Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts
Abstract
1. Introduction
2. Experimental Section
2.1. Samples and Data Collection
2.2. Immunosuppression Events and Subgroups
2.3. GST Analysis and Statistics
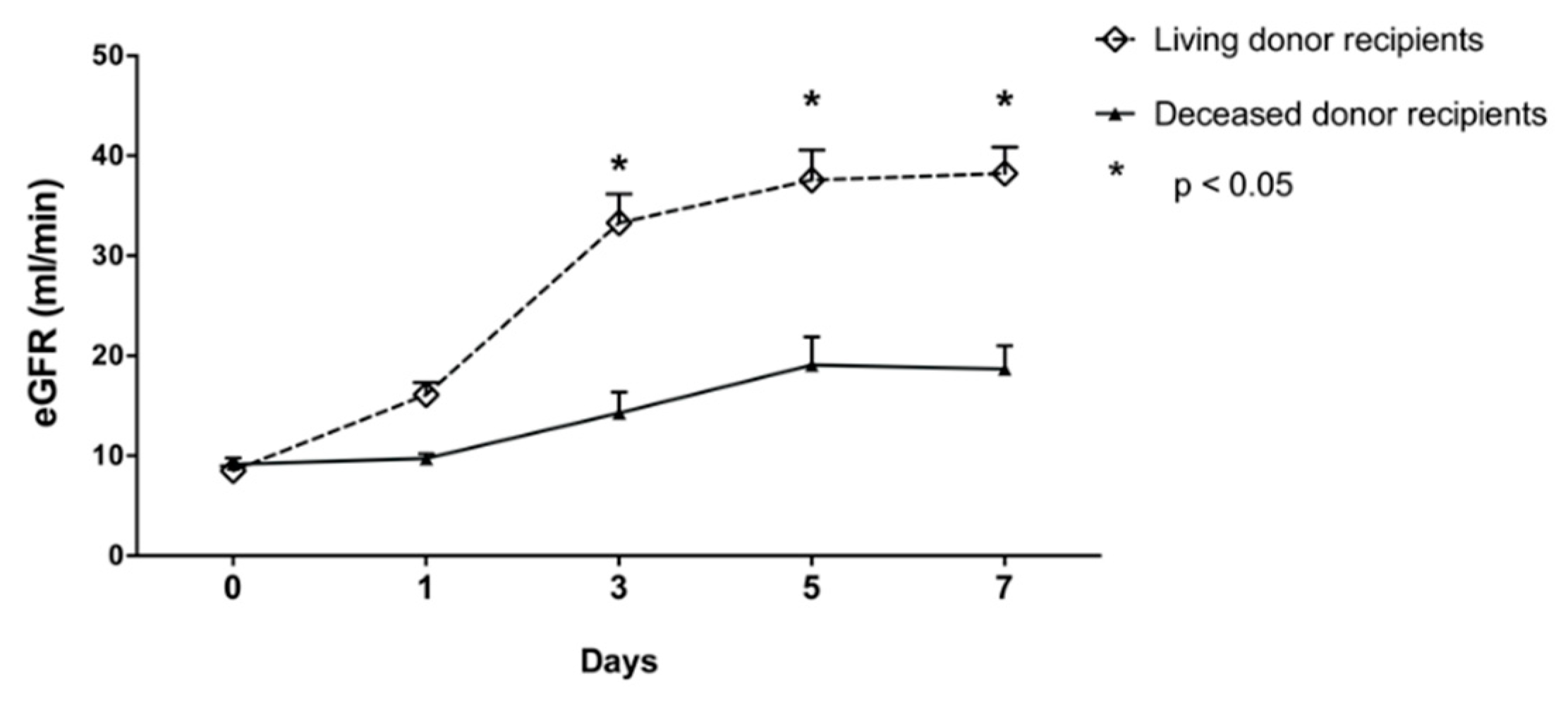
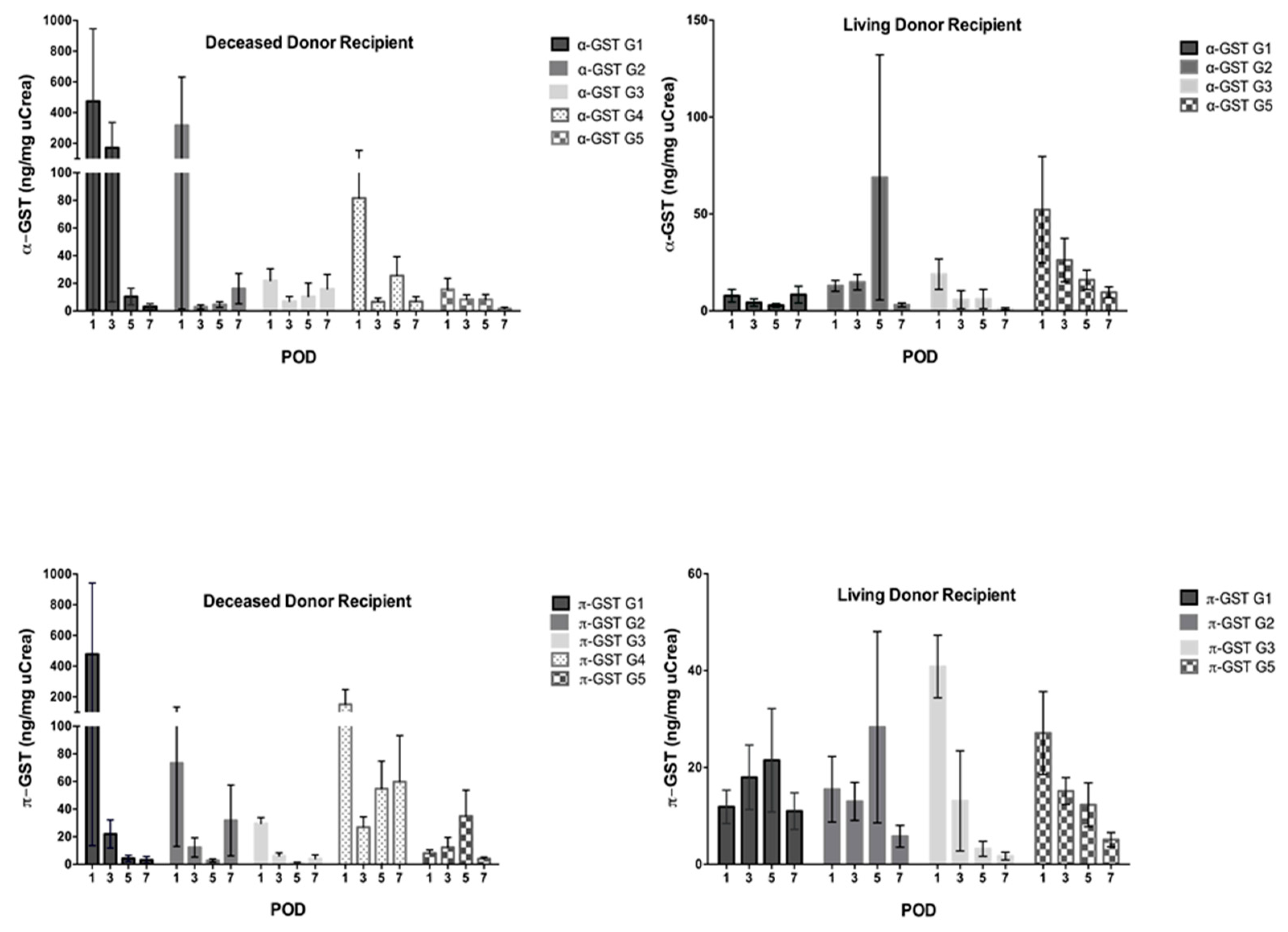
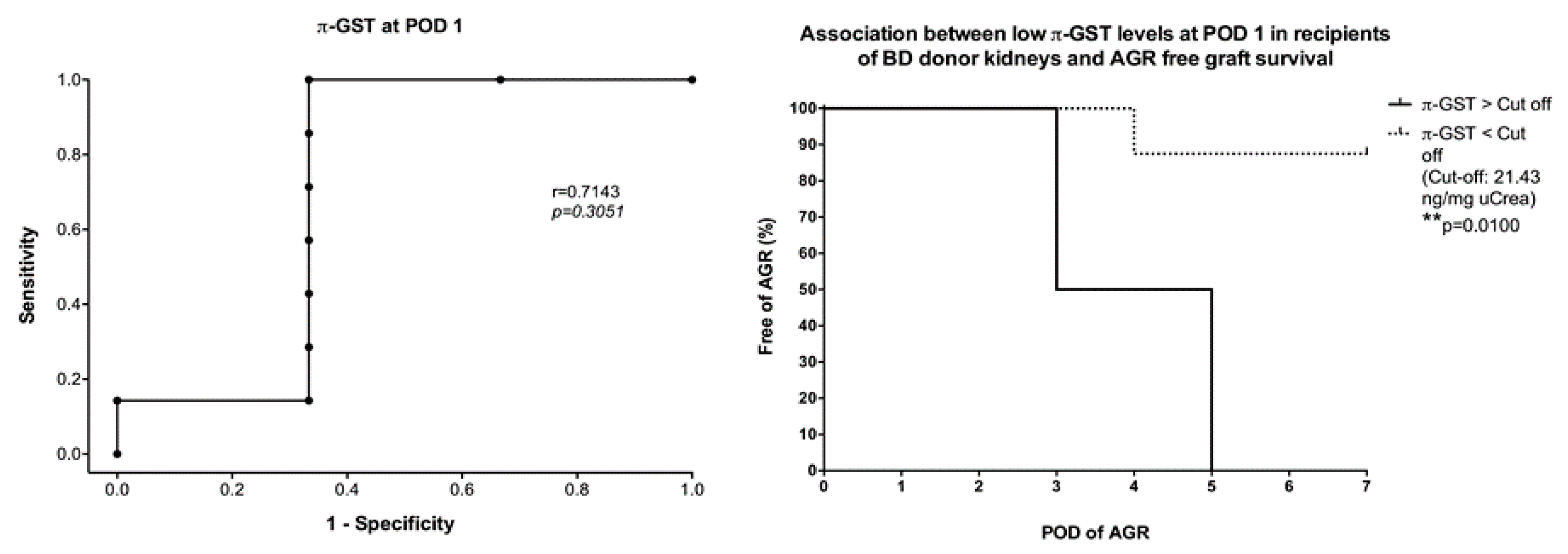
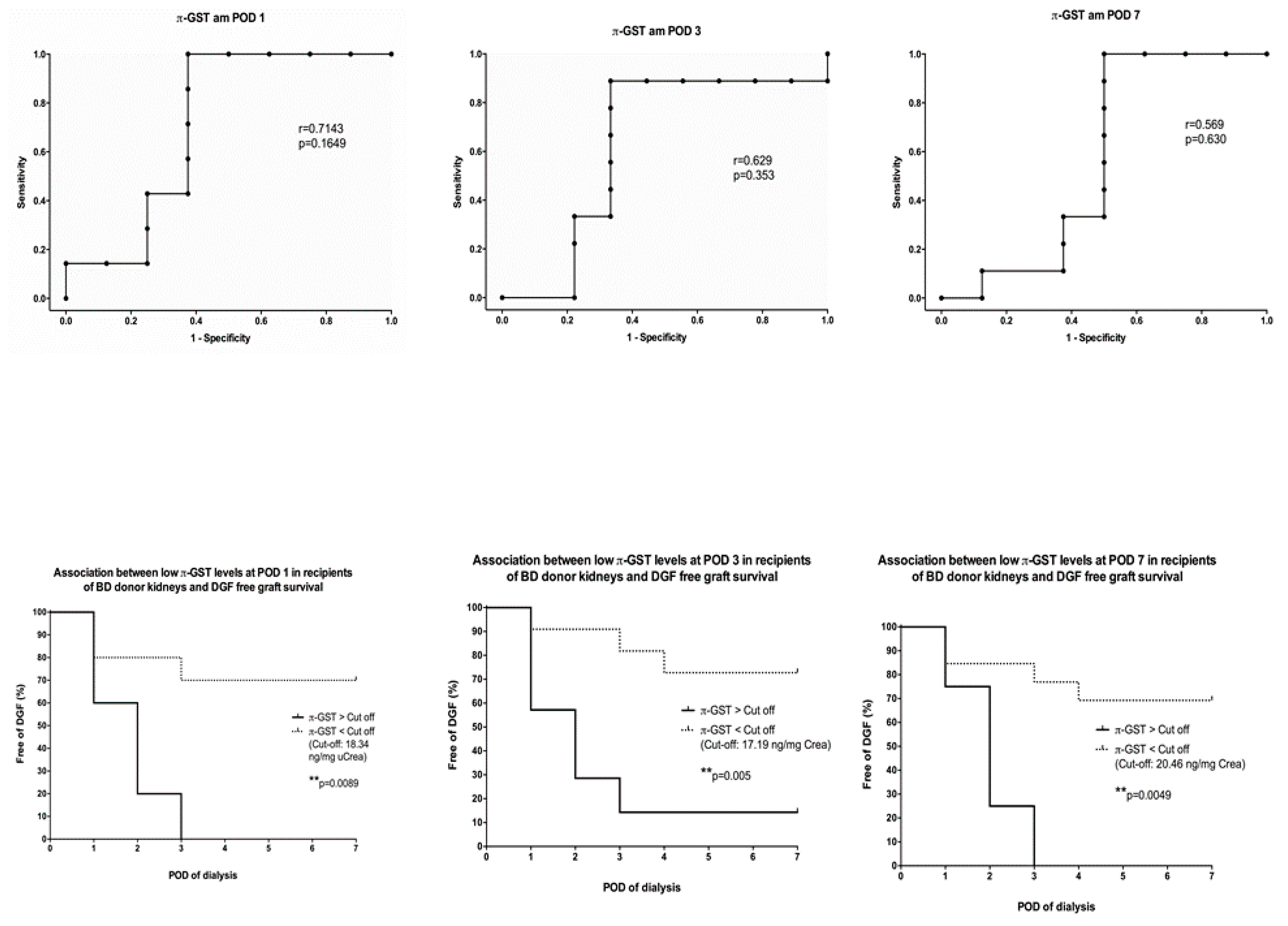
3. Results
3.1. Demographic Data
3.2. α- and π-GST in Recipients
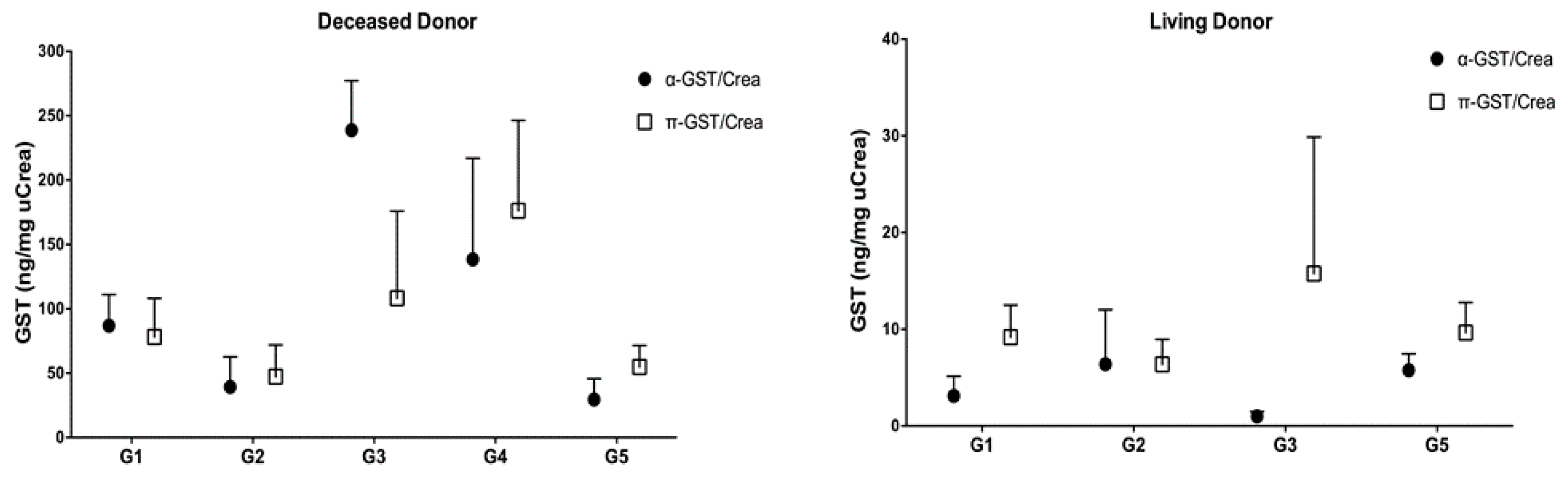
3.3. α- and π-GST in Donors
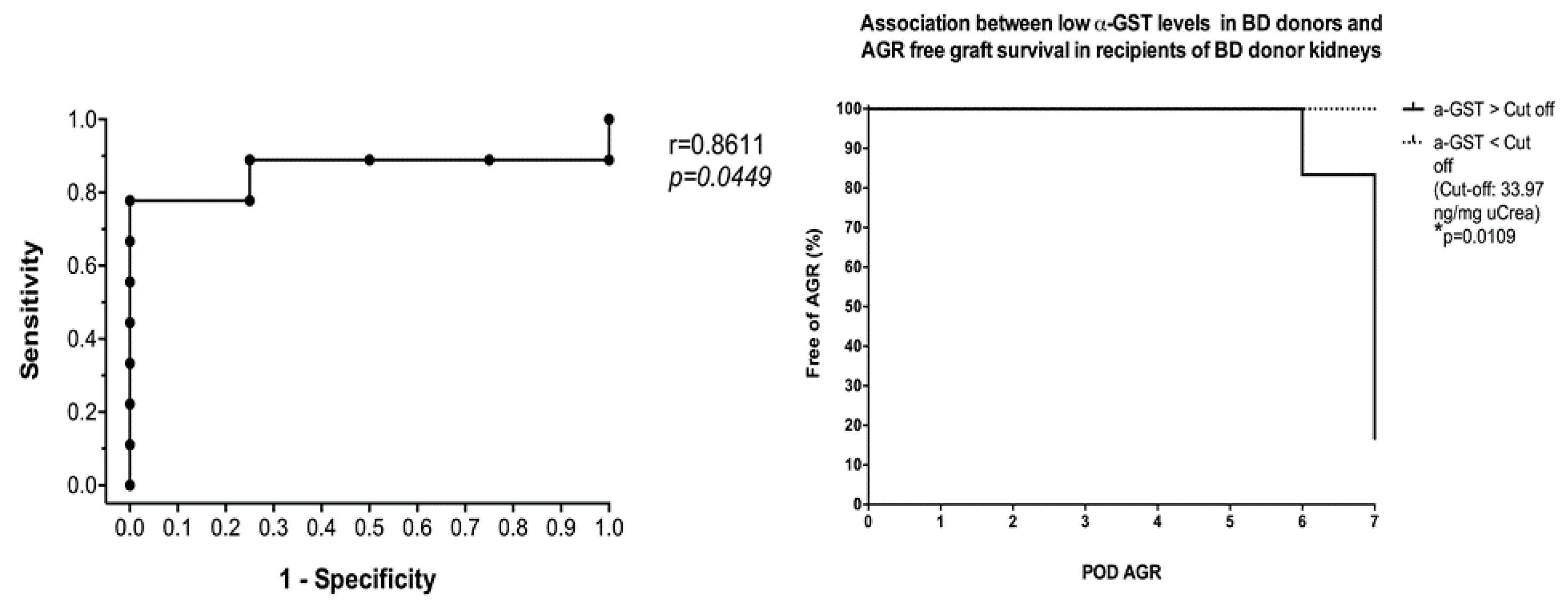
3.4. Six- and 12-Months Graft Survival
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, W.K.; Famure, O.; Li, Y.; Kim, S.J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015, 88, 851–858. [Google Scholar] [CrossRef]
- Marcen, R.; Fernandez-Rodriguez, A.; Rodriguez-Mendiola, N.; Ponte, B.; Galeano, C.; Villafruela, J.J.; Teruel, J.L.; Burgos, F.J.; Ortuno, J. Evolution of rejection rates and kidney graft survival: A historical analysis. Transplant Proc. 2009, 41, 2357–2359. [Google Scholar] [CrossRef]
