Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation
Abstract
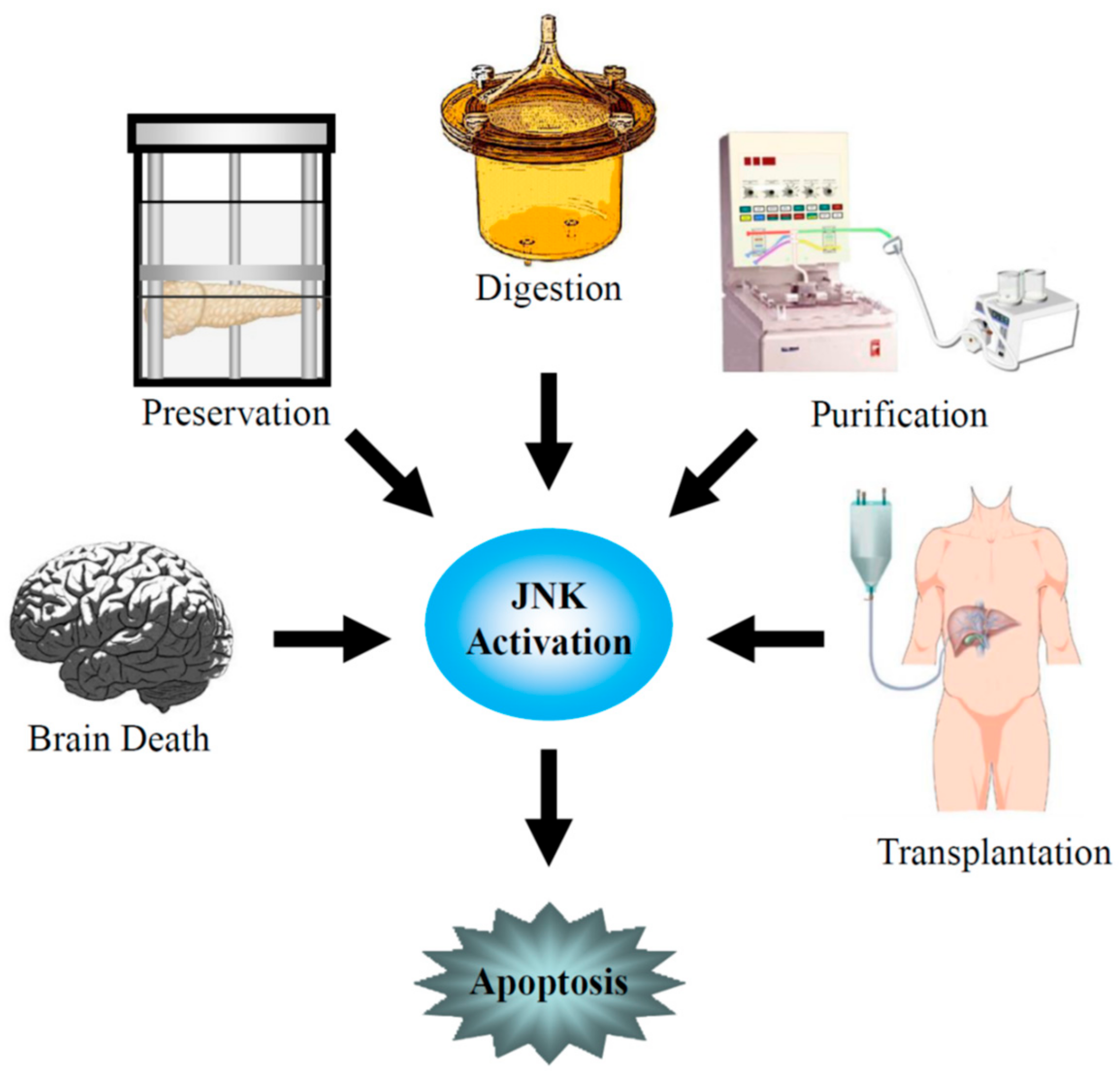
1. Introduction
2. Donor Organ
3. Pancreas Preservation
4. Islet Isolation and Culture
5. Islet Transplantation
6. JNK Inhibitors
7. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| JNK | c-Jun NH2-terminal kinase |
| MAPKs | mitogen-activated protein kinases |
| T1DM | type 1 diabetes mellitus |
| IL | interleukin |
| TNF | tumor necrosis factor |
| IFN | interferon |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| ER | endoplasmic reticulum |
| IBMIR | instant blood-mediated inflammatory reaction |
| ERKs | extracellular signal–regulated kinases |
| ATF-2 | activating transcription factor-2 |
| AP-1 | activator protein-1 |
| UW | University of Wisconsin solution |
| PFC | perfluorochemical |
| TLM | two-layer preservation method |
| ATP | adenosine triphosphate |
| EJ | extracellular-type/JNK inhibitor-containing solution |
| MKK | MAPK kinase |
| ICAM | intracellular adhesion molecule |
| APC | activated protein C |
| JIP1 | JNK-interacting protein-1 |
| IB1 | islet-brain-1 |
| JNKI | JNK inhibitory peptide |
| 11R | 11-arginine |
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed. 2015. Available online: www.diabetesatlas.org (accessed on 1 June 2016).
