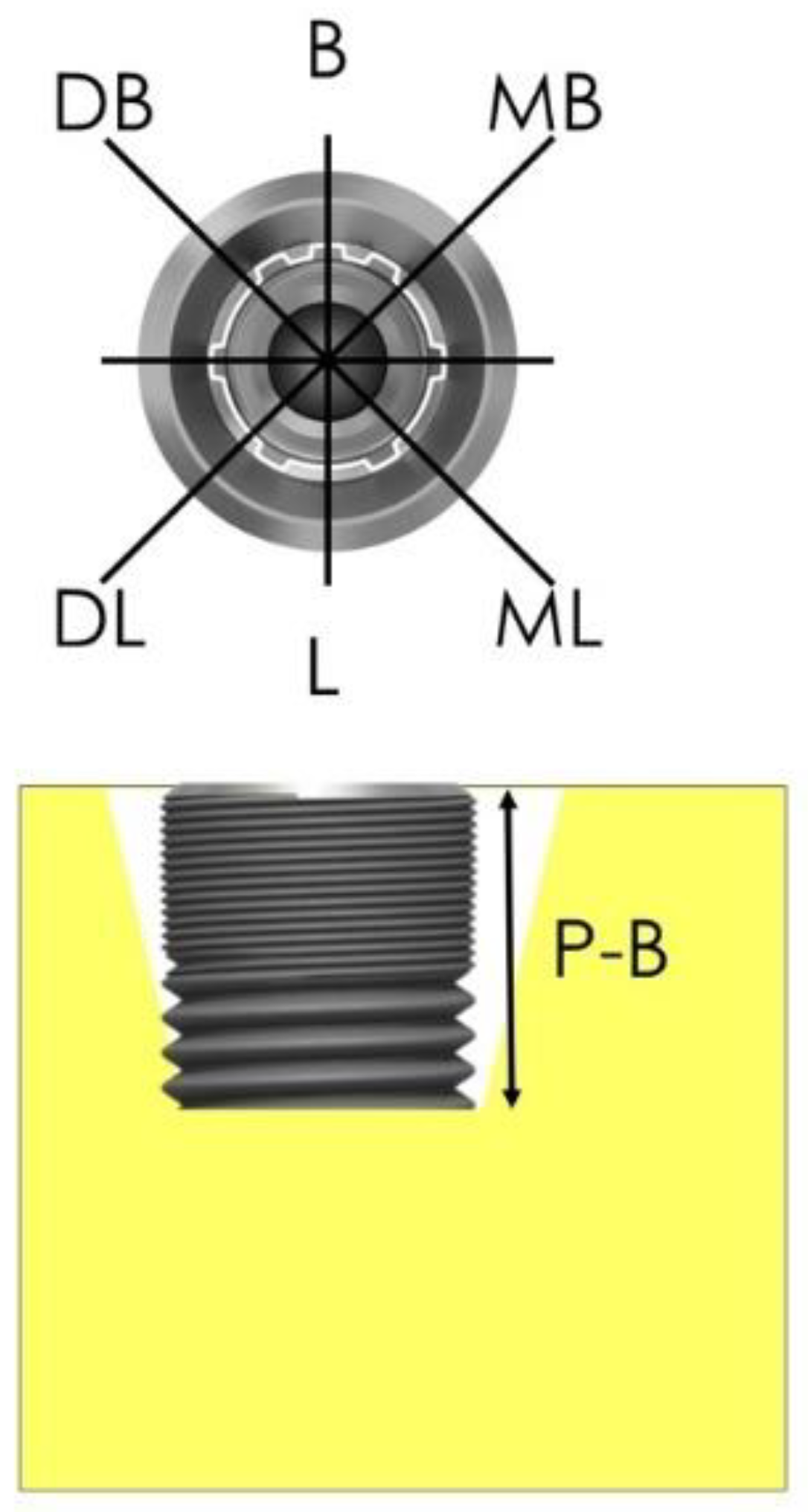
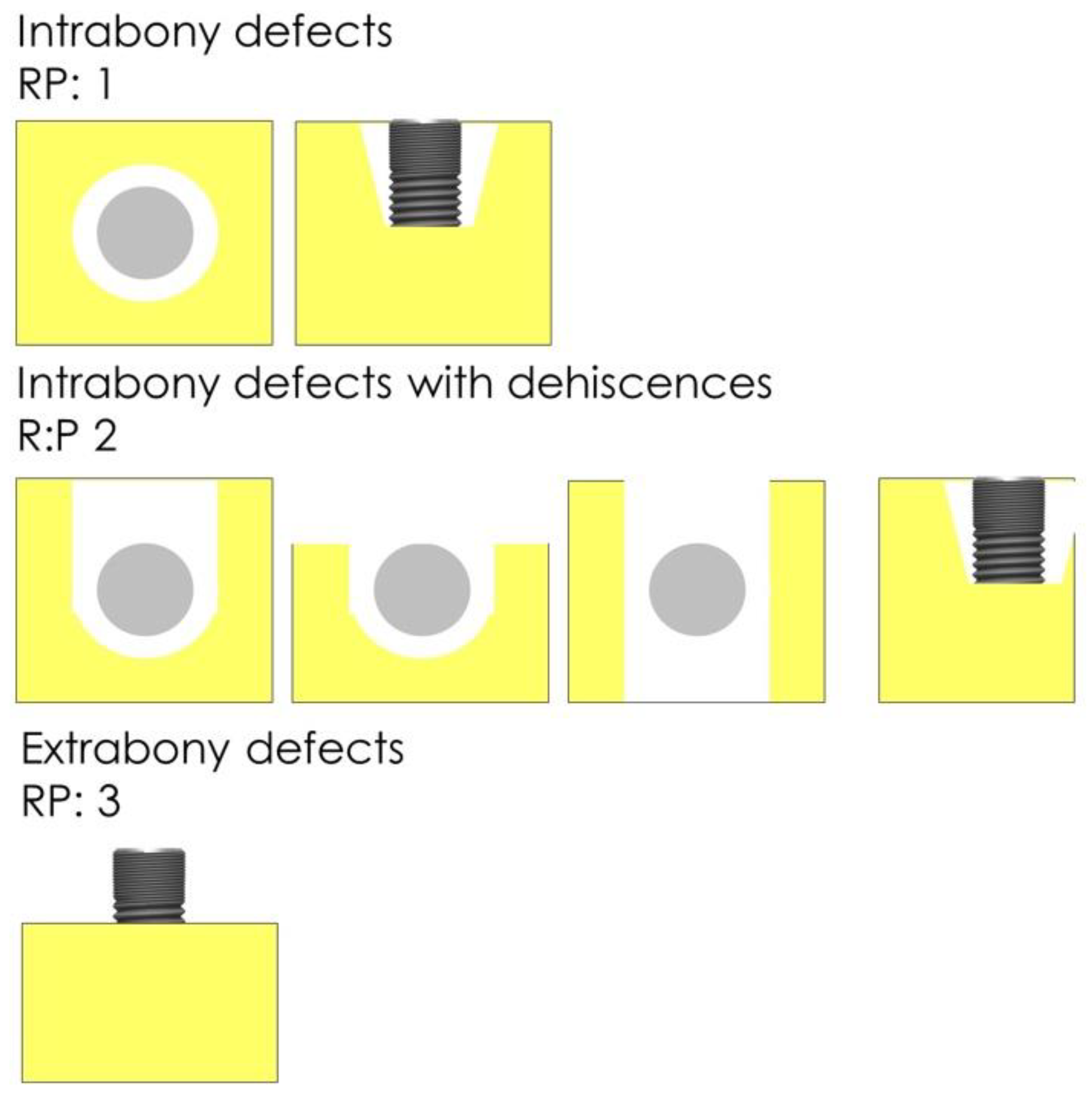
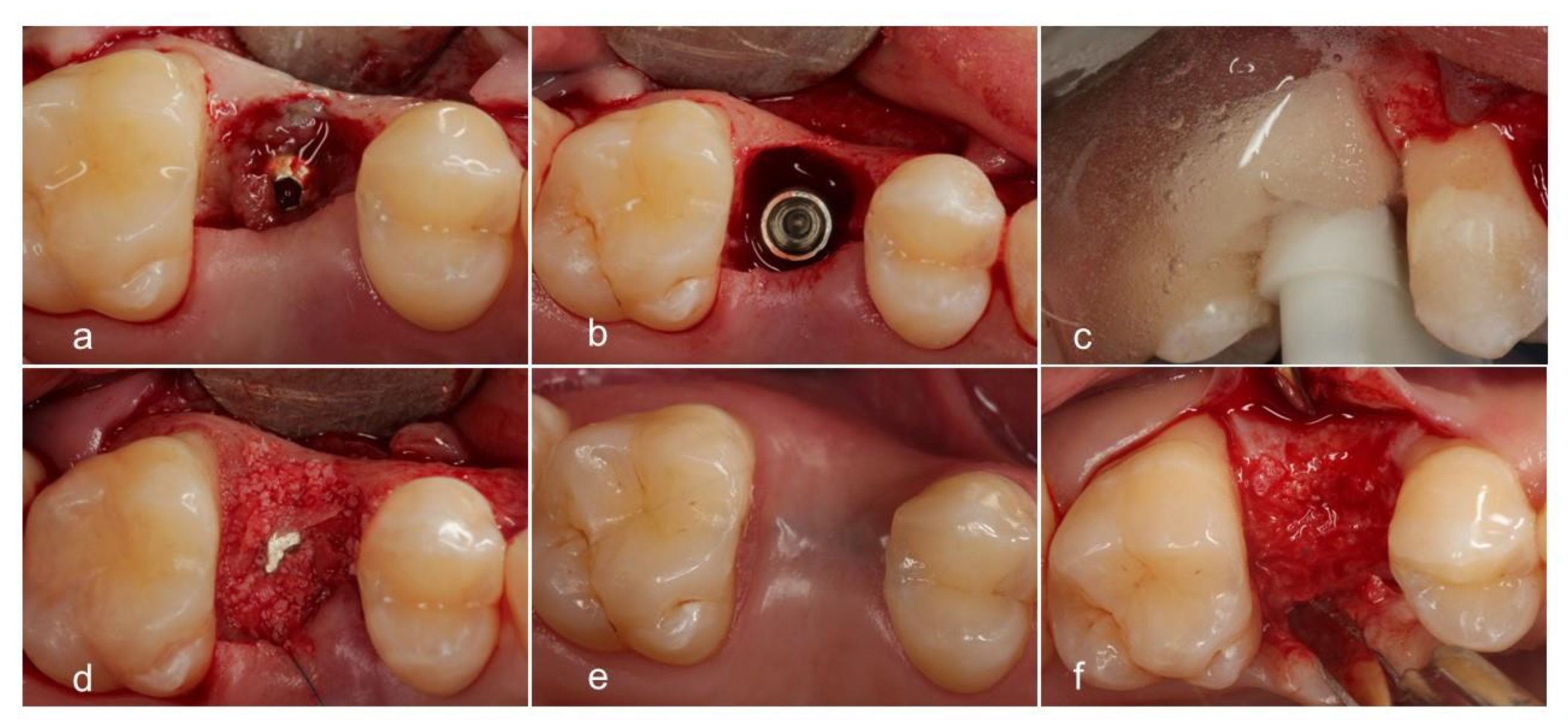
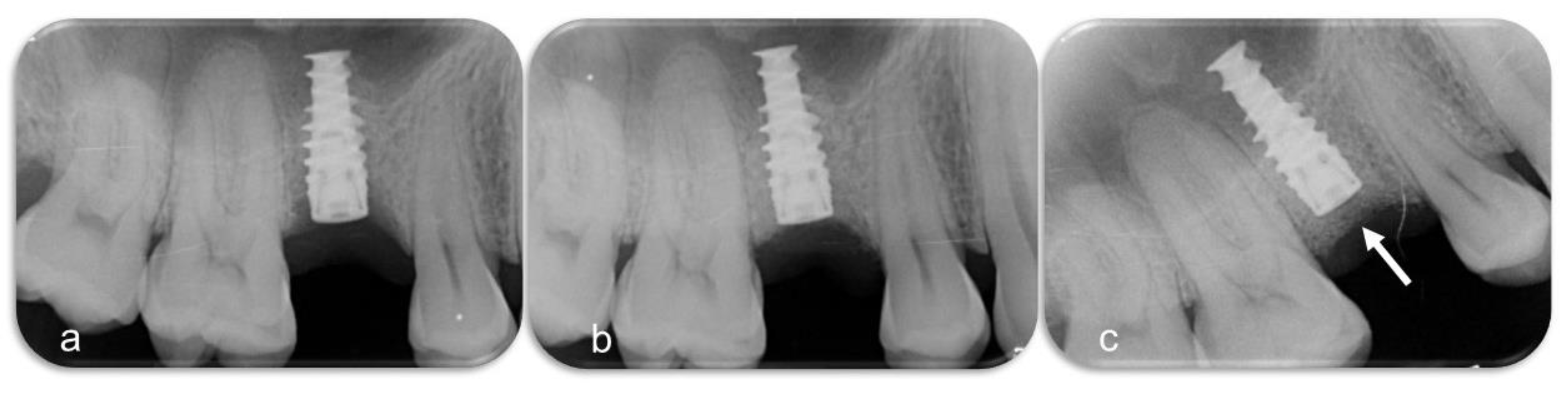
2.6. Procedures and Measurements
2.7. Statistics
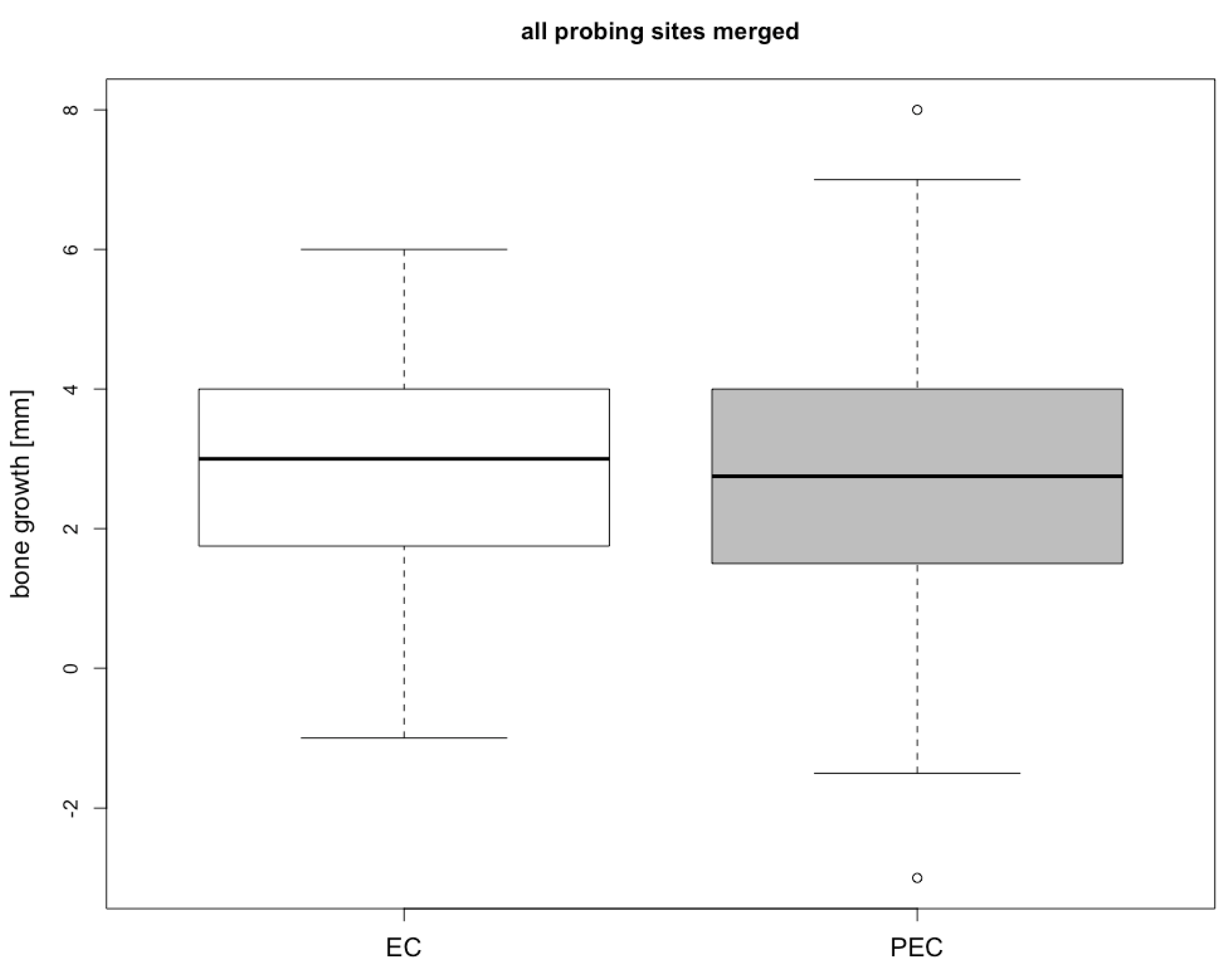
3. Results
4. Discussion
4.1. Design of Study
4.2. Our Results
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sendyk, D.I.; Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A.; Deboni, Z.; Cristina, M. Does Surgical Experience Influence Implant Survival Rate? A Systematic Review and Meta-Analysis. Int. J. Prosthodont. 2017, 30, 341–347. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Tastepe, C.S.; van Waas, R.; Liu, Y.; Wismeijer, D. Air powder abrasive treatment as an implant surface cleaning method: A literature review. Int. J. Oral Maxillofac. Implant. 2012, 27, 1461–1473. [Google Scholar]
- Claffey, N.; Clarke, E.; Polyzois, I.; Renvert, S. Surgical treatment of peri-implantitis. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur. J. Oral Implantol. 2012, 5, S21–S41. [Google Scholar]
