Is Complete Re-Osseointegration of an Infected Dental Implant Possible? Histologic Results of a Dog Study: A Short Communication
Abstract
1. Introduction
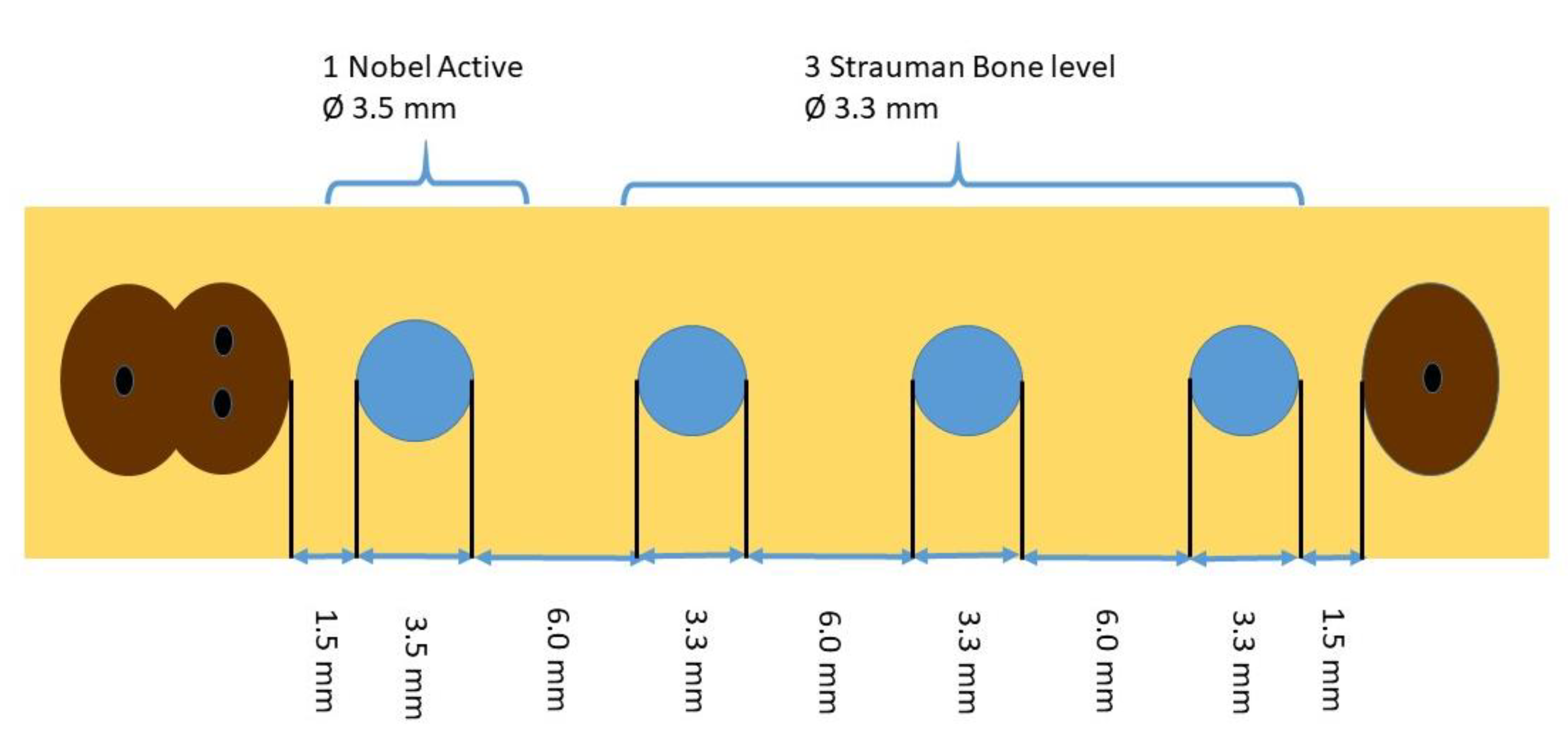
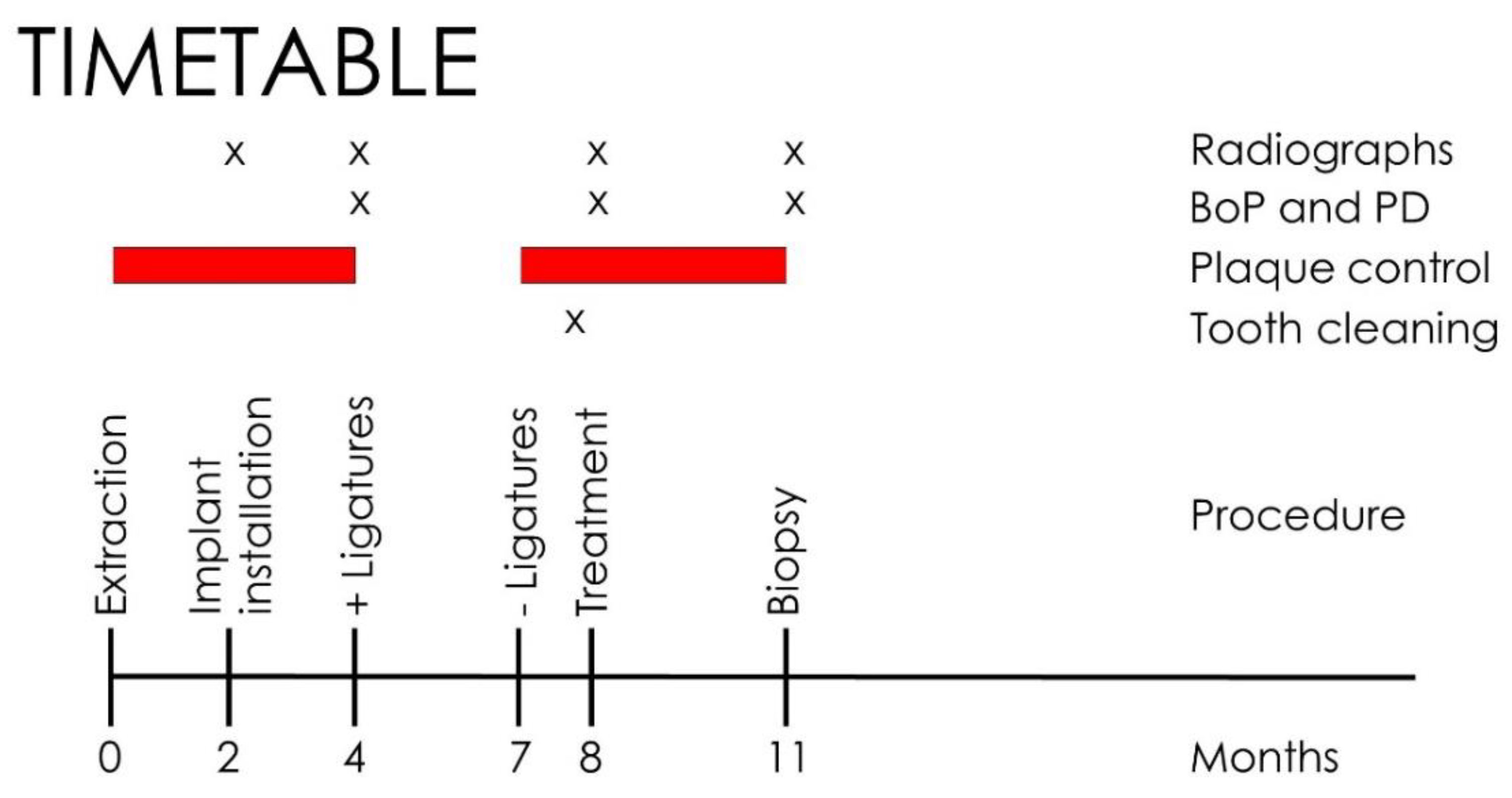
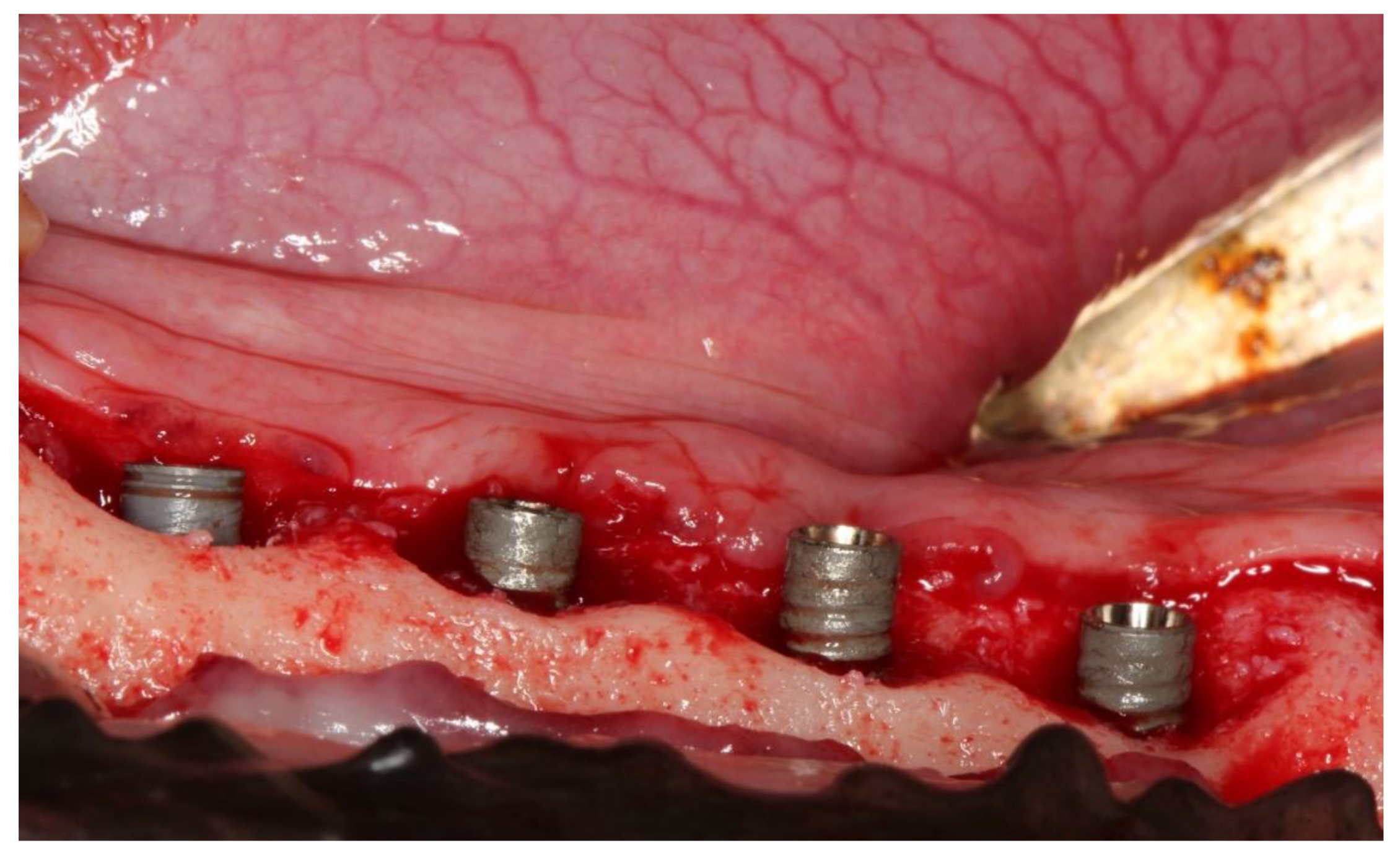
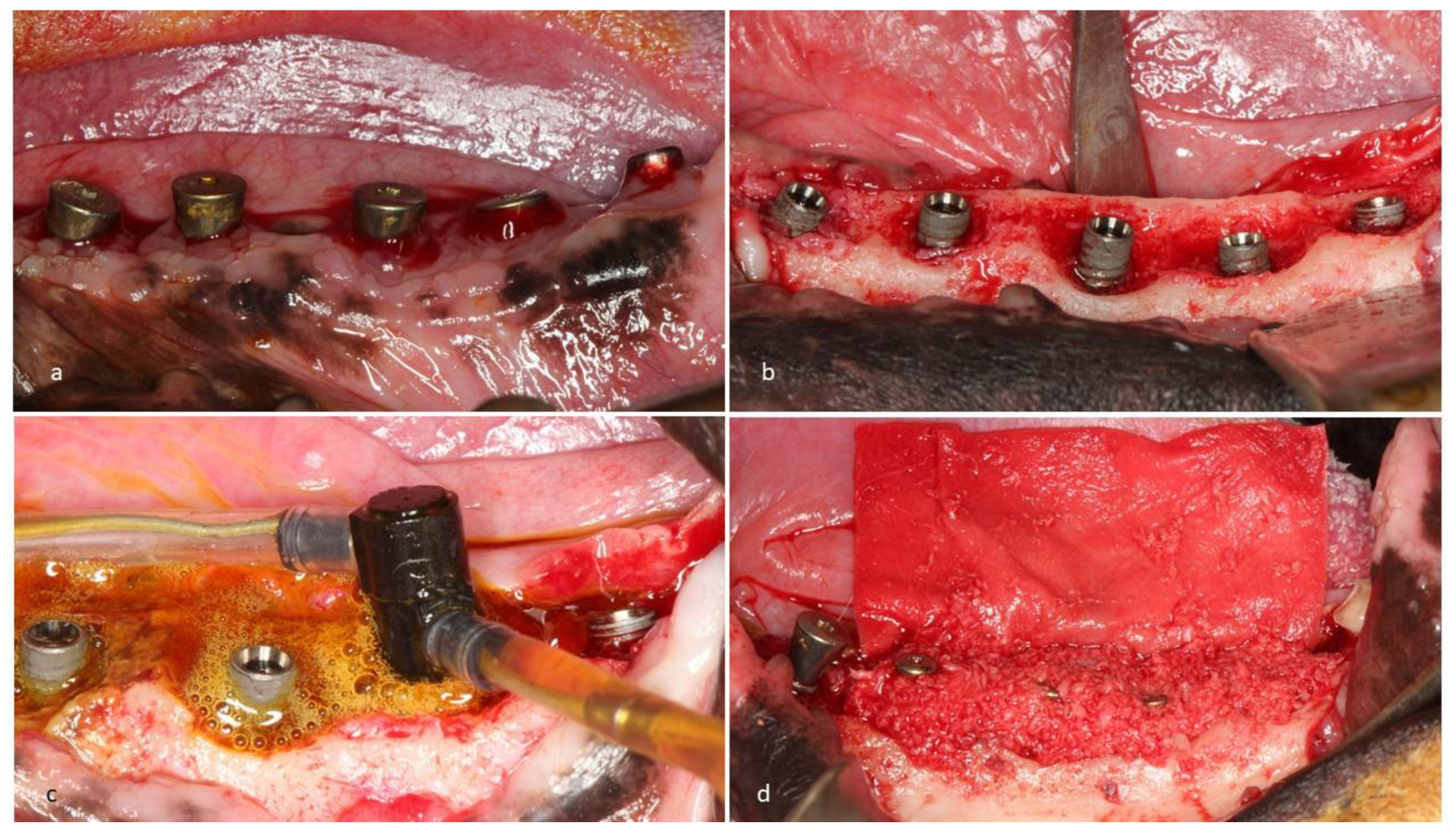
2. Experimental Section
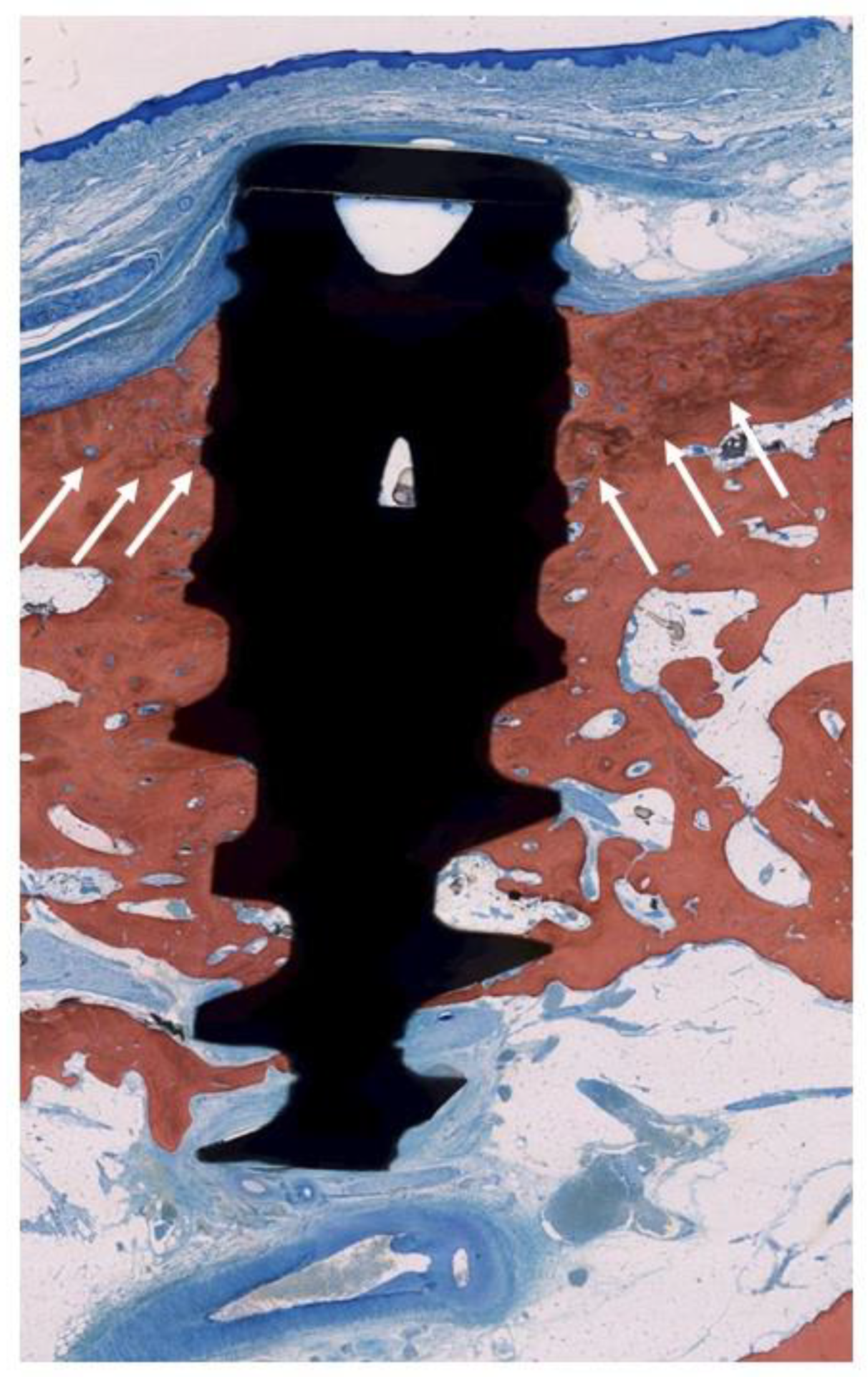
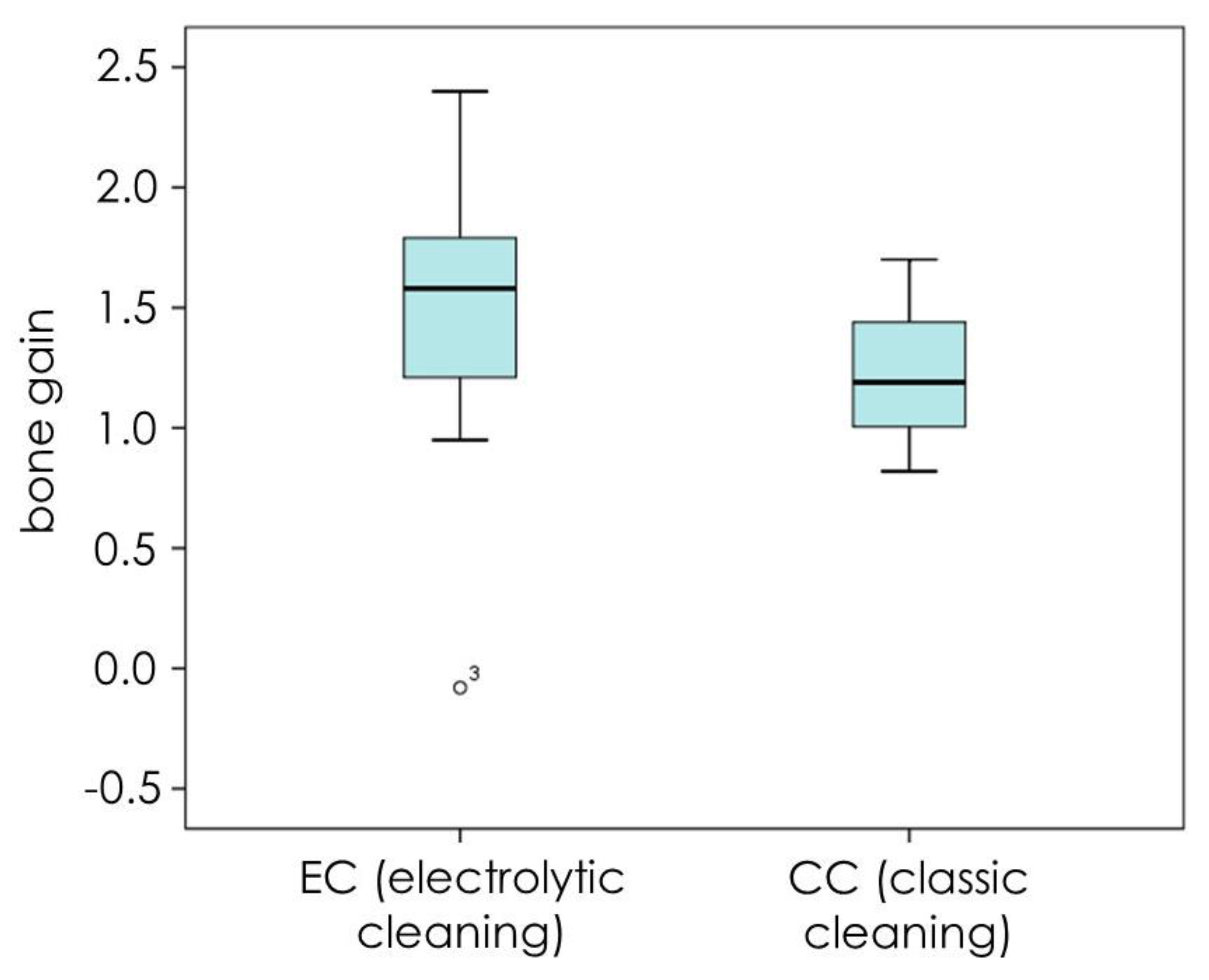
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implants Res. 2007, 18, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J.; Kotsakis, G.; Huh, J.