Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Participants
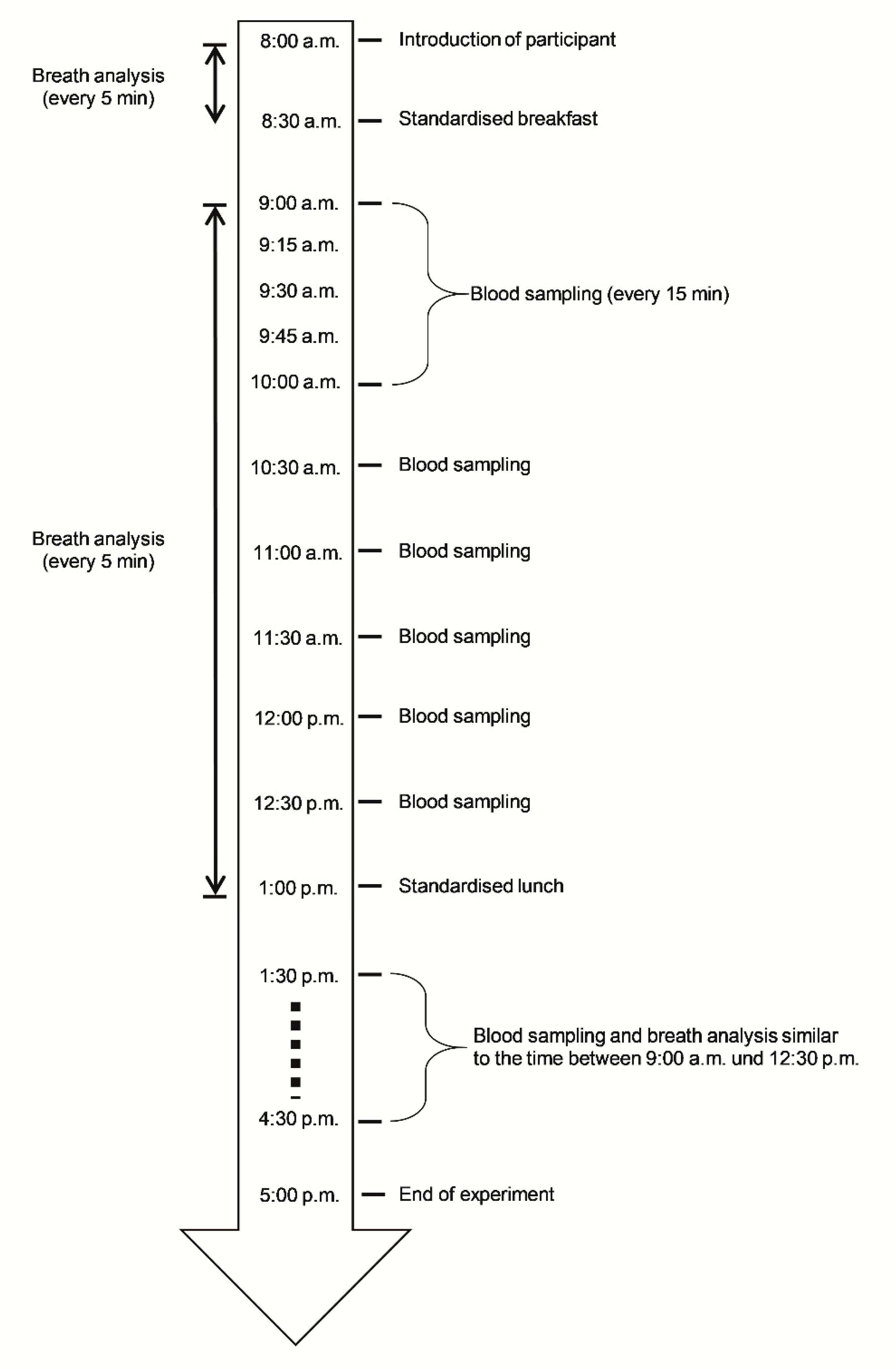
2.2. Procedures
2.3. Breath Analysis
2.4. Statistical Analysis
3. Results
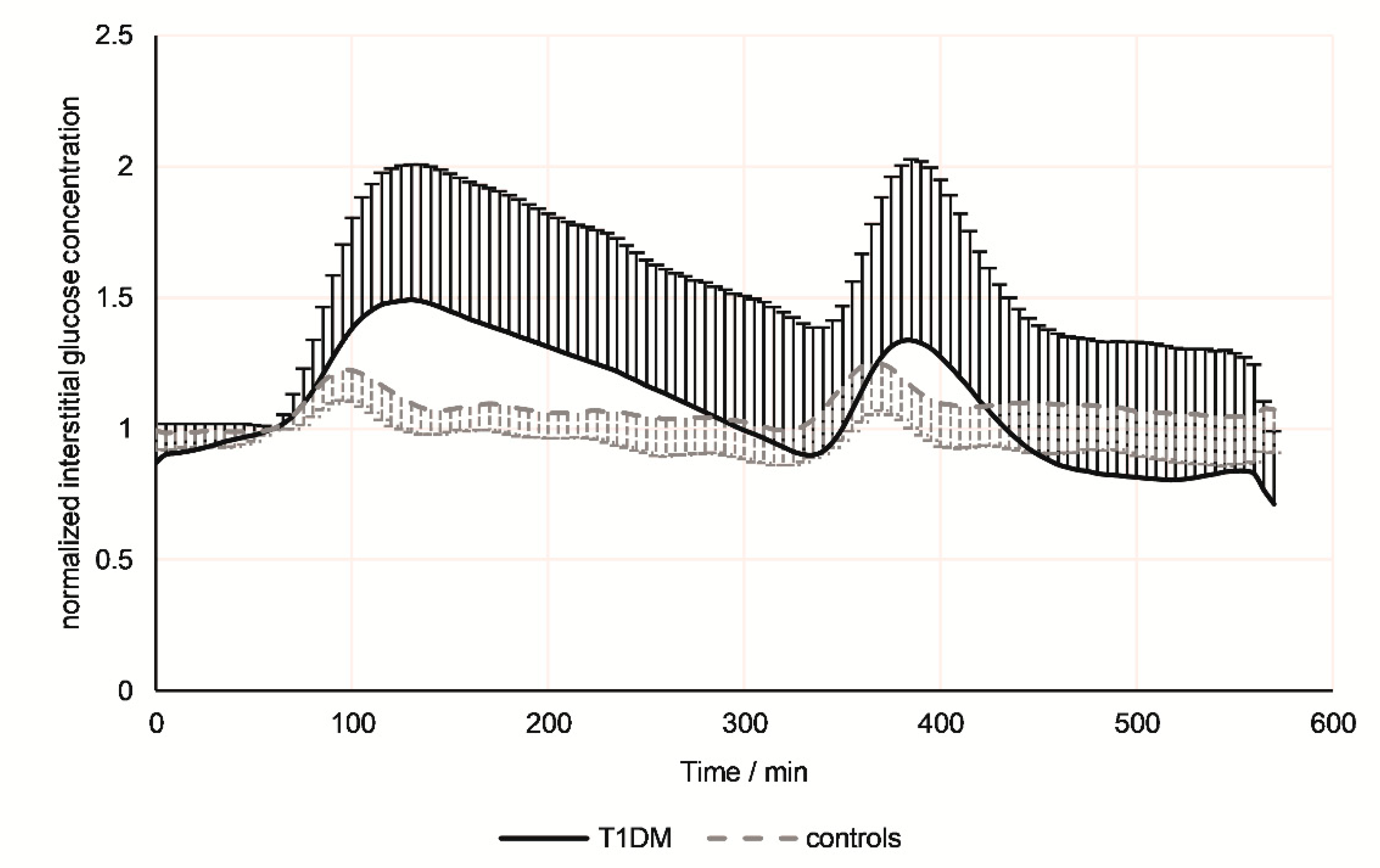
3.1. Continuous Glucose Monitoring
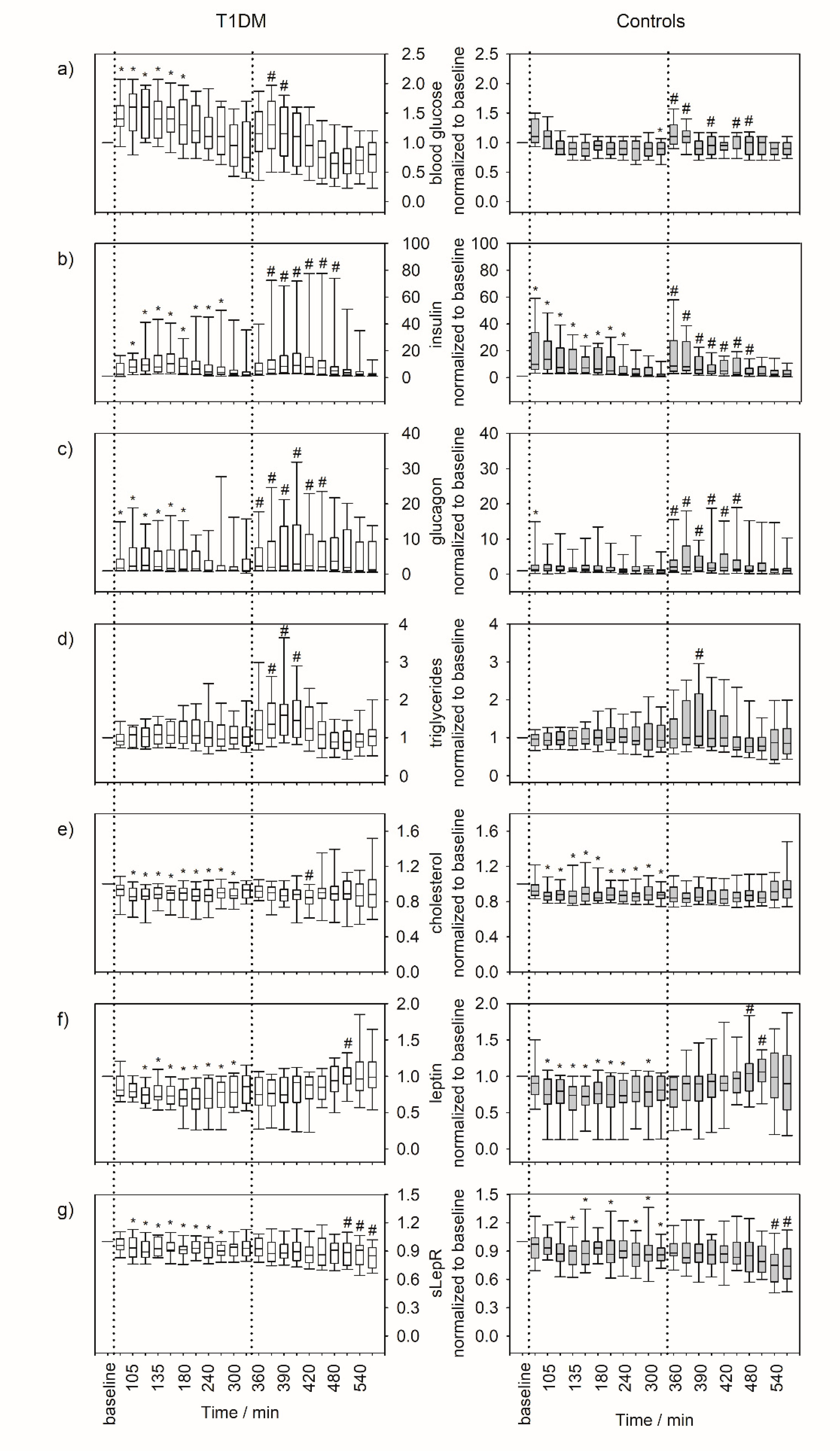
3.2. Serum Parameters
3.3. Breath VOCs
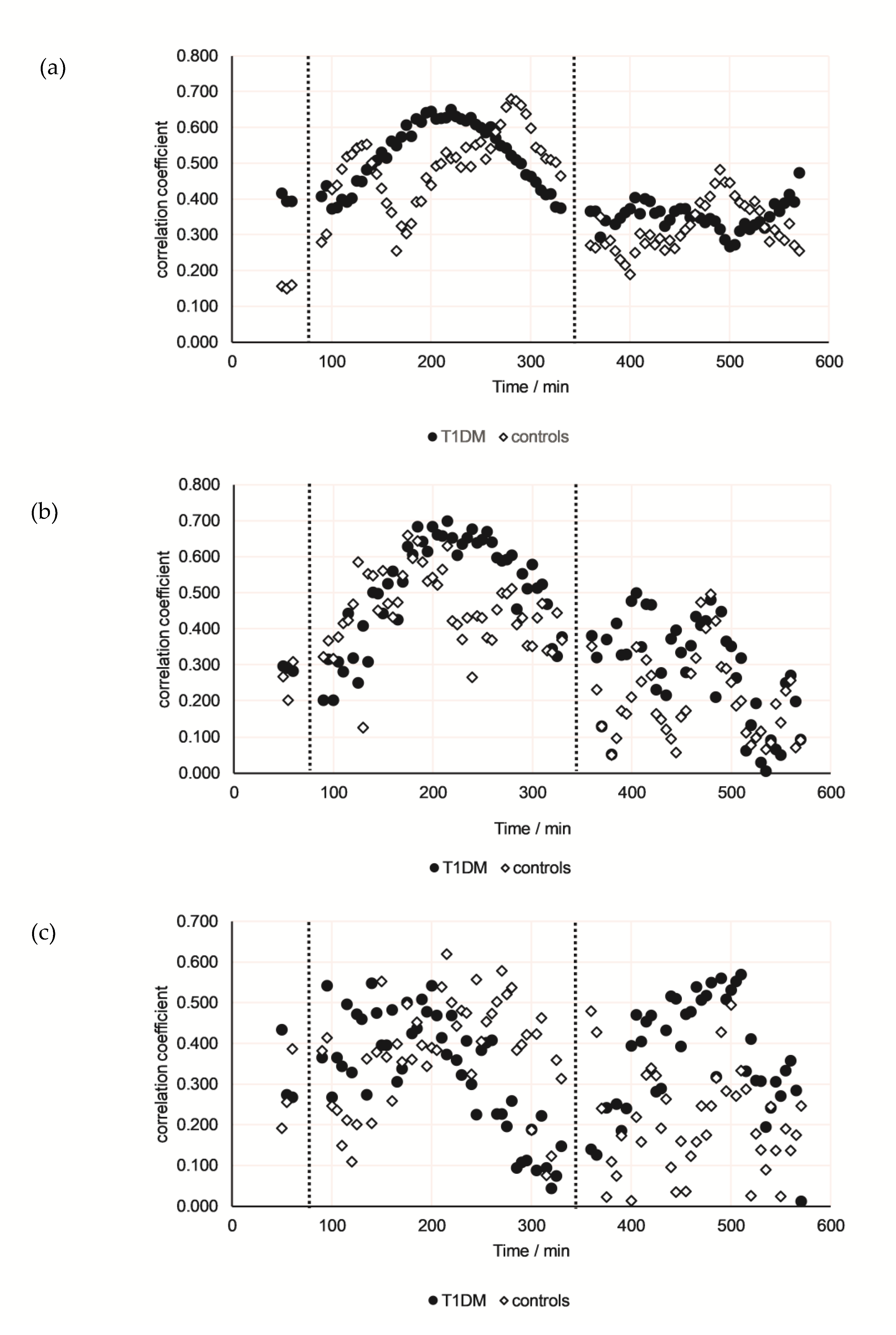
3.4. Correlations Between Breath VOCs and Either Serum Parameters or CGM Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Roche, E.F.; McKenna, A.M.; Ryder, K.J.; Brennan, A.A.; O’Regan, M.; Hoey, H.M. Is the incidence of type 1 diabetes in children and adolescents stabilising? The first 6 years of a National Register. Eur. J. Pediatr. 2016, 175, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.J.; Wherrett, D.K. Care of diabetes in children and adolescents: Controversies, changes, and consensus. Lancet 2015, 385, 2096–2106. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar] [PubMed]
- Wang, M.Y.; Chen, L.; Clark, G.O.; Lee, Y.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Charron, M.J.; Newgard, C.B.; et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.A. Leptin for type 1 diabetes: Coming onto stage to be (or not?). Pediatr. Diabetes 2012, 13, 68–73. [Google Scholar] [CrossRef][Green Version]
- Wasserfall, C.H.; Mathews, C.E.; Schatz, D.A. The use of leptin as treatment for type 1 diabetes mellitus: Counterpoint. Pediatr. Diabetes 2012, 13, 74–76. [Google Scholar] [CrossRef]
- McNeilly, A.D.; McCrimmon, R.J. The Scylla and Charybdis of glucose control in childhood type 1 diabetes? Pediatr. Diabetes 2015, 16, 235–241. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Tossavainen, P.H.; Dunger, D.B. Prevention and treatment of microvascular disease in childhood type 1 diabetes. Br. Med. Bull. 2010, 94, 145–164. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Colette, C.; Monnier, L. Acute glucose fluctuations and chronic sustained hyperglycemia as risk factors for cardiovascular diseases in patients with type 2 diabetes. Horm. Metab. Res. 2007, 39, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Hirsch, I.B. Glycemic variability: A hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006, 295, 1707–1708. [Google Scholar] [CrossRef]
- Zaccardi, F.; Pitocco, D.; Ghirlanda, G. Glycemic risk factors of diabetic vascular complications: The role of glycemic variability. Diabetes Metab. Res. Rev. 2009, 25, 199–207. [Google Scholar] [CrossRef]
- Kilpatrick, E.S. Arguments for and against the role of glucose variability in the development of diabetes complications. J. Diabetes Sci. Technol. 2009, 3, 649–655. [Google Scholar] [CrossRef]
