Progressive Pulmonary Artery Dilatation is Associated with Type B Aortic Dissection in Patients with Marfan Syndrome
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Magnetic Resonance Imaging
2.3. Measurements
2.4. Statistics
3. Results
4. Discussion
Key messages
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Elefteriades, J.A. Natural history of thoracic aortic aneurysms: Indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg. 2002, 74, S1877–S1880. [Google Scholar] [CrossRef]
- Trimarchi, S.; Nienaber, C.A.; Rampoldi, V.; Myrmel, T.; Suzuki, T.; Bossone, E.; Tolva, V.; Deeb, M.G.; Upchurch, G.R.; Cooper, J.V.; et al. Role and results of surgery in acute type B aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006, 114, I357–I364. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Fattori, R.; Trimarchi, S.; Isselbacher, E.; Myrmel, T.; Evangelista, A.; Hutchison, S.; Sechtem, U.; Cooper, J.V.; Smith, D.E.; et al. Long-term survival in patients presenting with type B acute aortic dissection: Insights from the International Registry of Acute Aortic Dissection. Circulation 2006, 114, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Schleich, J.M. Images in cardiology. Development of the human heart: Days 15–21. Heart 2002, 87, 487. [Google Scholar] [CrossRef] [PubMed]
- Van Kimmenade, R.R.; Kempers, M.; de Boer, M.J.; Loeys, B.L.; Timmermans, J. A clinical appraisal of different Z-score equations for aortic root assessment in the diagnostic evaluation of Marfan syndrome. Genet. Med. 2013, 15, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; de Paepe, A.; Danks, D.; Finidori, G.; Gedde-Dahl, T.; Goodman, R.; Hall, J.G.; Hollister, D.W.; Horton, W.; McKusick, V.A.; et al. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am. J. Med. Genet. 1988, 29, 581–594. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Devereux, R.B.; Dietz, H.C.; Hennekam, R.C.; Pyeritz, R.E. Revised diagnostic criteria for the Marfan syndrome. Am. J. Med. Genet. 1996, 62, 417–426. [Google Scholar] [CrossRef]