- Martins, L.; Ventura, A.; Branco, A.; Carvalho, M.J.; Henriques, A.C.; Dias, L.; Sarmento, A.M.; Amil, M. Cyclosporine versus tacrolimus in kidney transplantation: Are there differences in nephrotoxicity? Transplant Proc. 2004, 36, 877–879. [Google Scholar] [CrossRef]
- Ojo, A.O.; Wolfe, R.A.; Held, P.J.; Port, F.K.; Schmouder, R.L. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 1997, 63, 968–974. [Google Scholar] [CrossRef]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef]
- Klintmalm, G.; Sawe, J.; Ringden, O.; von Bahr, C.; Magnusson, A. Cyclosporine plasma levels in renal transplant patients. Association with renal toxicity and allograft rejection. Transplantation 1985, 39, 132–137. [Google Scholar] [CrossRef]
- Wilczek, H.E. Percutaneous needle biopsy of the renal allograft. A clinical safety evaluation of 1129 biopsies. Transplantation 1990, 50, 790–797. [Google Scholar] [CrossRef]
- Branten, A.J.; Mulder, T.P.; Peters, W.H.; Assmann, K.J.; Wetzels, J.F. Urinary excretion of glutathione S transferases alpha and pi in patients with proteinuria: Reflection of the site of tubular injury. Nephron 2000, 85, 120–126. [Google Scholar] [CrossRef]