- Siegelaar, S.E.; Holleman, F.; Hoekstra, J.B.; DeVries, J.H. Glucose variability; does it matter? Endocr. Rev. 2010, 31, 171–182. [Google Scholar] [CrossRef]
- Wentholt, I.M.; Kulik, W.; Michels, R.P.; Hoekstra, J.B.; DeVries, J.H. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia 2008, 51, 183–190. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Costello, B.d.L.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Sehnert, S.S.; Jiang, L.; Burdick, J.F.; Risby, T.H. Breath biomarkers for detection of human liver diseases: Preliminary study. Biomarkers 2002, 7, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; McCulloch, M.; Jackson, J.; McKee, D.L.; Walker, J.C.; Solouki, T. Evidence for Cancer Biomarkers in Exhaled Breath. IEEE Sens. J. 2010, 10, 185–210. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Sliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef]
- Siegel, A.P.; Daneshkhah, A.; Hardin, D.S.; Shrestha, S.; Varahramyan, K.; Agarwal, M. Analyzing breath samples of hypoglycemic events in type 1 diabetes patients: Towards developing an alternative to diabetes alert dogs. J. Breath Res. 2017, 11, 026007. [Google Scholar] [CrossRef]
- Pabst, F.; Miekisch, W.; Fuchs, P.; Kischkel, S.; Schubert, J.K. Monitoring of oxidative and metabolic stress during cardiac surgery by means of breath biomarkers: An observational study. J. Cardiothorac. Surg. 2007, 2, 37. [Google Scholar] [CrossRef]
- Patterson, C.S.; McMillan, L.C.; Stevenson, K.; Radhakrishnan, K.; Shiels, P.G.; Padgett, M.J.; Skeldon, K.D. Dynamic study of oxidative stress in renal dialysis patients based on breath ethane measured by optical spectroscopy. J. Breath Res. 2007, 1, 026005. [Google Scholar] [CrossRef]
- Scholpp, J.; Schubert, J.K.; Miekisch, W.; Geiger, K. Breath markers and soluble lipid peroxidation markers in critically ill patients. Clin. Chem. Lab. Med. 2002, 40, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Herbig, J.; Muller, M.; Schallhart, S.; Titzmann, T.; Graus, M.; Hansel, A. On-line breath analysis with PTR-TOF. J. Breath Res. 2009, 3, 027004. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Schmidt, M.; Oertel, P.; Obermeier, J.; Brock, B.; Kamysek, S.; Dunkl, J.; Zimmermann, R.; Schubert, J.K.; Miekisch, W. Continuous Real Time Breath Gas Monitoring in the Clinical Environment by Proton-Transfer-Reaction-Time-of-Flight-Mass Spectrometry. Anal. Chem. 2013, 85, 10321–10329. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Spanel, P.; Herbig, J.; Beauchamp, J. Mass spectrometry for real-time quantitative breath analysis. J. Breath Res. 2014, 8, 027101. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Trefz, P.; Kamysek, S.; Schubert, J.K.; Miekisch, W. Instant effects of changing body positions on compositions of exhaled breath. J. Breath Res. 2015, 9, 047105. [Google Scholar] [CrossRef]
