Involution of Breast Lobules, Mammographic Breast Density and Prognosis Among Tamoxifen-Treated Estrogen Receptor-Positive Breast Cancer Patients
Abstract
1. Introduction
2. Methods
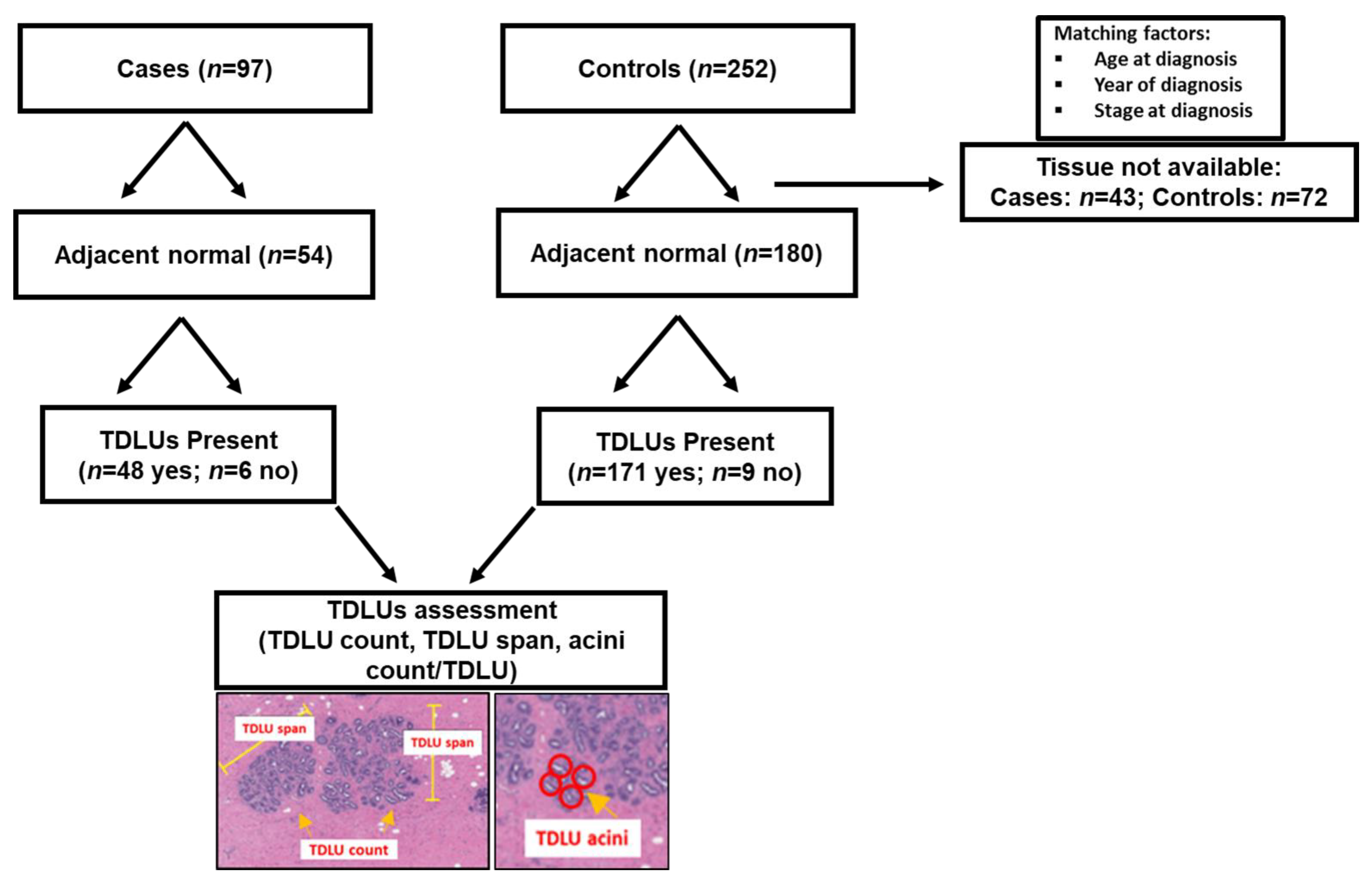
2.1. Study Population
2.2. Breast Tissue Collection
2.3. TDLU Histologic Assessment
2.4. Mammographic Breast Density (MD) Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Relationships between Participant Characteristics and TDLU Measurements
3.3. Association between MD and Measures of TDLU Involution
3.4. Influence of TDLU Measurements on the Relationship between MD Decline and Reduced Risk of Breast Cancer-Specific Death among ER-positive Patients Treated with Tamoxifen
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Abbreviations
| BMI | Body mass index |
| ER | Estrogen receptor |
| H&E | Hematoxylin and eosin |
| KPNW | Kaiser Permanente Northwest |
| MD | Mammographic breast density |
| OR | Odds ratio |
| TDLU | Terminal duct lobular unit |
| SERM | Selective estrogen receptor modulator |
References
- Rosenberg, P.S.; Barker, K.A.; Anderson, W.F. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl. Cancer Inst. 2015, 107, 9. [Google Scholar] [CrossRef] [PubMed]
- Bodai, B.I.; Tuso, P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 2015, 19, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.W.; Chew, G.L.; Britt, K.L.; Ingman, W.V.; Henderson, M.A.; Hopper, J.L.; Thompson, E.W. Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat 2014, 144, 479–502. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Silva, I.D.S. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Mullooly, M.; Pfeiffer, R.M.; Nyante, S.J.; Heckman-Stoddard, B.M.; Perloff, M.; Jatoi, I.; Brinton, L.A.; Aiello Bowles, E.J.; Hoover, R.N.; Glass, A.; et al. Mammographic density as a biosensor of tamoxifen effectiveness in adjuvant endocrine treatment of breast cancer: Opportunities and implications. J. Clin. Oncol. 2016, 34, 2093–2097. [Google Scholar] [CrossRef]
- Nyante, S.J.; Sherman, M.E.; Pfeiffer, R.M.; Berrington de Gonzalez, A.; Brinton, L.A.; Aiello Bowles, E.J.; Hoover, R.N.; Glass, A.; Gierach, G.L. Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J. Natl. Cancer Inst. 2015, 107, 3. [Google Scholar] [CrossRef]
- Li, J.; Humphreys, K.; Eriksson, L.; Edgren, G.; Czene, K.; Hall, P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J. Clin. Oncol. 2013, 31, 2249–2256. [Google Scholar] [CrossRef]
- Mullooly, M.; Gierach, G.L. The potential for mammographic breast density change as a biosensor of adjuvant tamoxifen therapy adherence and response. JNCI Cancer Spectr. 2018, 2, pky072. [Google Scholar] [CrossRef]