- Groenink, M.; Lohuis, T.A.; Tijssen, J.G.; Naeff, M.S.; Hennekam, R.C.; van der Wall, E.E.; Mulder, B.J. Survival and complication free survival in Marfan’s syndrome: Implications of current guidelines. Heart 1999, 82, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.I.; Burton, K.J.; Gray, J.; Bosner, M.S.; Kouchoukos, N.T.; Roman, M.J.; Boxer, M.; Devereux, R.B.; Tsipouras, P. Life expectancy in the Marfan syndrome. Am. J. Cardiol. 1995, 75, 157–160. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. Corrigendum to: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur. Heart J. 2015, 36, 2779. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, S.; Jonker, F.H.; Hutchison, S.; Isselbacher, E.M.; Pape, L.A.; Patel, H.J.; Froehlich, J.B.; Muhs, B.E.; Rampoldi, V.; Grassi, V.; et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2011, 142, e101–e107. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.S.; Bischoff, M.S.; Lin, H.M.; Oyfe, I.; Lookstein, R.; Griepp, R.B.; Di Luozzo, G. Predicting the risk for acute type B aortic dissection in hypertensive patients using anatomic variables. JACC Cardiovasc. Imaging 2013, 6, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, A.W.; Franken, R.; Zwinderman, A.H.; Timmermans, J.; Scholte, A.J.; van den Berg, M.P.; de Waard, V.; Pals, G.; Mulder, B.J.; Groenink, M. The risk for type B aortic dissection in Marfan syndrome. J. Am. Coll. Cardiol. 2015, 65, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nollen, G.J.; van Schijndel, K.E.; Timmermans, J.; Groenink, M.; Barentsz, J.O.; van der Wall, E.E.; Stoker, J.; Mulder, B.J. Pulmonary artery root dilatation in Marfan syndrome: Quantitative assessment of an unknown criterion. Heart 2002, 87, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, S.; De