-K.; Jeong, S.C.; Nam, K.Y.; Kim, J.R.; Heo, Y.C.; Kim, H.-C.; Zhang, L.; Evans, M.D.; et al. Clinical and microbiologic investigation of an expedited peri-implantitis dog model: An animal study. BMC Oral Health 2019, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sculean, A.; Engebretson, S.P.; Becker, J.; Sager, M. Animal models for peri-implant mucositis and peri-implantitis. Periodontol. 2000 2015, 68, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Maguire, R. Re-osseointegration on previously contaminated surfaces: A systematic review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ratka, C.; Weigl, P.; Henrich, D.; Koch, F.; Schlee, M.; Zipprich, H. The Effect of In Vitro Electrolytic Cleaning on Biofilm-Contaminated Implant Surfaces. J. Clin. Med. 2019, 8, 1397. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Zipprich, H. Treatment of periimplantitis-electrolytic cleaning versus mechanical and electrolytic cleaning—A randomized controlled clinical trial—6 month results. J. Clin. Med. 2019, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.A.; Kenney, E.B.; Carranza, F.A.; Donath, K. The regenerative potential of plaque-induced peri-implant bone defects treated by a submerged membrane technique: An experimental study. Int. J. Oral Maxillofac Implants 1993, 8, 13–18. [Google Scholar] [PubMed]
- Linkevicius, T.; Puisys, A.; Linkeviciene, L.; Peciuliene, V.; Schlee, M. Crestal Bone Stability around Implants with Horizontally Matching Connection after Soft Tissue Thickening: A Prospective Clinical Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Cho, S.C.; Wallace, S.S. The effect of inter-implant distance on the height of inter-implant bone crest. J. Periodontol. 2000, 71, 546–549. [Google Scholar] [CrossRef] [PubMed]



| Dog | Quadrant | Straumann | Nobel | Straumann Not Treated | Nobel Not Treated | Total | Not Placed |