- Mandrup-Poulsen, T.; Bendtzen, K.; Nerup, J.; Dinarello, C.A.; Svenson, M.; Nielsen, J.H. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia 1986, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.A.; Kwon, G.; Marino, M.H.; Rodi, C.P.; Sullivan, P.M.; Turk, J.; McDaniel, M.L. Tyrosine kinase inhibitors prevent cytokine-induced expression of iNOS and COX-2 by human islets. Am. J. Physiol. 1996, 270, C1581–C1587. [Google Scholar] [CrossRef] [PubMed]
- Ankarcrona, M.; Dypbukt, J.M.; Brüne, B.; Nicotera, P. Interleukin-1 beta-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp. Cell Res. 1994, 213, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Fujii, J.; Seo, H.G.; Suzuki, K.; Matsuoka, T.; Nakamura, M.; Tatsumi, H.; Yamasaki, Y.; Kamada, T.; Taniguchi, N. Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes 1995, 44, 733–738. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Matsumoto, S.; Okitsu, T.; Iwanaga, Y.; Noguchi, H.; Nagata, H.; Yonekawa, Y.; Yamada, Y.; Fukuda, K.; Tsukiyama, K.; Suzuki, H.; et al. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet 2005, 365, 1642–1644. [Google Scholar] [CrossRef]
- Fiorina, P.; Shapiro, A.M.; Ricordi, C.; Secchi, A. The clinical impact of islet transplantation. Am. J. Transplant. 2008, 8, 1990–1997. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Gallant, H.L.; Hao, E.G.; Lakey, J.R.; McCready, T.; Rajotte, R.V.; Yatscoff, R.W.; Kneteman, N.M. The portal immunosuppressive storm: Relevance to islet transplantation? Ther. Drug Monit. 2005, 27, 35–37. [Google Scholar] [CrossRef]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front. Endocrinol. 2017, 8, 343. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Kotlabova, K.; Lim, J.H.; Mitroulis, I.; Ferreira, A.; Chen, L.S.; Gercken, B.; Steffen, A.; Kemter, E.; Klotzsche-von Ameln, A.; et al. Developmental endothelial locus-1 modulates platelet-monocyte interactions and instant blood-mediated inflammatory reaction in islet transplantation. Thromb. Haemost. 2016, 115, 781–788. [Google Scholar] [CrossRef]
- Nilsson, B.; Ekdahl, K.N.; Korsgren, O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr. Opin. Organ. Transplant. 2011, 16, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Davalli, A.M.; Scaglia, L.; Zangen, D.H.; Hollister, J.; Bonner-Weir, S.; Weir, G.C. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996, 45, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Wang, R.; Paraskevas, S.; Maysinger, D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery 1999, 126, 393–398. [Google Scholar] [CrossRef]
- Thomas, D.; Yang, H.; Boffa, D.J.; Ding, R.; Sharma, V.K.; Lagman, M.; Li, B.; Hering, B.; Mohanakumar, T.; Lakey, J.; et al. Proapoptotic Bax is hyperexpressed in isolated human islets compared with antiapoptotic Bcl-2. Transplantation 2002, 74, 1489–1496. [Google Scholar] [CrossRef]
- Rehman, K.K.; Bertera, S.; Bottino, R.; Balamurugan, A.N.; Mai, J.C.; Mi, Z.; Trucco, M.; Robbins, P.D. Protection of islets by in situ peptide-mediated transduction of the Ikappa B kinase inhibitor Nemo-binding domain peptide. J. Biol. Chem. 2003, 278, 9862–9868. [Google Scholar] [CrossRef]
- Abdelli, S.; Ansite, J.; Roduit, R.; Borsello, T.; Matsumoto, I.; Sawada, T.; Allaman-Pillet, N.; Henry, H.; Beckmann, J.S.; Hering, B.J.; et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004, 53, 2815–2823. [Google Scholar] [CrossRef][Green Version]
- Ammendrup, A.; Maillard, A.; Nielsen, K.; Aabenhus Andersen, N.; Serup, P.; Dragsbaek Madsen, O.; Mandrup-Poulsen, T.; Bonny, C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 2000, 49, 1468–1476. [Google Scholar] [CrossRef]
- Saldeen, J.; Lee, J.C.; Welsh, N. Role of p38 mitogen-activated protein kinase (p38 MAPK) in cytokine-induced rat islet cell apoptosis. Biochem. Pharmacol. 2001, 61, 1561–1569. [Google Scholar] [CrossRef]
- Bonny, C.; Oberson, A.; Negri, S.; Sauser, C.; Schorderet, D.F. Cell-permeable peptide inhibitors of JNK: Novel blockers of beta-cell death. Diabetes 2001, 50, 77–82. [Google Scholar] [CrossRef]