- Schreiver, C.; Jacoby, U.; Watzer, B.; Thomas, A.; Haffner, D.; Fischer, D.C. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): A cross-sectional cohort study. Clin. Endocrinol. 2013, 79, 641–647. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; Von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Trefz, P.; Schubert, J.K.; Miekisch, W. Effects of humidity, CO2 and O2 on real-time quantitation of breath biomarkers by means of PTR-ToF-MS. J. Breath Res. 2018, 12, 026016. [Google Scholar] [CrossRef]
- Blaikie, T.P.; Edge, J.A.; Hancock, G.; Lunn, D.; Megson, C.; Peverall, R.; Richmond, G.; Ritchie, G.A.; Taylor, D. Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J. Breath Res. 2014, 8, 046010. [Google Scholar] [CrossRef]
- Wang, C.J.; Mbi, A.; Shepherd, M. A Study on Breath Acetone in Diabetic Patients Using a Cavity Ringdown Breath Analyzer: Exploring Correlations of Breath Acetone with Blood Glucose and Glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Nelson, N.; Lagesson, V.; Nosratabadi, A.R.; Ludvigsson, J.; Tagesson, C. Exhaled Isoprene and Acetone in Newborn Infants and in Children with Diabetes Mellitus. Pediatr. Res. 1998, 44, 363. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Dummer, J.; Lunt, H.; Scotter, J.; McCartin, F.; Cook, J.; Swanney, M.; Kendall, D.; Logan, F.; Epton, M. Measurement of breath acetone concentrations by selected ion flow tube mass spectrometry in type 2 diabetes. J. Breath Res. 2011, 5, 046011. [Google Scholar] [CrossRef]
- Schwarz, K.; Pizzini, A.; Arendacka, B.; Zerlauth, K.; Filipiak, W.; Schmid, A.; Dzien, A.; Neuner, S.; Lechleitner, M.; Scholl-Burgi, S.; et al. Breath acetone-aspects of normal physiology related to age and gender as determined in a PTR-MS study. J. Breath Res. 2009, 3, 027003. [Google Scholar] [CrossRef]
- Turner, C. Potential of breath and skin analysis for monitoring blood glucose concentration in diabetes. Expert Rev. Mol. Diagn. 2011, 11, 497–503. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The Clinical Potential of Exhaled Breath Analysis for Diabetes Mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Jones, A.W.; Sagarduy, A.; Ericsson, E.; Arnqvist, H.J. Concentrations of acetone in venous blood samples from drunk drivers, type-I diabetic outpatients, and healthy blood donors. J. Anal. Toxicol. 1993, 17, 182–185. [Google Scholar] [CrossRef]