Backer, J.; Gorgan, N.R.; Rybczynski, M.; Hillebrand, M.; Schuler, H.; Bernhardt, A.M.; Koschyk, D.; Bannas, P.; Keyser, B.; et al. The main pulmonary artery in adults: A controlled multicenter study with assessment of echocardiographic reference values, and the frequency of dilatation and aneurysm in Marfan syndrome. Orphanet. J. Rare Dis. 2014, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Lundby, R.; Rand-Hendriksen, S.; Hald, J.K.; Pripp, A.H.; Smith, H.J. The pulmonary artery in patients with Marfan syndrome: A cross-sectional study. Genet. Med. 2012, 14, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Stark, V.C.; Huemmer, M.; Olfe, J.; Mueller, G.C.; Kozlik-Feldmann, R.; Mir, T.S. The Pulmonary Artery in Pediatric Patients with Marfan Syndrome: An Underestimated Aspect of the Disease. Pediatr. Cardiol. 2018, 39, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
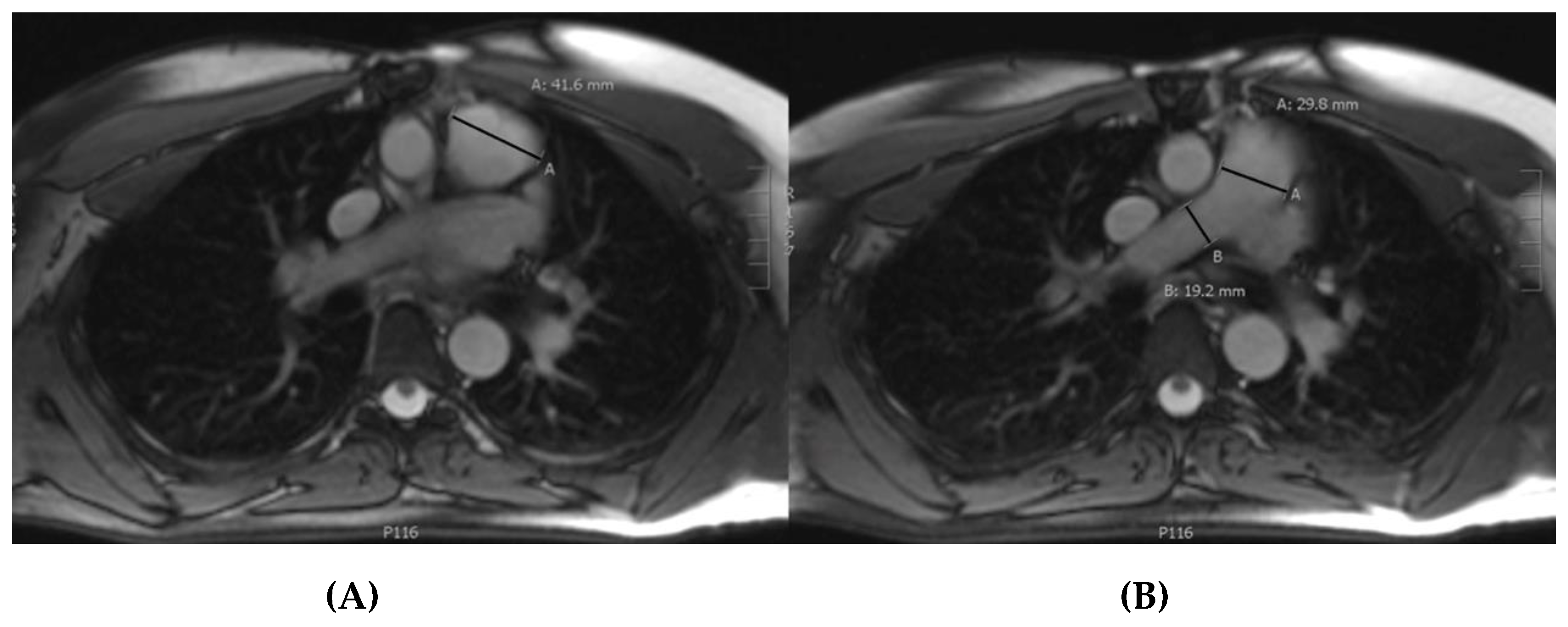
| Whole Population (n = 162) | Type B Dissection (n = 10) | Type A Dissection (n = 14) | Both Type A and B (n = 4) | No Dissection (n = 134) | |
|---|---|---|---|---|---|
| Baseline Characteristics | |||||
| Male, number (%) | 86 (53.1%) | 7 (70%) | 11 (79%) | 1 (25%) | 67 (50.0%) |
| Age (y), mean (SD) | 34.7 (12.8) | 36.6 (12.6) | 42.8 (9.0) | 33.9 (10.2) | 33.8 (13.0) |
| Body weight (kg), mean (SD) | 81.5 (18.2) | 84.1 (14.2) | 91.8 (19.0) | 83.3 (25.4) | 80.1 (18.0) |
| BMI, mean (SD) | 23.3 (5.3) | 22.6 (2.6) | 26.3 (5.8) | 23.2 (7.0) | 23.0 (5.3) |
| Systolic blood pressure (mmHg), mean (SD) | 125.8 (17.2) | 134.7 (23.7) | 132.5(12.1) | 129.5 (20.8) | 124.3 (16.8) |
| Diastolic blood pressure (mmHg), mean (SD) | 75.4 (10.5) | 75.5 (11.6) | 76.9 (9.4) | 74.3 (4.5) | 75.3 (10.7) |
| Time of follow-up (between two MRI’s, years) | 8.6 (2.7) | 10.9 (1.6) | 8.9 (2.9) | 7.5 (1.9) | 8.4 (2.7) |
| Aorta and Pulmonary Artery Diameters | |||||
| Aortic root (mm), mean (SD) | 34.7 (5.4) | 36.7 (4.8) | 31.4 (6.2) | 32.5 (2.9) | 35.0 (5.3) |