- Clarke, R.; Tyson, J.J.; Dixon, J.M. Endocrine resistance in breast cancer—An overview and update. Mol. Cell. Endocrinol. 2015, 418 Pt 3, 220–234. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef]
- Chew, G.L.; Huo, C.W.; Huang, D.; Blick, T.; Hill, P.; Cawson, J.; Frazer, H.; Southey, M.C.; Hopper, J.L.; Britt, K.; et al. Effects of tamoxifen and oestrogen on histology and radiographic density in high and low mammographic density human breast tissues maintained in murine tissue engineering chambers. Breast Cancer Res. Treat. 2014, 148, 303–314. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.N.; Thompson, H.J. Quantitative assessment of mammary gland density in rodents using digital image analysis. Biol. Proced. Online 2011, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. Development of the human breast. Maturitas 2004, 49, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Pfeiffer, R.M.; Patel, D.A.; Linville, L.; Brinton, L.A.; Gierach, G.L.; Yang, X.R.; Papathomas, D.; Visscher, D.; Mies, C.; et al. Terminal duct lobular unit involution of the normal breast: Implications for breast cancer etiology. J. Natl. Cancer Inst. 2014, 106, 10. [Google Scholar] [CrossRef] [PubMed]
- Baer, H.J.; Collins, L.C.; Connolly, J.L.; Colditz, G.A.; Schnitt, S.J.; Tamimi, R.M. Lobule type and subsequent breast cancer risk: Results from the Nurses’ Health Studies. Cancer 2009, 115, 1404–1411. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, S.; Hillman, D.W.; et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef]
- Milanese, T.R.; Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Degnim, A.C.; Vachon, C.M.; Reynolds, C.A.; et al. Age-Related lobular involution and risk of breast cancer. J. Natl. Cancer. Inst. 2006, 98, 1600–1607. [Google Scholar] [CrossRef]
- McKian, K.P.; Reynolds, C.A.; Visscher, D.W.; Nassar, A.; Radisky, D.C.; Vierkant, R.A.; Degnim, A.C.; Boughey, J.C.; Ghosh, K.; Anderson, S.S.; et al. Novel breast tissue feature strongly associated with risk of breast cancer. J. Clin. Oncol. 2009, 27, 5893–5898. [Google Scholar] [CrossRef]
- Radisky, D.C.; Visscher, D.W.; Frank, R.D.; Vierkant, R.A.; Winham, S.; Stallings-Mann, M.; Hoskin, T.L.; Nassar, A.; Vachon, C.M.; Denison, L.A.; et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res. Treat. 2016, 155, 423–430. [Google Scholar] [CrossRef]
- Cuzick, J.; Warwick, J.; Pinney, E.; Duffy, S.W.; Cawthorn, S.; Howell, A.; Forbes, J.F.; Warren, R.M. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J. Natl. Cancer Inst. 2011, 103, 744–752. [Google Scholar] [CrossRef]