- de Almeida, J.M.; Matheus, H.R.; Rodrigues Gusman, D.J.; Faleiros, P.L.; Januário de Araújo, N.; Noronha Novaes, V.C. Effectiveness of Mechanical Debridement Combined With Adjunctive Therapies for Nonsurgical Treatment of Periimplantitis: A Systematic Review. Implant Dent. 2017, 26, 137–144. [Google Scholar] [CrossRef]
- del Pozo, J.L.; Rouse, M.S.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. The electricidal effect: Reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob. Agents Chemother. 2009, 53, 41–45. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, S202–S213. [Google Scholar] [CrossRef]
- Mohn, D.; Zehnder, M.; Stark, W.J.; Imfeld, T. Electrochemical disinfection of dental implants—A proof of concept. PLoS ONE 2011, 6, e16157. [Google Scholar] [CrossRef]
- Zipprich, H.; Ratka, C.; Schlee, M.; Brodbeck, U.; Lauer, H.C.; Seitz, O. Periimplantitistherapie: Durchbruch mit neuer Reinigungsmethode. Dentalmagazin 2013, 31, 14–17. [Google Scholar]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef]
- Ratka, C.; Weigl, P.; Henrich, D.; Koch, F.; Schlee, M.; Zipprich, H. The Effect of In Vitro Electrolytic Cleaning on Biofilm-Contaminated Implant Surfaces. J. Clin. Med. 2019, 8, 1397. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implant. Res. 2007, 18, 161–170. [Google Scholar] [CrossRef]
- Alomrani, A.N.; Hermann, J.S.; Jones, A.A.; Buser, D.; Schoolfield, J.; Cochran, D.L. The effect of a machined collar on coronal hard tissue around titanium implants: A radiographic study in the canine mandible. Int. J. Oral Maxillofac. Implant. 2005, 20, 677–686. [Google Scholar]
- Laurell, L.; Lundgren, D. Marginal bone level changes at dental implants after 5 years in function: A meta-analysis. Clin. Implant Dent. Relat. Res. 2011, 13, 19–28. [Google Scholar] [CrossRef]