- Baker, M.S.; Chen, X.; Cao, X.C.; Kaufman, D.B. Expression of a dominant negative inhibitor of NF-kappaB protects MIN6 beta-cells from cytokine-induced apoptosis. J. Surg. Res. 2001, 97, 117–122. [Google Scholar] [CrossRef]
- Keesler, G.A.; Bray, J.; Hunt, J.; Johnson, D.A.; Gleason, T.; Yao, Z.; Wang, S.W.; Parker, C.; Yamane, H.; Cole, C.; et al. Purification and activation of recombinant p38 isoforms alpha, beta, gamma, and delta. Protein Expr. Purif. 1998, 14, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the c-Jun N-terminal kinase. Biochem. Soc. Symp. 1999, 64, 1–12. [Google Scholar] [PubMed]
- Boulton, T.G.; Nye, S.H.; Robbins, D.J.; Ip, N.Y.; Radziejewska, E.; Morgenbesser, S.D.; DePinho, R.A.; Panayotatos, N.; Cobb, M.H.; Yancopoulos, G.D. ERKs: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991, 65, 663–675. [Google Scholar] [CrossRef]
- Raingeaud, J.; Gupta, S.; Rogers, J.S.; Dickens, M.; Han, J.; Ulevitch, R.J.; Davis, R.J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995, 270, 7420–7426. [Google Scholar] [CrossRef]
- Clerk, A.; Fuller, S.J.; Michael, A.; Sugden, P.H. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998, 273, 7228–7234. [Google Scholar] [CrossRef]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos. Trans. R Soc. Lond. B Biol. Sci. 1996, 351, 127–134. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; MacGibbon, G.; Dragunow, M.; Cooper, G.J. Increased expression and activation of c-Jun contributes to human amylin-induced apoptosis in pancreatic islet beta-cells. J. Mol. Biol. 2002, 324, 271–285. [Google Scholar] [CrossRef]
- Wang, J.; Van De Water, T.R.; Bonny, C.; de Ribaupierre, F.; Puel, J.L.; Zine, A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J. Neurosci. 2003, 23, 8596–8607. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Cecka, J.M.; Gjertson, D.W.; Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 1995, 333, 333–336. [Google Scholar] [CrossRef]
- Pratschke, J.; Wilhelm, M.J.; Kusaka, M.; Laskowski, I.; Tilney, N.L. A model of gradual onset brain death for transplant-associated studies in rats. Transplantation 2000, 69, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nadeau, K.C.; Hancock, W.W.; Mackenzie, H.S.; Shaw, G.D.; Waaga, A.M.; Chandraker, A.; Sayegh, M.H.; Tilney, N.L. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation 1998, 65, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, M.; Pratschke, J.; Wilhelm, M.J.; Ziai, F.; Zandi-Nejad, K.; Mackenzie, H.S.; Hancock, W.W.; Tilney, N.L. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation 2000, 69, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J.; Pratschke, J.; Beato, F.; Taal, M.; Kusaka, M.; Hancock, W.W.; Tilney, N.L. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation 2000, 102, 2426–2433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contreras, J.L.; Eckstein, C.; Smyth, C.A.; Sellers, M.T.; Vilatoba, M.; Bilbao, G.; Rahemtulla, F.G.; Young, C.J.; Thompson, J.A.; Chaudry, I.H.; et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 2003, 52, 2935–2942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pratschke, J.; Wilhelm, M.J.; Kusaka, M.; Hancock, W.W.; Tilney, N.L. Activation of proinflammatory genes in somatic organs as a consequence of brain death. Transplant. Proc. 1999, 31, 1003–1005. [Google Scholar] [CrossRef]
- Lakey, J.R.; Burridge, P.W.; Shapiro, A.M. Technical aspects of islet preparation and transplantation. Transpl. Int. 2003, 16, 613–632. [Google Scholar] [CrossRef]
- Toyama, H.; Takada, M.; Suzuki, Y.; Kuroda, Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant. 2003, 12, 27–32. [Google Scholar] [CrossRef]
- Angele, M.K.; Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Effect of gender and sex hormones on immune responses following shock. Shock 2000, 14, 81–90. [Google Scholar] [CrossRef]
- Jarrar, D.; Wang, P.; Knöferl, M.W.; Kuebler, J.F.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery 2000, 128, 246–252. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Köditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef] [PubMed]