- Harrison, D.J.; Kharbanda, R.; Cunningham, D.S.; McLellan, L.I.; Hayes, J.D. Distribution of glutathione S-transferase isoenzymes in human kidney: Basis for possible markers of renal injury. J. Clin. Pathol. 1989, 42, 624–628. [Google Scholar] [CrossRef]
- Backman, L.; Appelkvist, E.L.; Ringden, O.; Dallner, G. Glutathione transferase in the urine: A marker for post-transplant tubular lesions. Kidney Int. 1988, 33, 571–577. [Google Scholar] [CrossRef]
- Kuzniar, J.; Marchewka, Z.; Krasnowski, R.; Boratynska, M.; Dlugosz, A.; Klinger, M. Enzymuria and low molecular weight protein excretion as the differentiating marker of complications in the early post kidney transplantation period. Int. Urol. Nephrol. 2006, 38, 753–758. [Google Scholar] [CrossRef]
- Polak, W.P.; Kosieradzki, M.; Kwiatkowski, A.; Danielewicz, R.; Lisik, W.; Michalak, G.; Paczek, L.; Lao, M.; Walaszewski, J.; Rowinski, W.A. Activity of glutathione S-transferases in the urine of kidney transplant recipients during the first week after transplantation. Ann. Transplant. 1999, 4, 42–45. [Google Scholar]
- Sundberg, A.G.; Appelkvist, E.L.; Backman, L.; Dallner, G. Urinary Pi-Class Glutathione Transferase as an Indicator of Tubular Damage in the Human Kidney. Nephron 1994, 67, 308–316. [Google Scholar] [CrossRef]