|---|---|---|---|---|---|---|---|
| 1 | right | 2 | 1 | 3 | 1 | ||
| left | 3 | 1 | 1 | 5 | |||
| 2 | right | 3 | 1 | 4 | |||
| left | 3 | 1 | 1 | 5 | |||
| 3 | right | 3 | 1 | 4 | |||
| left | 2 | 1 | 1 | 4 | |||
| 4 | right | 3 | 0 | 1 | 4 | ||
| left | 3 | 1 | 4 | ||||
| 5 | right | 3 | 1 | 4 | |||
| left | 3 | 0 | 1 | 4 | |||
| 6 | right | 3 | 1 | 1 | 5 | ||
| left | 3 | 1 | 4 | ||||
| 7 | right | 2 | 1 | 3 | 1 | ||
| left | 2 | 1 | 1 | 4 | |||
| 8 | right | 3 | 1 | 4 | |||
| left | 2 | 1 | 1 | 4 | |||
| total | 43 | 14 | 6 | 2 | 65 | 2 | |
| Straumann | 49 | ||||||
| Nobel | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlee, M.; Naili, L.; Rathe, F.; Brodbeck, U.; Zipprich, H. Is Complete Re-Osseointegration of an Infected Dental Implant Possible? Histologic Results of a Dog Study: A Short Communication. J. Clin. Med. 2020, 9, 235. https://doi.org/10.3390/jcm9010235
Schlee M, Naili L, Rathe F, Brodbeck U, Zipprich H. Is Complete Re-Osseointegration of an Infected Dental Implant Possible? Histologic Results of a Dog Study: A Short Communication. Journal of Clinical Medicine. 2020; 9(1):235. https://doi.org/10.3390/jcm9010235
Chicago/Turabian StyleSchlee, Markus, Loubna Naili, Florian Rathe, Urs Brodbeck, and Holger Zipprich. 2020. "Is Complete Re-Osseointegration of an Infected Dental Implant Possible? Histologic Results of a Dog Study: A Short Communication" Journal of Clinical Medicine 9, no. 1: 235. https://doi.org/10.3390/jcm9010235
APA StyleSchlee, M., Naili, L., Rathe, F., Brodbeck, U., & Zipprich, H. (2020). Is Complete Re-Osseointegration of an Infected Dental Implant Possible? Histologic Results of a Dog Study: A Short Communication. Journal of Clinical Medicine, 9(1), 235. https://doi.org/10.3390/jcm9010235