- Albrektsson, T.; Canullo, L.; Cochran, D.; de Bruyn, H. “Peri-Implantitis”: A Complication of a Foreign Body or a Man-Made “Disease”. Facts and Fiction. Clin. Implant Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Petzold, M.; Berglundh, T. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Bieling, K.; Becker, J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: A four-year clinical follow-up report. J. Clin. Periodontol. 2009, 36, 807–814. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Pittoni, D.; Roccuzzo, A.; Charrier, L.; Dalmasso, P. Surgical treatment of peri-implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7-year-results. Clin. Oral Implant. Res. 2017, 28, 1577–1583. [Google Scholar] [CrossRef]
- Coli, P.; Christiaens, V.; Sennerby, L.; de Bruyn, H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol. 2000 2017, 73, 203–217. [Google Scholar] [CrossRef]
- Schlee, M.; Naili, L.; Rathe, F.; Brodbeck, U.; Zipprich, H. Is complete re-osseointegration of an infected dental implant possible—Histological results of a dog study. A short communication. J. Clin. Med. 2019. Submitted. [Google Scholar]
- Ericsson, I.; Lindhe, J. Probing depth at implants and teeth. An experimental study in the dog. J. Clin. Periodontol. 1993, 20, 623–627. [Google Scholar] [CrossRef]
- Canullo, L.; Schlee, M.; Wagner, W.; Covani, U. International Brainstorming Meeting on Etiologic and Risk Factors of Peri-implantitis, Montegrotto (Padua, Italy), August 2014. Int. J. Oral Maxillofac. Implant. 2015, 30, 1093–1104. [Google Scholar] [CrossRef]

| General Information | Specific Information | n/Mean Years | Percentage |
|---|---|---|---|
| gender | female | 12 | 50.00% |
| male | 12 | 50.00% | |
| age | female | 59.2 y | |
| male | 51.4 y | ||
| jaw | maxilla EL | 4 | 16.67% |
| maxilla PEL | 8 | 33.33% | |
| mandible EL | 8 | 33.33% | |
| mandible PEL | 4 | 16.67% | |
| maxilla total | 12 | 50.00% | |
| mandible total | 12 | 50.00% | |
| smokers | EL | 3 | 12.50% |