- Backman, L.; Appelkvist, E.L.; Ringden, O.; Dallner, G. Appearance of basic glutathione transferase in the urine during tubular complications in renal transplant recipients. Transplant. Proc. 1988, 20, 427–430. [Google Scholar]
- Westhuyzen, J.; Endre, Z.H.; Reece, G.; Reith, D.M.; Saltissi, D.; Morgan, T.J. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol. Dial. Transplant. 2003, 18, 543–551. [Google Scholar] [CrossRef]
- Jochum, C.; Beste, M.; Sowa, J.P.; Farahani, M.S.; Penndorf, V.; Nadalin, S.; Saner, F.; Canbay, A.; Gerken, G. Glutathione-S-transferase subtypes alpha and pi as a tool to predict and monitor graft failure or regeneration in a pilot study of living donor liver transplantation. Eur. J. Med. Res. 2011, 16, 34–40. [Google Scholar] [CrossRef]
- Daemen, J.W.; Oomen, A.P.; Janssen, M.A.; van de Schoot, L.; van Kreel, B.K.; Heineman, E.; Kootstra, G. Glutathione S-transferase as predictor of functional outcome in transplantation of machine-preserved non-heart-beating donor kidneys. Transplantation 1997, 63, 89–93. [Google Scholar] [CrossRef]