- Jones, A.W.; Rossner, S. False-positive breath-alcohol test after a ketogenic diet. Int. J. Obes. 2007, 31, 559–561. [Google Scholar] [CrossRef]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Cryer, P.E. Glucose Counterregulation in Man. Diabetes 1981, 30, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Vella, A.; Basu, A.; Basu, R.; Schwenk, W.F.; Rizza, R.A. Lack of Suppression of Glucagon Contributes to Postprandial Hyperglycemia in Subjects with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2000, 85, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Wagner, A.M.; Carreras, G.; Gimenez, G.; Sanchez-Quesada, J.L.; Rigla, M.; Gomez-Gerique, J.A.; Pou, J.M.; De Leiva, A. Prevalence and Phenotypic Distribution of Dyslipidemia in Type 1 Diabetes Mellitus: Effect of Glycemic Control. Arch. Intern. Med. 2000, 160, 2756–2762. [Google Scholar] [CrossRef] [PubMed]
- Verges, B. Dyslipidaemia in diabetes mellitus. Review of the main lipoprotein abnormalities and their consequences on the development of atherogenesis. Diabetes Metab. 1999, 25, 32–40. [Google Scholar]
- Perez, A.; Caixas, A.; Carreras, G.; Mauricio, D.; Pou, J.M.; Serrat, J.; Gomez-Gerique, J.; De Leiva, A. Lipoprotein compositional abnormalities in type I diabetes: Effect of improved glycaemic control. Diabetes Res. Clin. Pract. 1997, 36, 83–90. [Google Scholar] [CrossRef]
- Homma, T.K.; Endo, C.M.; Saruhashi, T.; Mori, A.P.; Noronha, R.M.; Monte, O.; Calliari, L.E. Dyslipidemia in young patients with type 1 diabetes mellitus. Arch. Endocrinol. Metab. 2015, 59, 215–219. [Google Scholar] [CrossRef]
- Logan, B.K.; Jones, A.W. Endogenous Ethanol ‘Auto-Brewery Syndrome’ as a Drunk-Driving Defence Challenge. Med. Sci. Law 2000, 40, 206–215. [Google Scholar] [CrossRef]
- Simic, M.; Ajdukovic, N.; Veselinovic, I.; Mitrovic, M.; Djurendic-Brenesel, M. Endogenous ethanol production in patients with Diabetes Mellitus as a medicolegal problem. Forensic Sci. Int. 2012, 216, 97–100. [Google Scholar] [CrossRef]
- Kaji, H.; Hisamura, M.; Saito, N.; Sakai, H.; Aikawa, T.; Kondo, T.; Ide, H.; Murao, M. Clinical application of breath analysis for dimethyl sulfide following ingestion of dl-methionine. Clin. Chim. Acta 1979, 93, 377–380. [Google Scholar] [CrossRef]
- Morita, M.; Wang, H.-L. Association between oral malodor and adult periodontitis: A review. J. Clin. Periodontol. 2001, 28, 813–819. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Extra-oral halitosis: An overview. J. Breath Res. 2010, 4, 017003. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Teschl, S.; Teschl, G.; Miekisch, W.; Schubert, J.; Hinterhuber, H.; Amann, A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009, 3, 027006. [Google Scholar] [CrossRef] [PubMed]