| PEL | 1 | 4.17% | |
| BoP | 24 | 100.00% | |
| pus | 24 | 100.00% |
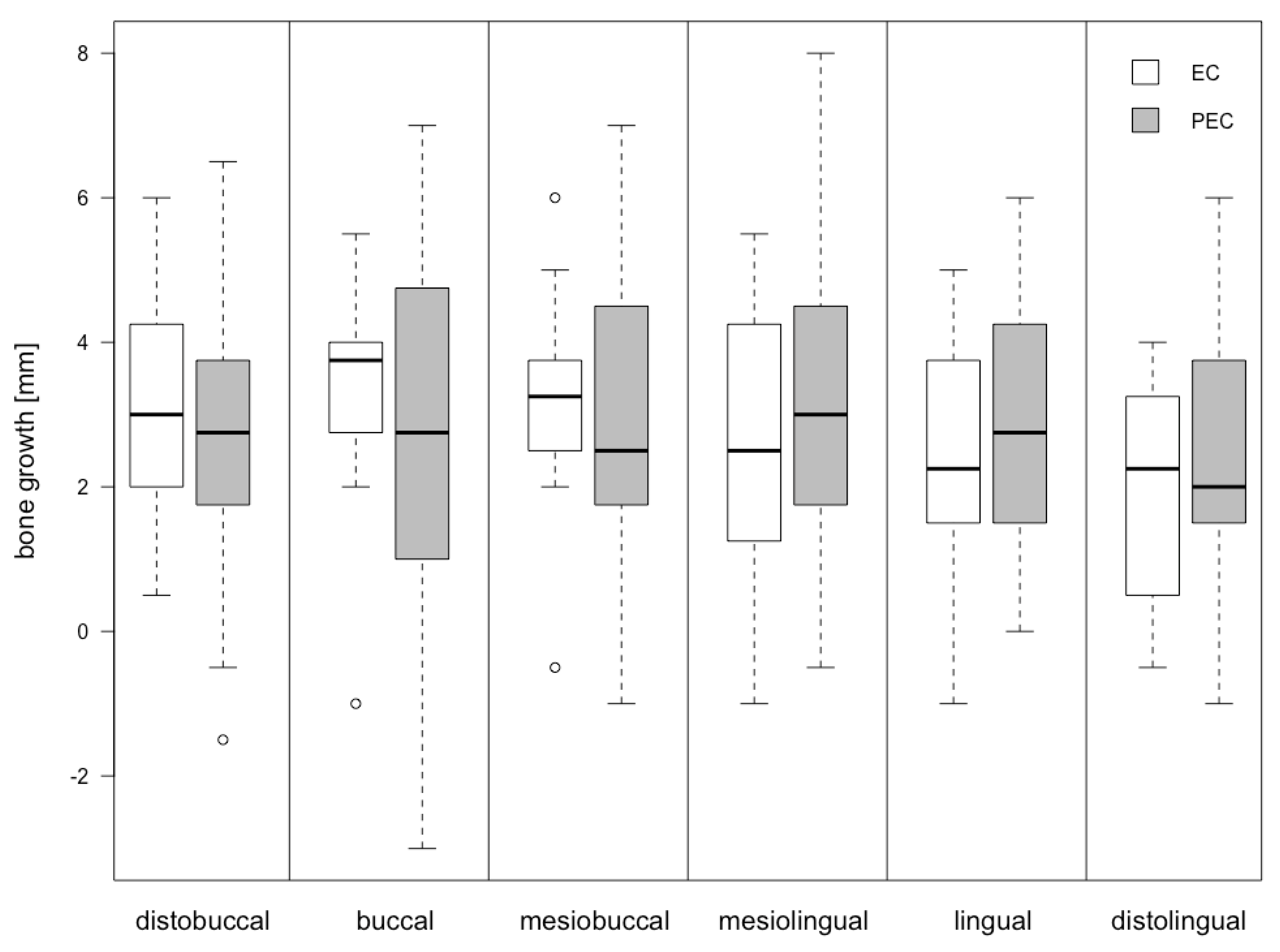
| Location | EC Group [mm] | PEC Group [mm] | Differences | |
|---|---|---|---|---|
| AEL-EL | p-Value | |||
| db | 3.00 ± 1.67 | 2.50 ± 2.10 | −0.50 | 0.52 |
| b | 3.25 ± 1.63 | 2.83 ± 2.94 | −0.42 | 0.71 |
| mb | 3.17 ± 1.61 | 3.00 ± 2.12 | −0.17 | 0.83 |
| ml | 2.58 ± 1.84 | 3.13 ± 2.25 | 0.55 | 0.53 |
| l | 2.29 ± 1.79 | 2.96 ± 1.86 | 0.67 | 0.38 |
| dl | 1.96 ± 1.57 | 2.46 ± 1.83 | 0.50 | 0.48 |
| Median | 2.71 ± 1.70 | 2.81 ± 2.15 | 0.10 | 0.87 |
| Type of Implant | Number |
|---|---|
| Astra TX | 5 |
| Astra EV | 2 |
| Straumann tissue level | 2 |
| Straumann bone level | 1 |
| Conelog | 2 |
| Camlog | 2 |
| Ankylos | 2 |
| Sky | 1 |
| Branemark | 2 |
| Xive | 1 |
| Steri Oss | 1 |
| Zimmer | 2 |
| Nobel Active | 1 |
| RP Class | Bone Gain |
|---|---|
| RP1 | 4.02 ± 0.96 |
| RP2 | 2.64 ± 1.58 |
| RP3 | 2.34 ± 1.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Weigl, P.; Zipprich, H. Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results. J. Clin. Med. 2019, 8, 1909. https://doi.org/10.3390/jcm8111909
Schlee M, Rathe F, Brodbeck U, Ratka C, Weigl P, Zipprich H. Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results. Journal of Clinical Medicine. 2019; 8(11):1909. https://doi.org/10.3390/jcm8111909
Chicago/Turabian StyleSchlee, Markus, Florian Rathe, Urs Brodbeck, Christoph Ratka, Paul Weigl, and Holger Zipprich. 2019. "Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results" Journal of Clinical Medicine 8, no. 11: 1909. https://doi.org/10.3390/jcm8111909
APA StyleSchlee, M., Rathe, F., Brodbeck, U., Ratka, C., Weigl, P., & Zipprich, H. (2019). Treatment of Peri-Implantitis—Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning—A Randomized Controlled Clinical Trial—Six-Month Results. Journal of Clinical Medicine, 8(11), 1909. https://doi.org/10.3390/jcm8111909