- Sundberg, A.; Appelkvist, E.L.; Dallner, G.; Nilsson, R. Glutathione transferases in the urine—Sensitive methods for detection of kidney damage-induced by nephrotoxic agents in humans. Environ. Health Perspect. 1994, 102, 293–296. [Google Scholar]
- Min, D.I.; Perry, P.J.; Chen, H.Y.; Hunsicker, L.G. Cyclosporine trough concentrations in predicting allograft rejection and renal toxicity up to 12 months after renal transplantation. Pharmacotherapy 1998, 18, 282–287. [Google Scholar]
- Bottiger, Y.; Brattstrom, C.; Tyden, G.; Sawe, J.; Groth, C.G. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br. J. Clin. Pharmacol. 1999, 48, 445–448. [Google Scholar] [CrossRef]
- Schiff, J.; Cole, E.; Cantarovich, M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin. J. Am. Soc. Nephrol. 2007, 2, 374–384. [Google Scholar] [CrossRef]
- Undre, N.A.; van Hooff, J.; Christiaans, M.; Vanrenterghem, Y.; Donck, J.; Heeman, U.; Kohnle, M.; Zanker, B.; Land, W.; Morales, J.M.; et al. Stevenson, P. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant. Proc. 1999, 31, 296–298. [Google Scholar] [CrossRef]