- Filser, J.G.; Csanady, G.A.; Denk, B.; Hartmann, M.; Kauffmann, A.; Kessler, W.; Kreuzer, P.E.; Pütz, C.; Shen, J.H.; Stei, P. Toxicokinetics of isoprene in rodents and humans. Toxicology 1996, 113, 278–287. [Google Scholar] [CrossRef]
- Sukul, P.; Schubert, J.K.; Oertel, P.; Kamysek, S.; Taunk, K.; Trefz, P.; Miekisch, W. FEV manoeuvre induced changes in breath VOC compositions: An unconventional view on lung function tests. Sci. Rep. 2016, 6, 28029. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Trefz, P.; Schubert, J.K.; Miekisch, W. Immediate effects of breath holding maneuvers onto composition of exhaled breath. J. Breath Res. 2014, 8, 037102. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Schwoebel, H.; Mau-Moeller, A.; Behrens, M.; Fuchs, P.; Sklorz, M.; Schubert, J.K.; Bruhn, S.; Miekisch, W. Metabolic monitoring and assessment of anaerobic threshold by means of breath biomarkers. Metabolomics 2012, 8, 1069–1080. [Google Scholar] [CrossRef]
- O’Hara, M.E.; Fernández Del Río, R.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Limonene in exhaled breath is elevated in hepatic encephalopathy. J. Breath Res. 2016, 10, 046010. [Google Scholar] [CrossRef]
- Del Río, R.F.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile Biomarkers in Breath Associated with Liver Cirrhosis—Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef]
- Leeds, J.S.; Forman, E.M.; Morley, S.; Scott, A.R.; Tesfaye, S.; Sanders, D.S. Abnormal liver function tests in patients with Type 1 diabetes mellitus: Prevalence, clinical correlations and underlying pathologies. Diabet. Med. 2009, 26, 1235–1241. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Seufert, J. Leptin Effects on Pancreatic β-Cell Gene Expression and Function. Diabetes 2004, 53, S152–S158. [Google Scholar] [CrossRef] [PubMed]
- Kratzsch, J.; Deimel, A.; Galler, A.; Kapellen, T.; Klinghammer, A.; Kiess, W. Increased serum soluble leptin receptor levels in children and adolescents with type 1 diabetes mellitus. Eur. J. Endocrinol. 2004, 151, 475–481. [Google Scholar] [CrossRef] [PubMed]

| Controls (13m/9f) | T1DM (Type 1 Diabetes) Patients (11m/11f) | |
|---|---|---|
| Age [years] | 14.2 (11.9–16.6) | 14.2 (11.9–17.1) |
| Anthropometric data | ||
| Height | ||
| absolute [cm] | 168.0 (143.4–187.6) | 163.7 (146.8–184.3) |
| normalized [Z-Score} | 0.27 (−1.43–2.30) | 0.17 (–1.40–1.82) |
| Weight | ||
| absolute [kg] | 58.6 (32.8–58.6) | 55.6 (36.7 - 96.2) |
| normalized [Z-Score] | 0.15 (–1.86–1.68) | 0.13 (–1.52–1.72) |
| BMI | ||
| absolute [kg/m2] | 20.3 (16–33.7) | 21.7 (16.4–28.9) |
| normalized [Z-Score] | 0.00 (−1.26–2.16) | 0.6 (–1.30–1.63) |