- Gierach, G.L.; Patel, D.A.; Pfeiffer, R.M.; Figueroa, J.D.; Linville, L.; Papathomas, D.; Johnson, J.M.; Chicoine, R.E.; Herschorn, S.D.; Shepherd, J.A.; et al. Relationship of terminal duct lobular unit involution of the breast with area and volume mammographic densities. Cancer Prev. Res. (Phila.) 2016, 9, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Hartmann, L.C.; Reynolds, C.; Visscher, D.W.; Brandt, K.R.; Vierkant, R.A.; Scott, C.G.; Radisky, D.C.; Sellers, T.A.; Pankratz, V.S.; et al. Association between mammographic density and age-related lobular involution of the breast. J. Clin. Oncol. 2010, 28, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Visscher, D.W.; Frost, M.H.; Hartmann, L.C.; Frank, R.D.; Vierkant, R.A.; McCullough, A.E.; Winham, S.J.; Vachon, C.M.; Ghosh, K.; Brandt, K.R.; et al. Clinicopathologic features of breast cancers that develop in women with previous benign breast disease. Cancer 2016, 122, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Figueroa, J.D.; Falk, R.T.; Zhang, H.; Pfeiffer, R.M.; Hewitt, S.M.; Lissowska, J.; Peplonska, B.; Brinton, L.; Garcia-Closas, M.; et al. Analysis of terminal duct lobular unit involution in luminal A and basal breast cancers. Breast Cancer Res. 2012, 14, R64. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Pfeiffer, R.M.; Brinton, L.A.; Palakal, M.M.; Degnim, A.C.; Radisky, D.; Hartmann, L.C.; Frost, M.H.; Stallings Mann, M.L.; Papathomas, D.; et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: A nested case-Control study. Breast Cancer Res. Treat. 2016, 159, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rosebrock, A.; Caban, J.J.; Figueroa, J.; Gierach, G.; Linville, L.; Hewitt, S.; Sherman, M. Quantitative analysis of TDLUs using adaptive morphological shape techniques. Proc. SPIE Int. Soc. Opt. Eng. 2013, 8676, 8676N. [Google Scholar]
- Byng, J.W.; Boyd, N.F.; Fishell, E.; Jong, R.A.; Yaffe, M.J. The quantitative analysis of mammographic densities. Phys. Med. Biol. 1994, 39, 1629–1638. [Google Scholar] [CrossRef]
- Ginsburg, O.M.; Martin, L.J.; Boyd, N.F. Mammographic density, lobular involution, and risk of breast cancer. Br. J. Cancer 2008, 99, 1369–1374. [Google Scholar] [CrossRef]
- Engmann, N.J.; Scott, C.G.; Jensen, M.R.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A.P.; Malkov, S.; Whaley, D.H.; Hruska, C.B.; Wu, F.F.; et al. Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors. Cancer Epidemiol. Biomark. Prev. 2017, 26, 930–937. [Google Scholar] [CrossRef]
- Guo, C.; Sung, H.; Zheng, S.; Guida, J.; Li, E.; Li, J.; Hu, N.; Deng, J.; Figueroa, J.D.; Sherman, M.E.; et al. Age-related terminal duct lobular unit involution in benign tissues from Chinese breast cancer patients with luminal and triple-negative tumors. Breast Cancer Res. 2017, 19, 61. [Google Scholar] [CrossRef]