- Eckhoff, D.E.; Eckstein, C.; Smyth, C.A.; Vilatoba, M.; Bilbao, G.; Rahemtulla, F.G.; Young, C.J.; Anthony Thompson, J.; Chaudry, I.H.; Contreras, J.L. Enhanced isolated pancreatic islet recovery and functionality in rats by 17beta-estradiol treatment of brain death donors. Surgery 2004, 136, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.L.; Smyth, C.A.; Bilbao, G.; Young, C.J.; Thompson, J.A.; Eckhoff, D.E. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: Molecular mechanisms and islet functionality. Transplantation 2002, 74, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Eckhoff, D.E.; Smyth, C.A.; Eckstein, C.; Bilbao, G.; Young, C.J.; Thompson, J.A.; Contreras, J.L. Suppression of the c-Jun N-terminal kinase pathway by 17beta-estradiol can preserve human islet functional mass from proinflammatory cytokine-induced destruction. Surgery 2003, 134, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Matsumoto, S.; Onaca, N.; Naziruddin, B.; Jackson, A.; Ikemoto, T.; Shimoda, M.; Fujita, Y.; Chujo, D.; Iwanaga, Y.; et al. Ductal injection of JNK inhibitors before pancreas preservation prevents islet apoptosis and improves islet graft function. Hum. Gene Ther. 2009, 20, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Levy, M.F.; Kobayashi, N.; Matsumoto, S. Pancreas preservation by the two-layer method: Does it have a beneficial effect compared with simple preservation in University of Wisconsin solution? Cell Transplant. 2009, 18, 497–503. [Google Scholar] [CrossRef]
- Hiraoka, K.; Trexler, A.; Eckman, E.; Stage, A.; Nevile, S.; Sageshima, J.; Shibata, S.; Sutherland, D.E.; Hering, B.J. Successful pancreas preservation before islet isolation by the simplified two-layer cold storage method. Transplant. Proc. 2001, 33, 952–953. [Google Scholar] [CrossRef]
- Tanioka, Y.; Sutherland, D.E.; Kuroda, Y.; Gilmore, T.R.; Asaheim, T.C.; Kronson, J.W.; Leone, J.P. Excellence of the two-layer method (University of Wisconsin solution/perfluorochemical) in pancreas preservation before islet isolation. Surgery 1997, 122, 435–441. [Google Scholar] [CrossRef]
- Noguchi, H.; Ueda, M.; Nakai, Y.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Kobayashi, N.; Nakamura, T.; Wada, H.; et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am. J. Transplant. 2006, 6, 496–504. [Google Scholar] [CrossRef]
- Tanioka, Y.; Kuroda, Y.; Kim, Y.; Matsumoto, S.; Suzuki, Y.; Ku, Y.; Fujita, H.; Saitoh, Y. The effect of ouabain (inhibitor of an ATP-dependent Na+/K+ pump) on the pancreas graft during preservation by the two-layer method. Transplantation 1996, 62, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Fujino, Y.; Kawamura, T.; Suzuki, Y.; Fujiwara, H.; Saitoh, Y. Mechanism of oxygenation of pancreas during preservation by a two-layer (Euro-Collins’ solution/perfluorochemical) cold-storage method. Transplantation 1990, 49, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Fujino, Y.; Morita, A.; Ku, Y.; Saitoh, Y. Correlation between high adenosine triphosphate tissue concentration and good posttransplant outcome for the canine pancreas graft after preservation by the two-layer cold storage method. Transplantation 1991, 52, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Fujita, H.; Matsumoto, S.; Suzuki, Y.; Kim, Y.; Tanioka, Y.; Ku, Y. Protection of canine pancreatic microvascular endothelium against cold ischemic injury during preservation by the two-layer method. Transplantation 1997, 64, 948–953. [Google Scholar] [CrossRef]
- Matsuda, T.; Suzuki, Y.; Tanioka, Y.; Toyama, H.; Kakinoki, K.; Hiraoka, K.; Fujino, Y.; Kuroda, Y. Pancreas preservation by the 2-layer cold storage method before islet isolation protects isolated islets against apoptosis through the mitochondrial pathway. Surgery 2003, 134, 437–445. [Google Scholar] [CrossRef]