- Willicombe, M.; Rizzello, A.; Goodall, D.; Papalois, V.; McLean, A.G.; Taube, D. Risk factors and outcomes of delayed graft function in renal transplant recipients receiving a steroid sparing immunosuppression protocol. World J. Transplant. 2017, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Irish, W.D.; Ilsley, J.N.; Schnitzler, M.A.; Feng, S.; Brennan, D.C. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am. J. Transplant. 2010, 10, 2279–2286. [Google Scholar] [CrossRef]
- Sundberg, A.G.; Nilsson, R.; Appelkvist, E.L.; Dallner, G. Immunohistochemical Localization of Alpha-Class and Pi-Class Glutathione Transferases in Normal Human Tissues. Pharmacol. Toxicol. 1993, 72, 321–331. [Google Scholar] [CrossRef]
- Seabra, V.F.; Perianayagam, M.C.; Tighiouart, H.; Liangos, O.; dos Santos, O.F.; Jaber, B.L. Urinary alpha-GST and pi-GST for prediction of dialysis requirement or in-hospital death in established acute kidney injury. Biomarkers 2011, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.E.; Bhangoo, R.S.; Reese, P.P.; Doshi, M.D.; Weng, F.L.; Hong, K.; Lin, H.; Han, G.; Hasz, R.D.; Goldstein, M.J.; et al. Glutathione S-transferase iso-enzymes in perfusate from pumped kidneys are associated with delayed graft function. Am. J. Transplant. 2014, 14, 886–896. [Google Scholar] [CrossRef]