- Maskarinec, G.; Ju, D.; Horio, D.; Loo, L.W.; Hernandez, B.Y. Involution of breast tissue and mammographic density. Breast Cancer Res. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Guo, C.; Li, E.; Li, J.; Pfeiffer, R.M.; Guida, J.L.; Cora, R.; Hu, N.; Deng, J.; Figueroa, J.D.; et al. The relationship between terminal duct lobular unit features and mammographic density among Chinese breast cancer patients. Int. J. Cancer 2019, 145, 70–77. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Case Patients | Control Patients | Comparison of Case Patients and Control Patients | ||||
|---|---|---|---|---|---|---|---|
| (n = 234) | (n = 54) | (n = 180) | |||||
| Characteristic | n | % | n | % | n | % | p Value $ |
| Age at diagnosis (years) | 0.20 | ||||||
| <50 | 59 | 25.2 | 14 | 25.9 | 45 | 25.0 | |
| 50–59 | 65 | 27.8 | 10 | 18.5 | 55 | 30.6 | |
| >59 | 110 | 47.0 | 30 | 55.6 | 80 | 44.4 | |
| Race | 0.58* | ||||||
| White | 229 | 97.9 | 175 | 97.2 | 54 | 100.0 | |
| Non-white | 4 | 1.7 | 4 | 2.2 | 0 | 0.0 | |
| Missing | 1 | 0.4 | 1 | 0.6 | 0 | 0.0 | |
| Smoking status at baseline | 0.02 | ||||||
| Never | 128 | 54.7 | 22 | 40.7 | 106 | 58.9 | |
| Ever | 106 | 45.3 | 32 | 59.3 | 74 | 41.1 | |
| BMI at diagnosis (kg/m2) | 0.16 | ||||||
| <25 | 72 | 30.8 | 11 | 20.4 | 61 | 33.9 | |
| 25 to <30 | 64 | 27.4 | 16 | 29.6 | 48 | 26.7 | |
| ≥30 | 67 | 28.6 | 19 | 35.2 | 48 | 26.7 | |
| Missing | 31 | 13.2 | 8 | 14.8 | 23 | 12.8 | |
| Hormone therapy use at baseline | 0.79 | ||||||
| Non-user | 92 | 39.3 | 22 | 40.7 | 70 | 38.9 | |
| Current user | 104 | 44.4 | 22 | 40.7 | 82 | 45.6 | |
| Former user | 38 | 16.2 | 10 | 18.5 | 28 | 15.6 | |
| SEER summary stage at diagnosis | 0.90 | ||||||
| Localized | 98 | 41.9 | 23 | 42.6 | 75 | 41.7 | |
| Regional/distant/unknown | 136 | 58.1 | 31 | 57.4 | 105 | 58.3 | |
| Year of diagnosis | 0.16 | ||||||
| 1990–1996 | 93 | 39.7 | 23 | 42.6 | 70 | 38.9 | |
| 1997–2000 | 79 | 33.8 | 22 | 40.7 | 57 | 31.7 | |
| 2001–2008 | 62 | 26.5 | 9 | 16.7 | 53 | 29.4 | |
| Progesterone receptor | 0.02 | ||||||
| Negative | 48 | 20.5 | 17 | 31.5 | 31 | 17.2 | |
| Positive | 185 | 79.1 | 37 | 68.5 | 148 | 82.2 | |
| Missing | 1 | 0.4 | 0 | 0.0 | 1 | 0.6 | |
| Tumor size (mm) | 0.08 | ||||||
| ≤2 | 141 | 60.3 | 26 | 48.1 | 115 | 63.9 | |
| >2 | 84 | 35.9 | 24 | 44.4 | 60 | 33.3 | |
| Missing | 9 | 3.8 | 4 | 7.4 | 5 | 2.8 | |
| Tumor differentiation | <0.01 * | ||||||
| Well differentiated | 44 | 18.8 | 1 | 1.9 | 43 | 23.9 | |
| Moderately differentiated | 123 | 52.6 | 32 | 59.3 | 91 | 50.6 | |
| Poorly differentiated | 54 | 23.1 | 17 | 31.5 | 37 | 20.6 | |