- Jin, S.M.; Kim, K.S.; Lee, S.Y.; Gong, C.H.; Park, S.K.; Shin, J.S.; Park, C.G.; Kim, S.J. The sequential combination of a JNK inhibitor and simvastatin protects porcine islets from peritransplant apoptosis and inflammation. Cell Transplant. 2011, 20, 1139–1151. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kitamura, S.; Kobayashi, N.; Saitoh, I.; et al. A Novel Preservation Solution Containing a JNK Inhibitory Peptide Efficiently Improves Islet Yield for Porcine Islet Isolation. Transplantation 2019, 103, 344–352. [Google Scholar] [CrossRef]
- Davalli, A.M.; Ogawa, Y.; Ricordi, C.; Scharp, D.W.; Bonner-Weir, S.; Weir, G.C. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995, 59, 817–820. [Google Scholar] [CrossRef]
- Brancho, D.; Tanaka, N.; Jaeschke, A.; Ventura, J.J.; Kelkar, N.; Tanaka, Y.; Kyuuma, M.; Takeshita, T.; Flavell, R.A.; Davis, R.J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003, 17, 1969–1978. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Baltimore, D. I kappa B: A specific inhibitor of the NF-kappa B transcription factor. Science 1988, 242, 540–546. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): Differences revealed by gene targeting. Bioessays 2006, 28, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, S.; Puyal, J.; Bielmann, C.; Buchillier, V.; Abderrahmani, A.; Clarke, P.G.; Beckmann, J.S.; Bonny, C. JNK3 is abundant in insulin-secreting cells and protects against cytokine-induced apoptosis. Diabetologia 2009, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Varona-Santos, J.L.; Pileggi, A.; Molano, R.D.; Sanabria, N.Y.; Ijaz, A.; Atsushi, M.; Ichii, H.; Pastori, R.L.; Inverardi, L.; Ricordi, C.; et al. c-Jun N-terminal kinase 1 is deleterious to the function and survival of murine pancreatic islets. Diabetologia 2008, 51, 2271–2280. [Google Scholar] [CrossRef][Green Version]
- Moberg, L.; Johansson, H.; Lukinius, A.; Berne, C.; Foss, A.; Källen, R.; Østraat, Ø.; Salmela, K.; Tibell, A.; Tufveson, G.; et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002, 360, 2039–2045. [Google Scholar] [CrossRef]
- Bennet, W.; Sundberg, B.; Growth, C.G.; Brendel, M.D.; Brandhorst, D.; Brandhorst, H.; Bretzel, R.G.; Elgue, G.; Larsson, R.; Nilsson, B.; et al. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 1999, 48, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Moberg, L.; Olsson, A.; Berne, C.; Felldin, M.; Foss, A.; Källen, R.; Salmela, K.; Tibell, A.; Tufveson, G.; Nilsson, B.; et al. Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: Implications for clinical islet transplantation. Transplantation 2003, 76, 1285–1288. [Google Scholar] [CrossRef]
- Badet, L.; Titus, T.; Metzen, E.; Handa, A.; McShane, P.; Chang, L.W.; Giangrande, P.; Gray, D.W. The interaction between primate blood and mouse islets induces accelerated clotting with islet destruction. Xenotransplantation 2002, 9, 91–96. [Google Scholar] [CrossRef]
- Bottino, R.; Fernandez, L.A.; Ricordi, C.; Lehmann, R.; Tsan, M.F.; Oliver, R.; Inverardi, L. Transplantation of allogeneic islets of Langerhans in the rat liver: Effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 1998, 47, 316–323. [Google Scholar] [CrossRef]
- Fernandez, L.; Bottino, R.; Oliver, R.; Lehmann, R.; Ricordi, C.; Fu Tsan, M.; Inverardi, L. Endothelial cell dysfunction after intraportal islet cell transplant in rats. Transplant. Proc. 1997, 29, 2064–2065. [Google Scholar] [CrossRef]
- Fontaine, M.J.; Blanchard, J.; Rastellini, C.; Lazda, V.; Herold, K.C.; Pollak, R. Pancreatic islets activate portal vein endothelial cells in vitro. Ann. Clin. Lab. Sci. 2002, 32, 352–361. [Google Scholar]
- Arita, S.; Une, S.; Ohtsuka, S.; Atiya, A.; Kasraie, A.; Shevlin, L.; Mullen, Y. Prevention of primary islet isograft nonfunction in mice with pravastatin. Transplantation 1998, 65, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.B.; Gores, P.F.; Field, M.J.; Farney, A.C.; Gruber, S.A.; Stephanian, E.; Sutherland, D.E. Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes 1994, 43, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kenmochi, T.; Miyamoto, M.; Mullen, Y. Protection of mouse islet isografts from nonspecific inflammatory damage by recipient treatment with nicotinamide and 15-deoxyspergualin. Cell Transplant. 1996, 5, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xenos, E.S.; Stevens, R.B.; Sutherland, D.E.; Lokeh, A.; Ansite, J.D.; Casanova, D.; Gores, P.F.; Platt, J.L. The role of nitric oxide in IL-1 beta-mediated dysfunction of rodent islets of Langerhans. Implications for the function of intrahepatic islet grafts. Transplantation 1994, 57, 1208–1212. [Google Scholar] [CrossRef]