| Carbohydrate intake with standardized meals [g/kg] | 3.86 (2.43–5.87) | 4.37 (2.42–6.98) |
| HbA1c | ||
| at time of examination [%] | 5.3 (4.8–5.9) | 9.5 (6.3–11.5) |
| mean over the past year [%] | 9.1 (6.4–11.6) | |
| Duration of disease [years] | - | 6.2 (2.3–11.4) |
| Duration of insulin therapy [years] | - | 5.1 (1.9–10.8) |
| Daily dose of basal insulin [IU/kg] | - | 0.37 (0.20–0.57) |
| T1DM Patients | |||||||
| Time/min | Glucose /mmol/L | Insulin /mU/L | Glucagon /pg/mL | Triglycerides /mmol/L | Cholesterol /mmol/L | Leptin /ng/mL | sLepR /ng/mL |
| baseline | 9.85 | 10.16 | 19.15 | 0.50 | 3.10 | 7.43 | 30.20 |
| 90 | 14.85 * | 30.14 | 99.82 * | 0.46 | 2.91 | 5.95 | 31.39 |
| 105 | 16.00 * | 70.41 * | 96.39 * | 0.51 | 2.73 * | 6.08 | 30.51 * |
| 120 | 16.10 * | 90.24 * | 81.36 * | 0.52 | 2.61 * | 5.15 * | 30.02 * |
| 135 | 14.25 * | 105.26 * | 81.76 * | 0.55 | 2.73 * | 5.38 * | 27.91 * |
| 150 | 14.35 * | 110.32 * | 78.21 * | 0.60 | 2.67 * | 4.91 * | 31.23 * |
| 180 | 14.00 * | 85.20 * | 79.43 * | 0.62 | 2.73 * | 4.49 * | 28.28 * |
| 210 | 12.40 | 80.49 * | 76.89 | 0.63 | 2.73 * | 4.20 * | 28.87 * |
| 240 | 12.30 | 57.29 * | 74.97 | 0.52 | 2.69 * | 4.40 * | 28.90 * |
| 270 | 12.15 | 56.42 * | 75.63 | 0.52 | 2.83 * | 4.67 * | 28.42 * |
| 300 | 10.40 | 45.35 | 68.94 | 0.51 | 2.80 * | 5.38 * | 29.37 |
| 330 | 8.95 | 34.75 | 66.21 | 0.55 | 2.87 | 5.33 | 30.06 |
| 360 | 11.90 | 48.43 | 97.71 # | 0.63 | 2.97 | 5.00 | 32.19 |
| 375 | 12.70 # | 86.49 # | 102.42 # | 0.77 # | 2.82 | 5.46 | 32.04 |
| 390 | 11.80 # | 103.97 # | 104.22 # | 0.88 # | 2.71 | 5.01 | 30.23 |
| 405 | 10.25 | 100.61 # | 101.40 # | 0.75 # | 2.68 | 4.81 | 28.62 |
| 420 | 10.05 | 100.18 # | 98.22 # | 0.64 | 2.56 # | 5.62 | 28.13 |
| 450 | 7.90 | 77.76 # | 92.53 # | 0.51 | 2.89 | 5.07 | 28.82 |
| 480 | 6.85 | 67.05 # | 82.33 | 0.43 | 2.79 | 5.81 | 28.94 |
| 510 | 6.90 | 46.59 | 80.75 | 0.43 | 2.84 | 6.41 # | 29.71 # |
| 540 | 6.85 | 38.43 | 70.20 | 0.46 | 2.64 | 7.29 | 27.94 # |
| 570 | 7.55 | 24.92 | 76.52 | 0.51 | 2.79 | 6.54 | 26.48 # |
| Healthy Controls | |||||||
| Time/min | Glucose /mmol/L | Insulin /mU/L | glucagon /pg/mL | Triglycerides /mmol/L | Cholesterol /mmol/L | Leptin /ng/mL | sLepR /ng/mL |
| baseline | 5.00 | 6.16 | 37.75 | 0.42 | 2.47 | 4.27 | 17.53 |
| 90 | 5.90 | 45.49 * | 73.03 * | 0.38 | 2.29 | 3.68 | 16.67 |
| 105 | 5.65 | 52.51 * | 62.63 | 0.40 | 2.32 * | 2.93 * | 15.26 |
| 120 | 4.75 | 38.64 * | 66.61 | 0.38 | 2.29 * | 2.80 * | 15.43 |
| 135 | 4.65 | 32.23 * | 59.03 | 0.35 | 2.21 * | 2.84 * | 14.16 * |
| 150 | 4.55 | 30.43 * | 62.74 | 0.36 | 2.27 * | 2.98 * | 15.94 * |
| 180 | 4.75 | 28.10 * | 62.79 | 0.37 | 2.12 * | 3.04 * | 16.76 |
| 210 | 4.70 | 25.41 * | 59.37 | 0.36 | 2.32 * | 2.32 * | 14.45 * |