| Deceased Donor Grafts | Living Donor Grafts | p Value | |
|---|---|---|---|
| Donor | 30 | 50 | |
| Age (years) | 58 ± 15 | 53 ± 10 | n.s. |
| Sex (male/female) | 11/19 | 22/28 | n.s. |
| BMI | 27.3 ± 6.5 | 25.3 ± 3.2 | n.s. |
| eGFR, d0 (mL/min) | 96 ± 39 | 97 ± 18 | n.s. |
| Diuresis in last hour (mL) | 152 ± 81 | - | |
| Recipient | 30 | 50 | |
| Age (years) | 58 ± 12 | 47 ± 15 | <0.05 |
| Sex (male/female) | 22/8 | 35/15 | n.s. |
| BMI | 26.4 ± 4 | 25.8 ± 4.9 | n.s. |
| eGFR, d0 (mL/min) | 9 ± 3 | 8 ± 4 | n.s. |
| Primary disease | |||
| Glomerulonephritis | 9 | 24 | n.s. |
| Hypertensive nephrosclerosis | 7 | 9 | n.s. |
| Polycystic kidney disease | 6 | 2 | n.s. |
| Autoimmune disease | 2 | 5 | n.s. |
| Diabetes | 2 | 3 | n.s. |
| Urologic disease | 2 | 2 | n.s. |
| Calcineurin-induced nephrotoxicity | 0 | 2 | n.s. |
| Others | 2 | 3 | n.s. |
| Dialysis | |||
| Hemodialysis | 29 | 28 | n.s. |
| Peritoneal dialysis | 1 | 3 | n.s. |
| No dialysis | 0 | 19 | <0.05 |
| Cold ischemia time (minutes) | 613 ± 269 | 193 ± 62 | <0.05 |