| Missing | 13 | 5.6 | 4 | 7.4 | 9 | 5.0 | |
| Duration of tamoxifen use (months) | |||||||
| Mean (SD) | 50.4 (19.9) | 42.6 (20.3) | 52.7 (19.3) | <0.01 # | |||
| Case patients (n = 54) | Control patients (n = 180) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDLU Count/100 mm2 | TDLU Span& (Microns) | Acini Count/TDLU& | TDLU Count/100 mm2 | TDLU Span& (Microns) | Acini Count/TDLU& | |||||||||||||
| Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | Median | IQR | p Value | |
| All patients | 5.9 | 1.3–10.0 | 416 | 284.0–665.5 | 21 | 14.0–42.3 | 7.0 | 2.7–16.3 | 465 | 312.5–631.5 | 24 | 11.5–46.5 | ||||||
| Age at diagnosis (years) | <0.01 | 0.01 | 0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||
| <50 | 14.7 | 8.7–27.8 | 677 | 409.8–928.5 | 43 | 30.0–65.0 | 11.4 | 5.5–21.7 | 594 | 477.0–768.5 | 40 | 24.3–56.8 | ||||||
| 50–59 | 7.5 | 3.6–22.9 | 406 | 331.5–606.5 | 23 | 12.5–49.0 | 12.5 | 4.6–25.5 | 487 | 368.0–655.0 | 28 | 15.0–51.0 | ||||||
| >59 | 3.5 | 1.0–7.5 | 348 | 245.0–465.0 | 18 | 12.0–22.5 | 3.9 | 2.0–9.1 | 342 | 271.0–496.0 | 14 | 8.5–25.5 | ||||||
| Race | 0.03 | 0.09 | 0.09 | |||||||||||||||
| White | 5.9 | 1.3–10.0 | − | 416 | 284.0–665.5 | − | 21 | 14.0–42.3 | − | 6.8 | 2.7–15.0 | 453 | 307.0–628.5 | 24 | 11.5–46.0 | |||
| Non-white | 0.0 | − | 0 | − | 0 | − | 27.8 | 13.9–44.0 | 650 | 515.8–929.3 | 50 | 33.5–61.8 | ||||||
| Smoking status at baseline | 0.16 | 0.61 | 0.51 | 0.18 | 0.06 | 0.14 | ||||||||||||
| Never | 3.8 | 1.0–9.5 | 399 | 269.5–606.5 | 19 | 12.0–40.0 | 8.3 | 2.7–18.6 | 512 | 328.5–687.3 | 25 | 11.5–53.5 | ||||||
| Ever | 8.3 | 2.5–10.6 | 421 | 331.5–671.0 | 22 | 14.5–43.5 | 6.2 | 2.8–11.3 | 425 | 299.5–575.5 | 20 | 10.5–40.5 | ||||||
| Missing | ||||||||||||||||||
| BMI at diagnosis (kg/m2) | 0.57 | 0.27 | 0.19 | 0.04 | 0.72 | 0.33 | ||||||||||||
| <25 | 7.1 | 2.3–11.1 | 380 | 227.5–448.0 | 16 | 8.0–27.0 | 8.0 | 4.6–19.5 | 518 | 307.0–675.5 | 25 | 14.0–53.5 | ||||||
| 25 to <30 | 4.7 | 1.4–9.2 | 353 | 245.0–494.5 | 16 | 12.0–21.0 | 7.4 | 2.8–15.6 | 422 | 298.0–614.5 | 20 | 10.5–51.0 | ||||||
| ≥30 | 3.9 | 0.9–9.9 | 432 | 308.8–648.3 | 23 | 16.0–44.8 | 6.1 | 2.1–10.6 | 477 | 323.0–655.0 | 28 | 11.5–40.5 | ||||||
| Missing | ||||||||||||||||||
| Hormone therapy use at baseline | 0.21 | 0.88 | 0.76 | 0.70 | 0.19 | 0.34 | ||||||||||||
| Non-user | 3.3 | 0.6–9.5 | 399 | 239.0–729.0 | 22 | 8.0–40.0 | 7.6 | 3.1–18.6 | 474 | 334.0–675.5 | 25 | 11.5–51.5 | ||||||
| Current user | 6.7 | 3.6–10.0 | 418 | 341.5–606.5 | 20 | 16.0–41.0 | 7.0 | 2.6–15.0 | 477 | 317.0–640.5 | 24 | 13.5–50.0 | ||||||
| Former user | 9.2 | 1.3–21.8 | 450 | 286.0–671.0 | 31 | 14.0–53.0 | 5.3 | 2.4–18.5 | 358 | 271.0–605.5 | 15 | 10.5–34.0 | ||||||