| 240 | 4.40 | 20.63 * | 41.26 | 0.37 | 2.08 * | 2.94 * | 14.76 |
| 270 | 4.70 | 18.36 | 58.83 | 0.33 | 2.08 * | 2.96 | 12.95 * |
| 300 | 4.65 | 13.94 | 6.37 | 0.39 | 2.22 * | 2.81 * | 13.82 * |
| 330 | 4.35 * | 9.21 | 20.82 | 0.42 | 2.13 * | 3.00 | 15.21 * |
| 360 | 5.75 # | 39.93 # | 72.82 # | 0.40 | 2.09 | 3.05 | 15.11 |
| 375 | 5.6 # | 41.48 # | 71.90 # | 0.45 | 2.07 | 3.37 | 14.98 |
| 390 | 4.65 | 24.61 # | 65.12 # | 0.49 # | 2.26 | 3.65 | 14.78 |
| 405 | 4.80 # | 22.38 # | 78.32 # | 0.46 | 2.08 | 3.32 | 14.81 |
| 420 | 4.80 | 18.70 # | 68.65 # | 0.45 | 2.12 | 3.40 | 14.18 |
| 450 | 4.90 # | 17.72 # | 67.62 # | 0.35 | 2.21 | 3.78 | 14.35 |
| 480 | 4.80 # | 17.55 # | 66.12 | 0.34 | 2.34 | 4.56 # | 13.96 |
| 510 | 4.60 | 14.37 | 52.96 | 0.30 | 2.28 | 4.13 # | 14.04 |
| 540 | 4.60 | 14.03 | 53.27 | 0.32 | 2.42 | 3.58 | 12.52 # |
| 570 | 4.40 | 11.43 | 35.45 | 0.36 | 2.49 | 2.62 | 11.75 # |
| T1DM Patients | |||||||
| Time/min | Acetone /ppbV | 2-Propanol /ppbV | Pentanal /ppbV | Ethanol /ppbV | Dimethyl Sulfide / ppbV | Isoprene /ppbV | limonene /ppbV |
| baseline | 824 | 1428 | 8.74 | 44.2 | 12.5 | 100.6 | 25.8 |
| 90 | 923 | 1275 | 7.05 | 72.2 | 11.4 | 107.5 | 48.0 * |
| 105 | 891 | 1597 | 9.83 | 55.1 | 11.7 | 105.8 | 38.1 * |
| 120 | 879 | 1533 | 7.81 | 64.2 | 11.4 | 88.5 | 36.5 * |
| 135 | 846 | 1360 | 7.20 | 62.6 * | 10.5 | 98.0 | 32.1 |
| 150 | 810 | 1499 | 8.03 | 71.6 * | 10.5 | 101.7 | 29.5 |
| 180 | 765 | 1468 | 6.80 | 76.8 | 11.3 | 89.0 | 26.1 |
| 210 | 724 | 1236 | 6.67 * | 62.2 | 11.6 | 100.9 | 26.1 |
| 240 | 661 | 1194 | 6.34 * | 65.6 | 11.7 | 104.2 | 23.9 |
| 270 | 622 * | 1124 | 5.41 * | 58.6 | 11.6 | 93.9 | 25.4 |
| 300 | 580 * | 959 * | 5.44 * | 66.6 | 12.0 | 122.7 | 22.6 |
| 330 | 552 * | 848 * | 5.03 * | 52.6 | 11.1 | 118.1 * | 22.9 |
| 360 | 574 | 964 | 4.47 | 68.8 | 11.8 | 125.4 | 24.7 |
| 375 | 521 | 935 | 4.98 | 135.9 # | 11.2 | 109.0 | 22.8 |
| 390 | 523 | 913 | 4.68 | 110.7 # | 11.7 | 126.3 | 23.0 |
| 405 | 452 # | 846 | 3.88 | 97.1 # | 12.4 | 112.0 | 22.0 |
| 420 | 477 # | 748 | 3.89 | 70.4 # | 12.7 # | 106.5 | 23.1 |
| 450 | 437 # | 790 | 3.69 # | 82.8 | 13.0 # | 96.5 | 23.8 |
| 480 | 386 # | 716 # | 3.21 # | 71.0 | 13.9 # | 115.3 | 26.1 |
| 510 | 365 # | 730 | 3.69 # | 80.5 | 13.5 # | 122.3 | 26.4 # |
| 540 | 332 # | 652 # | 3.31 # | 92.9 # | 14.0 # | 137.7 | 26.0 |
| 570 | 299 # | 667 # | 3.23 # | 75.3 | 13.4 # | 127.8 | 24.9 |
| Healthy Controls | |||||||
| Time/min | Acetone / ppbV | 2-Propanol /ppbV | Pentanal /ppbV | Ethanol / ppbV | dimethyl sulfide / ppbV | isoprene / ppbV | Limonene/ ppbV |
| baseline | 370 | 640 | 5.03 | 40.8 | 13.5 | 99.4 | 28.7 |