| Aorta ascendens (mm), mean (SD) | 28.5 (4.7) | 29.5 (5.6) | 33.2 (5.8) | 26.3 (2.2) | 28.0 (4.3) |
| Aortic arch (mm), mean (SD) | 22.3 (3.5) | 23.1 (1.6) | 27.7 (5.0) | 22.3 (1.5) | 21.6 (2.9) |
| Aorta descendens (mm), mean (SD) | 21.4 (4.8) | 27.3 (8.6) | 26.6 (6.0) | 21.3 (2.1) | 20.4 (3.6) |
| Pulmonary root (mm), mean (SD) | 32.8 (4.5) | 35.3 (4.1) | 35.9 (2.7) | 31.5 (4.7) | 32.4 (4.5) |
| Pulmonary bifurcation (mm), mean (SD) | 25.0 (3.4) | 24.8 (1.9) | 27.7 (1.5) | 25.8 (6.4) | 24.7(3.4) |
| Right pulmonary artery (mm), mean (SD) | 16.6 (3.2) | 17.6 (2.8) | 18.6 (2.9) | 14.5 (2.5) | 16.3 (3.2) |
| Left pulmonary artery (mm), mean (SD) | 17.2 (2.9) | 17.7 (2.4) | 19.8 (3.0) | 18.8 (3.9) | 16.8 (2.8) |
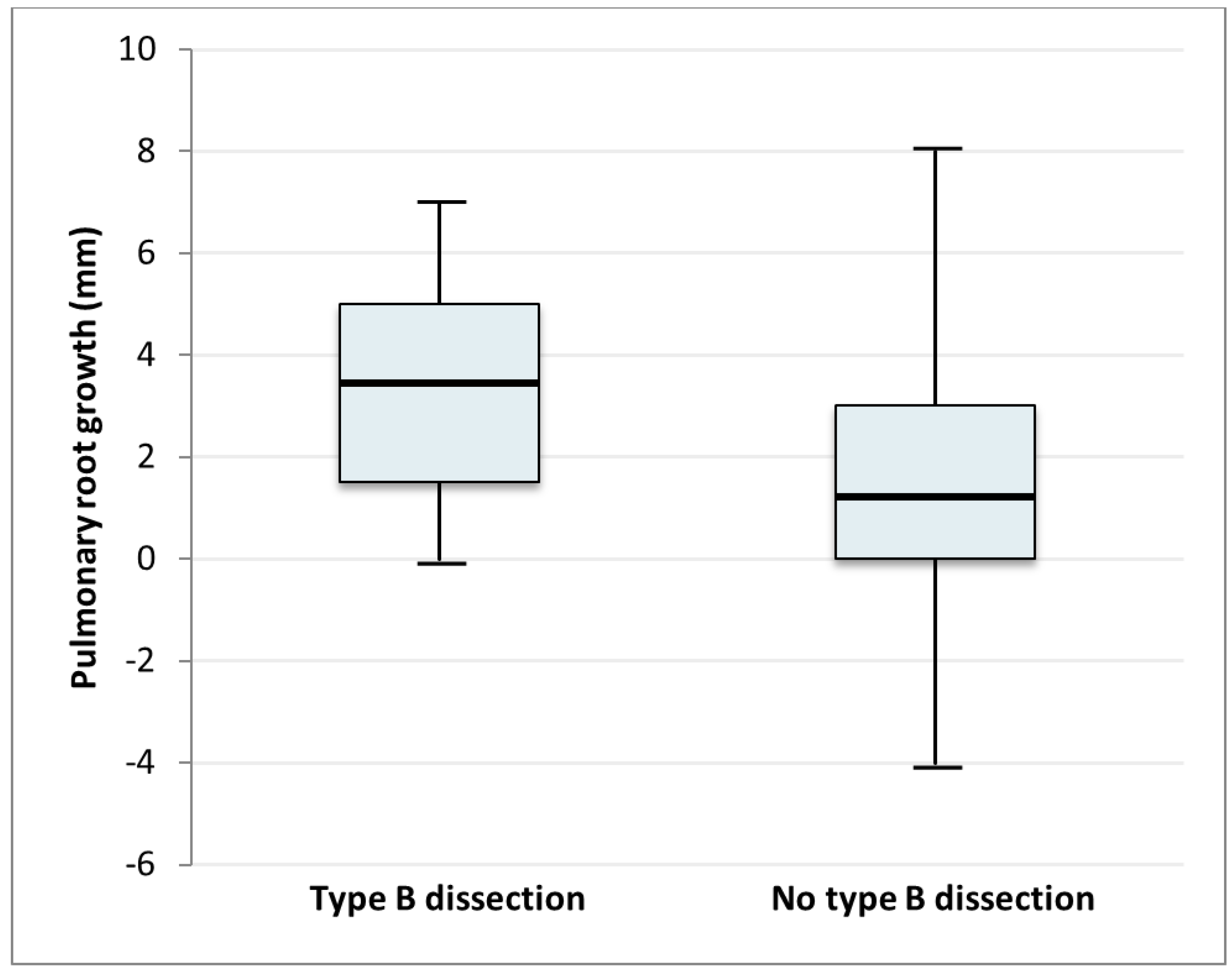
| Pulmonary root dilatation ≥2 mm | 71 (43.8%) | 7 (70.0%) | 7 (50.0%) | 4 (100%) | 53 (39.6%) |
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | ^R2 | OR | 95% CI | p-Value | ^R2 | |
| Type A Dissection | 0.074 | |||||||
| Sex (male) | 1.89 | 0.67; 5.32 | 0.23 | 0.019 | ||||
| Age (years) | 1.04 | 1.003; 1.08 | 0.04 | 0.054 | ||||
| Systolic blood pressure (mmHg) | 1.02 | 1.00; 1.05 | 0.12 | 0.029 | ||||
| Diameter ascending aorta (mm) | 1.15 | 1.04; 1.27 | <0.01 | 0.098 | 1.11 | 1.00; 1.24 | 0.08 | |
| Pulmonary root dilatation (mm) | 1.18 | 0.73; 1.92 | 0.50 | 0.011 | ||||
| Type B Dissection | 0.265 | |||||||
| Sex (male) | 1.20 | 0.40; 3.62 | 0.75 | 0.001 | ||||
| Age (years) | 1.01 | 0.97; 1.05 | 0.74 | 0.002 | ||||
| Systolic blood pressure (mmHg) | 1.03 | 0.10; 1.01 | 0.09 | 0.037 | 1.06 | 1.01; 1.11 | 0.02 | |
| Diameter descending aorta (mm) | 1.14 | 1.05; 1.24 | 0.003 | 0.114 | ||||
| Pulmonary root dilatation (mm) | 1.69 | 1.03; 2.77 | 0.04 | 0.108 | 1.85 | 1.10; 3.12 | 0.02 | |
| Both or Either | 0.345 | |||||||
| Sex (male) | 2.11 | 0.89; 5.00 | 0.09 | 0.031 | ||||