| Warm ischemia time (minutes) | 34 ± 11 | 23 ± 7 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katou, S.; Globke, B.; Morgul, M.H.; Vogel, T.; Struecker, B.; Otto, N.M.; Reutzel-Selke, A.; Marksteiner, M.; Brockmann, J.G.; Pascher, A.; et al. Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts. J. Clin. Med. 2019, 8, 1899. https://doi.org/10.3390/jcm8111899
Katou S, Globke B, Morgul MH, Vogel T, Struecker B, Otto NM, Reutzel-Selke A, Marksteiner M, Brockmann JG, Pascher A, et al. Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts. Journal of Clinical Medicine. 2019; 8(11):1899. https://doi.org/10.3390/jcm8111899
Chicago/Turabian StyleKatou, Shadi, Brigitta Globke, M. Haluk Morgul, Thomas Vogel, Benjamin Struecker, Natalie Maureen Otto, Anja Reutzel-Selke, Marion Marksteiner, Jens G. Brockmann, Andreas Pascher, and et al. 2019. "Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts" Journal of Clinical Medicine 8, no. 11: 1899. https://doi.org/10.3390/jcm8111899
APA StyleKatou, S., Globke, B., Morgul, M. H., Vogel, T., Struecker, B., Otto, N. M., Reutzel-Selke, A., Marksteiner, M., Brockmann, J. G., Pascher, A., & Schmitz, V. (2019). Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts. Journal of Clinical Medicine, 8(11), 1899. https://doi.org/10.3390/jcm8111899