| SEER summary stage at diagnosis | 0.97 | 0.75 | 0.77 | 0.16 | 0.05 | 0.03 | ||||||||||||
| Localized | 5.8 | 0.9–21.8 | 380 | 237.3–686.0 | 20 | 11.0–40.5 | 5.6 | 2.5–14.8 | 440 | 284.8–614.3 | 18 | 10.5–45.5 | ||||||
| Regional/distant/unknown | 6.0 | 1.5–9.7 | 422 | 325.3–610.0 | 21 | 14.0–44.8 | 8.4 | 3.2–17.4 | 487 | 344.5–658.5 | 28 | 14.5–51.0 | ||||||
| Year of Diagnosis | 0.67 | 0.21 | 0.40 | 0.62 | 0.09 | 0.25 | ||||||||||||
| 1990–1996 | 5.8 | 1.6–22.9 | 495 | 348.0–688.0 | 23 | 16.0–63.5 | 6.9 | 3.1–18.6 | 425 | 297.0–587.0 | 20 | 11.3–39.3 | ||||||
| 1997–2000 | 5.1 | 1.0–9.7 | 399 | 245.0–606.5 | 19 | 12.0–36.0 | 6.5 | 2.5–14.7 | 477 | 334.0–655.0 | 24 | 13.5–51.5 | ||||||
| 2001–2008 | 7.1 | 1.0–10.0 | 320 | 275.8–666.3 | 17 | 13.3–29.3 | 7.7 | 4.2–16.3 | 552 | 349.0–683.0 | 35 | 13.0–46.0 | ||||||
| Progesterone Receptor | 0.50 | 0.76 | 0.92 | 0.64 | 0.91 | 0.91 | ||||||||||||
| Negative | 5.4 | 1.3–9.7 | 424 | 331.5–497.0 | 19 | 14.5–27.5 | 8.2 | 3.4–24.1 | 468 | 302.0–640.50 | 26 | 15.0–41.5 | ||||||
| Positive | 7.1 | 1.6–11.1 | 411 | 269.5–684.0 | 22 | 12.0–49.0 | 7.0 | 2.7–14.9 | 466 | 318.0–627.3 | 24 | 11.5–50.5 | ||||||
| Missing | ||||||||||||||||||
| Tumor Size (cm) | 0.86 | 0.21 | 0.24 | 0.61 | 0.07 | 0.06 | ||||||||||||
| ≤2 | 6.7 | 2.7–21.2 | 421 | 282.0–683.0 | 19 | 14.5–46.0 | 6.7 | 3.1–14.8 | 449 | 298.0–615.5 | 23 | 11.0–45.0 | ||||||
| >2 | 4.7 | 0.7–9.6 | 399 | 269.5–671.0 | 22 | 12.0–41.0 | 8.3 | 2.6–18.7 | 542 | 346.0–655.0 | 29 | 17.0–51.5 | ||||||
| Missing | ||||||||||||||||||
| Tumor differentiation | 0.22 | 0.55 | 0.55 | 0.56 | 0.58 | 0.80 | ||||||||||||
| Well differentiated | 0.0 | − | 0 | − | 0 | − | 8.0 | 4.6–21.4 | 446 | 304.8–596.3 | 23 | 12.8–43.3 | ||||||
| Moderately differentiated | 7.3 | 3.1–14.6 | 399 | 245.0–671.0 | 19 | 12.0–46.0 | 7.3 | 2.5–15.0 | 518 | 303.3–676.8 | 25 | 11.5–51.5 | ||||||
| Poorly differentiated | 3.6 | 1.0–10.0 | 436 | 320.0–648.8 | 22 | 15.3–42.3 | 6.1 | 2.8–17.4 | 431 | 334.0–589.0 | 24 | 14.0–39.5 | ||||||
| Missing | ||||||||||||||||||
| Duration of tamoxifen use, mo | 0.24 | 0.52 | 0.17 | 0.46 | 0.06 | 0.16 | ||||||||||||
| ≤52 | 5.1 | 1.0–21.2 | 411 | 2860–729.0 | 23 | 16.0–49.0 | 9.4 | 3.2-19.9 | 558 | 396.0–745.0 | 29 | 16.0–54.5 | ||||||
| 53 to 61 | 1.5 | 1.0–4.2 | 354 | 245.0–448.0 | 15 | 9.5–21.5 | 7.3 | 2.9–13.3 | 429 | 304.0–606.5 | 24 | 11.5–45.5 | ||||||
| >61 | 8.7 | 6.0–9.7 | 458 | 319.0–513.5 | 20 | 12.0–31.0 | 5.2 | 2.5–18.8 | 465 | 297.0–614.5 | 20 | 11.5–41.0 | ||||||
| TDLU measure | Baseline Density (%) | Baseline Dense Area (cm2) | Baseline Non-Dense Area (cm2) | Follow-Up Density (%) | Breast Density Change (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | |
| Case patients | |||||||||||
| TDLU count/100 mm2 | |||||||||||
| 0 | 6 | 26.0 | (18.8, 33.2) | 43.2 | (30.5, 56.0) | 121.1 | (94.6, 147.7) | 17.1 | (11.5, 22.7) | −8.5 | (−15.3, −1.7) |
| 0–≤4.7 | 18 | 17.7 | (14.2, 21.3) | 31.5 | (24.9, 38.0) | 151.8 | (134.0, 169.6) | 18.4 | (14.9, 21.9) | 0.2 | (−3.9, 4.2) |
| >4.7–≤13 | 19 | 26.1 | (21.5, 30.6) | 30.7 | (24.0, 37.5) | 100.8 | (85.6, 116.0) | 22.8 | (18.8, 26.9) | −3.0 | (−7.3, 1.3) |
| >13 | 11 | 32.0 | (23.1, 40.8) | 55.6 | (39.6, 71.6) | 112.2 | (84.0, 140.4) | 26.3 | (18.6, 34.0) | −4.6 | (−12.1, 2.9) |
| p-value for trend | 0.15 | 0.61 | 0.11 | 0.07 | 0.75 | ||||||
| Median TDLU span (microns) | |||||||||||
| 0–≤352 | 18 | 17.8 | (14.4, 21.2) | 26.5 | (21.2, 31.7) | 133.7 | (114.6, 152.9) | 19.3 | (15.8, 22.7) | 1.5 | (−2.4, 5.4) |
| >352–≤578 | 15 | 26.3 | (22.0, 30.6) | 35.4 | (29.1, 41.8) | 109.3 | (91.1, 127.5) | 23.1 | (19.1, 27.1) | −3.6 | (−7.7, 0.5) |
| >578 | 15 | 33.6 | (26.7, 40.5) | 57.1 | (45.7, 68.6) | 114.1 | (87.9, 140.3) | 25.8 | (19.9, 31.8) | −6.5 | (−12.3, -0.7) |
| p-value for trend | <0.01 | <0.01 | 0.31 | 0.19 | 0.02 | ||||||
| Median acini count per TDLU | |||||||||||
| 0–≤15 | 15 | 20.5 | (16.2, 24.8) | 27.4 | (21.3, 33.5) | 121.6 | (100.8, 142.4) | 20.2 | (16.2, 24.2) | −0.5 | (−5.0, 4.1) |
| >15–≤36.5 | 19 | 22.7 | (18.6, 26.7) | 33.3 | (27.3, 39.2) | 122.0 | (103.4, 140.6) | 21.8 | (18.1, 25.5) | −1.3 | (−5.4, 2.8) |
| >36.5 | 14 | 33.1 | (25.7, 40.5) | 57.2 | (45.4, 69.0) | 116.2 | (88.9, 143.6) | 25.7 | (19.7, 31.8) | −6.1 | (−12.3, 0.0) |
| p-value for trend | 0.08 | <0.01 | 0.85 | 0.35 | 0.21 | ||||||
| Control patients | |||||||||||
| TDLU count/100 mm2 | |||||||||||
| 0 | 9 | 26.7 | (19.7, 33.6) | 30.8 | (20.6, 41.1) | 103.7 | (80.9, 126.5) | 22.5 | (16.4, 28.6) | −6.2 | (−12.6, 0.2) |
| 0–≤4.7 | 57 | 23.9 | (21.1, 26.7) | 33.5 | (28.9, 38.0) | 110.8 | (100.8, 120.9) | 19.6 | (17.2, 22.0) | −5.2 | (−7.9, −2.5) |
| >4.7–≤13 | 57 | 30.5 | (27.5, 33.5) | 40.6 | (35.8, 45.4) | 97.8 | (88.8, 106.8) | 25.9 | (23.3, 28.6) | −4.5 | (−7.1, −1.9) |
| >13 | 57 | 31.5 | (28.3, 34.8) | 39.5 | (34.4, 44.5) | 84.9 | (75.9, 93.9) | 22.4 | (19.8, 25.1) | −9.4 | (−12.2, −6.6) |
| p-value for trend | 0.03 | 0.15 | 0.02 | 0.36 | 0.09 | ||||||
| Median TDLU span (microns) | |||||||||||
| 0–≤352 | 57 | 25.7 | (22.6, 28.8) | 34.3 | (29.4, 39.3) | 105.5 | (95.5, 115.5) | 20.6 | (17.9, 23.3) | −6.0 | (−8.9, −3.1) |
| >352–≤578 | 57 | 28.3 | (25.4, 31.4) | 37.3 | (32.5, 42.1) | 94.2 | (85.4, 102.9) | 23.5 | (20.8, 26.1) | −4.6 | (−7.3, −1.9) |