| Age (years) | 1.03 | 1.00; 1.01 | 0.04 | 0.043 | ||||
| Systolic blood pressure (mmHg) | 1.03 | 1.00; 1.051 | 0.02 | 0.056 | 1.04 | 1.00; 1.10 | 0.11 | |
| Diameter ascending aorta (mm) | 1.13 | 1.04; 1.23 | 0.005 | 0.081 | ||||
| Diameter descending aorta (mm) | 1.27 | 1.14; 1.41 | <0.001 | 0.267 | 1.17 | 1.01; 1.37 | 0.04 | |
| Pulmonary root dilatation (mm) | 1.34 | 0.88; 2.04 | 0.17 | 0.039 | 1.50 | 1.00; 2.40 | 0.09 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouwer, C.; Bulut, H.; van Gemert, W.; Staal, A.H.; Cortenbach, K.; Snoeren, M.; Nijveldt, R.; Duijnhouwer, A.; Loeys, B.L.; van Royen, N.; et al. Progressive Pulmonary Artery Dilatation is Associated with Type B Aortic Dissection in Patients with Marfan Syndrome. J. Clin. Med. 2019, 8, 1848. https://doi.org/10.3390/jcm8111848
Brouwer C, Bulut H, van Gemert W, Staal AH, Cortenbach K, Snoeren M, Nijveldt R, Duijnhouwer A, Loeys BL, van Royen N, et al. Progressive Pulmonary Artery Dilatation is Associated with Type B Aortic Dissection in Patients with Marfan Syndrome. Journal of Clinical Medicine. 2019; 8(11):1848. https://doi.org/10.3390/jcm8111848
Chicago/Turabian StyleBrouwer, Christel, Haldun Bulut, Willemijn van Gemert, Alexander HJ Staal, Kim Cortenbach, Miranda Snoeren, Robin Nijveldt, Anthonie Duijnhouwer, Bart L Loeys, Niels van Royen, and et al. 2019. "Progressive Pulmonary Artery Dilatation is Associated with Type B Aortic Dissection in Patients with Marfan Syndrome" Journal of Clinical Medicine 8, no. 11: 1848. https://doi.org/10.3390/jcm8111848
APA StyleBrouwer, C., Bulut, H., van Gemert, W., Staal, A. H., Cortenbach, K., Snoeren, M., Nijveldt, R., Duijnhouwer, A., Loeys, B. L., van Royen, N., Timmermans, J., & van Kimmenade, R. R. (2019). Progressive Pulmonary Artery Dilatation is Associated with Type B Aortic Dissection in Patients with Marfan Syndrome. Journal of Clinical Medicine, 8(11), 1848. https://doi.org/10.3390/jcm8111848