- Eriksson, O.; Eich, T.; Sundin, A.; Tibell, A.; Tufveson, G.; Andersson, H.; Felldin, M.; Foss, A.; Kyllönen, L.; Langstrom, B.; et al. Positron emission tomography in clinical islet transplantation. Am. J. Transplant. 2009, 9, 2816–2824. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Larson, C.S.; Baker, M.S.; Kaufman, D.B. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation 2006, 81, 1421–1427. [Google Scholar] [CrossRef]
- Saudek, F.; Jirák, D.; Girman, P.; Herynek, V.; Dezortová, M.; Kríz, J.; Peregrin, J.; Berková, Z.; Zacharovová, K.; Hájek, M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010, 90, 1602–1606. [Google Scholar] [CrossRef]
- Eich, T.; Eriksson, O.; Lundgren, T.; Nordic Network for Clinical Islet Transplantation. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N. Engl. J. Med. 2007, 356, 2754–2755. [Google Scholar] [CrossRef]
- Wuillemin, W.A.; te Velthuis, H.; Lubbers, Y.T.; de Ruig, C.P.; Eldering, E.; Hack, C.E. Potentiation of C1 inhibitor by glycosaminoglycans: Dextran sulfate species are effective inhibitors of in vitro complement activation in plasma. J. Immunol. 1997, 159, 1953–1960. [Google Scholar]
- Fiorante, P.; Banz, Y.; Mohacsi, P.J.; Kappeler, A.; Wuillemin, W.A.; Macchiarini, P.; Roos, A.; Daha, M.R.; Schaffner, T.; Haeberli, A.; et al. Low molecular weight dextran sulfate prevents complement activation and delays hyperacute rejection in pig-to-human xenotransplantation models. Xenotransplantation 2001, 8, 24–35. [Google Scholar] [CrossRef]
- Johansson, H.; Goto, M.; Dufrane, D.; Siegbahn, A.; Elgue, G.; Gianello, P.; Korsgren, O.; Nilsson, B. Low molecular weight dextran sulfate: A strong candidate drug to block IBMIR in clinical islet transplantation. Am. J. Transplant. 2006, 6, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Magnusson, C.; Lundgren, T.; Korsgren, O.; Nilsson, B. Low molecular weight dextran sulfate is well tolerated in humans and increases endogenous expression of islet protective hepatocyte growth factor. Transplantation 2008, 86, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Von Zur-Mühlen, B.; Lundgren, T.; Bayman, L.; Berne, C.; Bridges, N.; Eggerman, T.; Foss, A.; Goldstein, J.; Jenssen, T.; Jorns, C.; et al. Open Randomized Multicenter Study to Evaluate Safety and Efficacy of Low Molecular Weight Sulfated Dextran in Islet Transplantation. Transplantation 2019, 103, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.L.; Eckstein, C.; Smyth, C.A.; Bilbao, G.; Vilatoba, M.; Ringland, S.E.; Young, C.; Thompson, J.A.; Fernández, J.A.; Griffin, J.H.; et al. Activated protein C preserves functional islet mass after intraportal transplantation: A novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes 2004, 53, 2804–2814. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.; Fernández, J.A. Activated protein C: Potential therapy for severe sepsis, thrombosis, and stroke. Semin. Hematol. 2002, 39, 197–205. [Google Scholar] [CrossRef]
- Joyce, D.E.; Gelbert, L.; Ciaccia, A.; DeHoff, B.; Grinnell, B.W. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J. Biol Chem. 2001, 276, 11199–11203. [Google Scholar] [CrossRef]
- White, B.; Schmidt, M.; Murphy, C.; Livingstone, W.; O’Toole, D.; Lawler, M.; O’Neill, L.; Kelleher, D.; Schwarz, H.P.; Smith, O.P. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br. J. Haematol. 2000, 110, 130–134. [Google Scholar] [CrossRef]
- Grey, S.T.; Tsuchida, A.; Hau, H.; Orthner, C.L.; Salem, H.H.; Hancock, W.W. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J. Immunol. 1994, 153, 3664–3672. [Google Scholar]
- Shibata, M.; Kumar, S.R.; Amar, A.; Fernandez, J.A.; Hofman, F.; Griffin, J.H.; Zlokovic, B.V. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 2001, 103, 1799–1805. [Google Scholar] [CrossRef]