| >578 | 57 | 32.8 | (29.6, 35.9) | 41.6 | (36.6, 46.5) | 87.7 | (79.4, 96.0) | 24.7 | (22.0, 27.3) | −8.5 | (−11.2, −5.9) |
| p-value for trend | 0.03 | 0.17 | 0.07 | 0.17 | 0.18 | ||||||
| Median acini count per TDLU | |||||||||||
| 0–≤15 | 57 | 24.1 | (21.3, 27.0) | 32.8 | (28.2, 37.4) | 109.7 | (100.0, 119.4) | 20.2 | (17.6, 22.7) | −4.8 | (−7.6, −2.0) |
| >15–≤36.5 | 56 | 29.1 | (26.1, 32.1) | 38.1 | (33.3, 42.9) | 93.7 | (85.2, 102.3) | 23.3 | (20.7, 25.9) | −5.9 | (−8.6, 3.2) |
| >36.5 | 57 | 34.1 | (30.8, 37.4) | 42.7 | (37.6, 47.9) | 83.8 | (75.6, 92.1) | 25.5 | (22.7, 28.3) | −8.6 | (−11.4, −5.9) |
| p-value for trend | <0.01 | 0.06 | <0.01 | 0.06 | 0.07 | ||||||
| OR (95% CI) | |
|---|---|
| Base model $ | 0.41 (0.18, 0.92) |
| Base model adjusted for TDLU count/mm2 | 0.41 (0.18, 0.94) |
| Base model adjusted for TDLU span & | 0.34 (0.14, 0.84) |
| Base model adjusted for acini count/TDLU & | 0.33 (0.13, 0.81) |
| Base model adjusted for TDLU count, span and acini/TDLU | 0.33 (0.13, 0.81) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullooly, M.; Nyante, S.J.; Pfeiffer, R.M.; Cora, R.; Butcher, D.; Sternberg, L.; Aiello Bowles, E.J.; Fan, S.; Figueroa, J.D.; Weinmann, S.; et al. Involution of Breast Lobules, Mammographic Breast Density and Prognosis Among Tamoxifen-Treated Estrogen Receptor-Positive Breast Cancer Patients. J. Clin. Med. 2019, 8, 1868. https://doi.org/10.3390/jcm8111868
Mullooly M, Nyante SJ, Pfeiffer RM, Cora R, Butcher D, Sternberg L, Aiello Bowles EJ, Fan S, Figueroa JD, Weinmann S, et al. Involution of Breast Lobules, Mammographic Breast Density and Prognosis Among Tamoxifen-Treated Estrogen Receptor-Positive Breast Cancer Patients. Journal of Clinical Medicine. 2019; 8(11):1868. https://doi.org/10.3390/jcm8111868
Chicago/Turabian StyleMullooly, Maeve, Sarah J. Nyante, Ruth M. Pfeiffer, Renata Cora, Donna Butcher, Lawrence Sternberg, Erin J. Aiello Bowles, Shaoqi Fan, Jonine D. Figueroa, Sheila Weinmann, and et al. 2019. "Involution of Breast Lobules, Mammographic Breast Density and Prognosis Among Tamoxifen-Treated Estrogen Receptor-Positive Breast Cancer Patients" Journal of Clinical Medicine 8, no. 11: 1868. https://doi.org/10.3390/jcm8111868
APA StyleMullooly, M., Nyante, S. J., Pfeiffer, R. M., Cora, R., Butcher, D., Sternberg, L., Aiello Bowles, E. J., Fan, S., Figueroa, J. D., Weinmann, S., Hoover, R. N., Brinton, L. A., Berrington de Gonzalez, A., Glass, A., Sherman, M. E., & Gierach, G. L. (2019). Involution of Breast Lobules, Mammographic Breast Density and Prognosis Among Tamoxifen-Treated Estrogen Receptor-Positive Breast Cancer Patients. Journal of Clinical Medicine, 8(11), 1868. https://doi.org/10.3390/jcm8111868