| 90 | 378 | 610 | 4.69 | 50.5 | 11.9 * | 101.1 | 53.1 * |
| 105 | 374 | 838 | 5.52 | 57.5 | 12.9 * | 83.4 | 43.9 * |
| 120 | 362 | 789 | 5.16 | 76.3 | 13.1 * | 89.6 | 38.3 * |
| 135 | 382 | 816 | 4.79 | 78.7 * | 12.7 | 81.8 | 29.3 |
| 150 | 365 | 887 | 4.03 | 77.1 * | 13.1 | 96.2 * | 28.6 |
| 180 | 326 | 698 | 3.47 * | 73.8 | 13.8 | 88.5 * | 28.3 |
| 210 | 319 * | 759 | 3.42 * | 47.9 | 12.6 | 105.2 | 24.5 |
| 240 | 287 * | 688 | 3.60 * | 56.8 | 13.9 | 117.3 | 25.4 |
| 270 | 280 * | 653 | 3.14 * | 45.2 | 13.4 | 108.4 | 27.4 |
| 300 | 272 * | 559 * | 3.14 * | 49.0 | 12.8 | 124.9 | 26.6 |
| 330 | 273 * | 580 | 2.87 * | 50.1 | 12.6 | 127.6 | 25.8 |
| 360 | 273 | 553 | 3.54 | 49.8 | 12.5 | 110.5 | 24.2 |
| 375 | 260 | 590 | 3.44 | 95.6 # | 12.8 | 105.3 # | 24.3 |
| 390 | 259 | 661 | 3.26 | 105.6 # | 13.5 | 122.6 | 24.6 |
| 405 | 256 | 599 | 2.82 | 111.4 # | 13.3 | 113.7 # | 24.0 |
| 420 | 248 # | 510 | 2.71 | 64.2 | 13.8 | 118.0 # | 24.0 |
| 450 | 232 # | 442 | 2.37 | 64.5 | 14.0 # | 122.0 | 23.4 |
| 480 | 224 # | 432 # | 2.46 # | 48.4 | 14.4 # | 132.5 | 24.2 |
| 510 | 227 # | 439 | 2.30 # | 45.6 | 14.3 # | 131.1 | 24.1 |
| 540 | 217 # | 421 # | 2.24 # | 68.1 | 15.0 # | 129.6 | 24.6 |
| 570 | 210 # | 458 # | 2.18 # | 51.4 | 14.7 # | 142.0 | 24.4 # |
| Acetone | 2-Propanol | Pentanal | ||
|---|---|---|---|---|
| sLepR | ||||
| overall | 0.561 | 0.487 | 0.542 | |
| T1DM patients | 0.364 | 0.321 | 0.424 | |
| controls | 0.500 | 0.321 | 0.374 | |
| Blood Glucose | ||||
| overall | 0.697 | 0.624 | 0.566 | |
| T1DM patients | 0.610 | 0.553 | 0.468 | |
| controls | 0.331 | 0.327 | 0.304 | |
| CGM data | ||||
| overall | 0.686 | 0.622 | 0.565 | |
| T1DM patients | 0.591 | 0.557 | 0.467 | |
| controls | 0.331 | 0.327 | 0.304 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trefz, P.; Schmidt, S.C.; Sukul, P.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus. J. Clin. Med. 2019, 8, 1797. https://doi.org/10.3390/jcm8111797
Trefz P, Schmidt SC, Sukul P, Schubert JK, Miekisch W, Fischer D-C. Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus. Journal of Clinical Medicine. 2019; 8(11):1797. https://doi.org/10.3390/jcm8111797
Chicago/Turabian StyleTrefz, Phillip, Sibylle C. Schmidt, Pritam Sukul, Jochen K. Schubert, Wolfram Miekisch, and Dagmar-Christiane Fischer. 2019. "Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus" Journal of Clinical Medicine 8, no. 11: 1797. https://doi.org/10.3390/jcm8111797
APA StyleTrefz, P., Schmidt, S. C., Sukul, P., Schubert, J. K., Miekisch, W., & Fischer, D.-C. (2019). Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus. Journal of Clinical Medicine, 8(11), 1797. https://doi.org/10.3390/jcm8111797