- Matsumoto, S.; Takita, M.; Chaussabel, D.; Noguchi, H.; Shimoda, M.; Sugimoto, K.; Itoh, T.; Chujo, D.; SoRelle, J.; Onaca, N.; et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant. 2011, 20, 1641–1647. [Google Scholar] [CrossRef]
- Noguchi, H.; Nakai, Y.; Ueda, M.; Masui, Y.; Futaki, S.; Kobayashi, N.; Hayashi, S.; Matsumoto, S. Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia 2007, 50, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A. Therapeutic promise of JNK ATP-noncompetitive inhibitors. Trends Mol. Med. 2005, 11, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Messoussi, A.; Feneyrolles, C.; Bros, A.; Deroide, A.; Daydé-Cazals, B.; Chevé, G.; Van Hijfte, N.; Fauvel, B.; Bougrin, K.; Yasri, A. Recent progress in the design, study, and development of c-Jun N-terminal kinase inhibitors as anticancer agents. Chem. Biol. 2014, 21, 1433–1443. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A.; Ngoei, K.R.; Zhao, T.T.; Yeap, Y.Y.; Ng, D.C. c-Jun N-terminal kinase (JNK) signaling: Recent advances and challenges. Biochim. Biophys. Acta 2010, 1804, 463–475. [Google Scholar] [CrossRef]
- Noguchi, H.; Matsushita, M.; Okitsu, T.; Moriwaki, A.; Tomizawa, K.; Kang, S.; Li, S.T.; Kobayashi, N.; Matsumoto, S.; Tanaka, K.; et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 2004, 10, 305–309. [Google Scholar] [CrossRef]
- Noguchi, H.; Sugimoto, K.; Miyagi-Shiohira, C.; Nakashima, Y.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, Y. RCAN-11R peptide provides immunosuppression for fully mismatched islet allografts in mice. Sci. Rep. 2017, 7, 3043. [Google Scholar] [CrossRef]
- Noguchi, H.; Matsushita, M.; Kobayashi, N.; Levy, M.F.; Matsumoto, S. Recent advances in protein transduction technology. Cell Transplant. 2010, 19, 649–654. [Google Scholar] [CrossRef]
- Matsushita, M.; Tomizawa, K.; Moriwaki, A.; Li, S.T.; Terada, H.; Matsui, H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J. Neurosci. 2001, 21, 6000–6007. [Google Scholar] [CrossRef]
- Noguchi, H.; Nakai, Y.; Matsumoto, S.; Kawaguchi, M.; Ueda, M.; Okitsu, T.; Iwanaga, Y.; Yonekawa, Y.; Nagata, H.; Minami, K.; et al. Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am. J. Transplant. 2005, 5, 1848–1855. [Google Scholar] [CrossRef]
- Fornoni, A.; Pileggi, A.; Molano, R.D.; Sanabria, N.Y.; Tejada, T.; Gonzalez-Quintana, J.; Ichii, H.; Inverardi, L.; Ricordi, C.; Pastori, R.L. Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia 2008, 51, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M. Modified cell-permeable JNK inhibitors efficiently prevents islet apoptosis and improves the outcome of islet transplantation. Sci. Rep. 2018, 8, 11082. [Google Scholar] [CrossRef] [PubMed]
- Borsello, T.; Clarke, P.G.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Ao, Z.; Kieffer, T.J.; Chen, H.; Safikhan, N.; Thompson, D.M.; Meloche, M.; Warnock, G.L.; Marzban, L. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: Implications in type 2 diabetes and islet transplantation. Diabetologia 2013, 56, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z.; Gou, W.; Adams, D.B.; Cui, W.; Morgan, K.A.; Strange, C.; Wang, H. α-1 Antitrypsin Enhances Islet Engraftment by Suppression of Instant Blood-Mediated Inflammatory Reaction. Diabetes 2017, 66, 970–980. [Google Scholar] [CrossRef]
- Nardelli, T.R.; Vanzela, E.C.; Benedicto, K.C.; Brozzi, F.; Fujita, A.; Cardozo, A.K.; Eizirik, D.L.; Boschero, A.C.; Ortis, F. Prolactin protects against cytokine-induced beta-cell death by NFκB and JNK inhibition. J. Mol. Endocrinol. 2018, 61, 25–36. [Google Scholar] [CrossRef]
- Wang, J.; Gou, W.; Kim, D.S.; Strange, C.; Wang, H. Clathrin-mediated Endocytosis of Alpha-1 Antitrypsin is Essential for its Protective Function in Islet Cell Survival. Theranostics 2019, 9, 3940–3951. [Google Scholar] [CrossRef]
- Kaneto, H.; Xu, G.; Fujii, N.; Kim, S.; Bonner-Weir, S.; Weir, G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002, 277, 30010–30018. [Google Scholar] [CrossRef]
- Kawamori, D.; Kajimoto, Y.; Kaneto, H.; Umayahara, Y.; Fujitani, Y.; Miyatsuka, T.; Watada, H.; Leibiger, I.B.; Yamasaki, Y.; Hori, M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 2003, 52, 2896–2904. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.M.; Davis, R.J. Targeting JNK for therapeutic benefit: From junk to gold? Nat. Rev. Drug Discov. 2003, 2, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Omori, K.; Vuong, T.; Pascual, M.; Valiente, L.; Ferreri, K.; Todorov, I.; Kuroda, Y.; Smith, C.V.; Kandeel, F.; et al. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am. J. Transplant. 2005, 5, 484–493. [Google Scholar] [CrossRef]
- Ito, T.; Omori, K.; Rawson, J.; Todorov, I.; Asari, S.; Kuroda, A.; Shintaku, J.; Itakura, S.; Ferreri, K.; Kandeel, F.; et al. Improvement of canine islet yield by donor pancreas infusion with a p38MAPK inhibitor. Transplantation 2008, 86, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Todorov, I.; Shintaku, J.; Rawson, J.; Al-Abdullah, I.H.; Higgins, L.S.; Medicherla, S.; Kandeel, F.; Mullen, Y. P38alpha-selective mitogen-activated protein kinase inhibitor for improvement of cultured human islet recovery. Pancreas 2010, 39, 436–443. [Google Scholar] [CrossRef]
- Collier, J.J.; Fueger, P.T.; Hohmeier, H.E.; Newgard, C.B. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 2006, 55, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Steer, S.A.; Scarim, A.L.; Chambers, K.T.; Corbett, J.A. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006, 3, e17. [Google Scholar]

| Agents | Administration Step | Effect | Year | Reference |
|---|---|---|---|---|
| 17β-estradiol | Brain death | Reduction in JNK activation, nuclear AP-1, c-fos, Jun-D, and ATF-2 activities Enhancement of islet viability and islet mass | 2003 | [46] |
| Cell-permeable peptide inhibitor (11R-JNKI) | Culture | Prevention of islet apoptosis Improvement of islet graft function | 2005 | [101] |
| Cell-permeable peptide inhibitor (11R-JNKI) | Transplantation | Prevention of islet graft loss Improvement of islet transplant outcome | 2007 | [92] |
| Cell-permeable TAT peptide inhibitor (L-JNKI) | Culture | Reduction of the islet loss in culture and protection from cell death regulation of AKT/GSK3B activity | 2008 | [102] |
| Cell-permeable peptide inhibitor (11R-JNKI), SP600125 | Pancreas preservation | Prevention of JNK activation during the isolation procedure Improvement of islet transplant outcome | 2009 | [47] |
| SP600125 (+ simvastatin) | Pancreas preservation | Increase of the β-cell viability index and islet survival rate | 2011 | [57] |
| GLP-1 1 receptor agonist (exenatide) | Culture | Lower JNK and caspase-3 activation and β-cell apoptosis | 2013 | [105] |
| α-1 antitrypsin | Transplantation | Suppression of JNK phosphorylation Suppression of blood-mediated coagulation pathways | 2017 | [106] |
| Prolactin | Culture | Prevention of the activation of JNK via AKT | 2018 | [107] |
| Cell-permeable peptide inhibitor (8R-sJNKI) | Culture | Prevention of islet apoptosis Improvement of islet graft function | 2018 | [103] |
| Cell-permeable peptide inhibitor (8R-sJNKI) | Pancreas preservation | Prevention of JNK activation during the isolation procedure Improvement of islet transplant outcome | 2019 | [58] |
| α-1 antitrypsin | i.p. injection 24 h before islet isolation | Suppression of JNK phosphorylation Suppression of caspase 9 activation | 2019 | [108] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, H. Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation. J. Clin. Med. 2019, 8, 1763. https://doi.org/10.3390/jcm8111763
Noguchi H. Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation. Journal of Clinical Medicine. 2019; 8(11):1763. https://doi.org/10.3390/jcm8111763
Chicago/Turabian StyleNoguchi, Hirofumi. 2019. "Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation" Journal of Clinical Medicine 8, no. 11: 1763. https://doi.org/10.3390/jcm8111763
APA StyleNoguchi, H. (2019). Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation. Journal of Clinical Medicine, 8(11), 1763. https://doi.org/10.3390/jcm8111763





