A 3D Follow-Up Study of Cranial Asymmetry from Early Infancy to Toddler Age after Preterm versus Term Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. 3D Image Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Academy of Pediatrics AAP. Task Force on Infant Positioning and SIDS: Positioning and SIDS. Pediatrics 1992, 89, 1120–1126. [Google Scholar]
- Mawji, A.; Vollman, A.R.; Hatfield, J.; McNeil, D.A.; Sauvé, R. The Incidence of Positional Plagiocephaly: A Cohort Study. Pediatrics 2013, 132, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mawji, A.; Vollman, A.R.; Fung, T.; Hatfield, J.; McNeil, D.A.; Sauvé, R. Risk factors for positional plagiocephaly and appropriate time frames for prevention messaging. Paediatr. Child Health 2014, 19, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Van Vlimmeren, L.A.; Takken, T.; Van Adrichem, L.N.; Van Der Graaf, Y.; Helders, P.J.; Engelbert, R.H. Plagiocephalometry: A non-invasive method to quantify asymmetry of the skull; a reliability study. Eur. J. Pediatr. 2006, 165, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Van Vlimmeren, L.A.; Engelbert, R.H.; Pelsma, M.; Groenewoud, H.M.; Boere-Boonekamp, M.M.; Nijhuis-van der Sanden, M.W. The course of skull deformation from birth to 5 years of age: A prospective cohort study. Eur. J. Pediatr. 2017, 176, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, P.; Böhm, H.; Linz, C.; Kochel, J.; Blecher, C.; Keil, N.; Stellzig-Eisenhauer, A.; Schweitzer, T. Spectrum of positional deformities—Is there a real difference between plagiocephaly and brachycephaly? J. Cranio-Maxillofac. Surg. 2014, 42, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Aarnivala, H.; Vuollo, V.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A. Preventing deformational plagiocephaly through parent guidance: A randomized, controlled trial. Eur. J. Pediatr. 2015, 174, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Aarnivala, H.; Vuollo, V.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Holmström, L.; Valkama, A. The course of positional cranial deformation from 3 to 12 months of age and associated risk factors: A follow-up with 3D imaging. Eur. J. Pediatr. 2016, 175, 1893–1903. [Google Scholar] [CrossRef]
- De Bock, F.; Braun, V.; Renz-Polster, H. Deformational plagiocephaly in normal infants: A systematic review of causes and hypotheses. Arch. Dis. Child. 2017, 102, 535–542. [Google Scholar] [CrossRef]
- Ballardini, E.; Sisti, M.; Basaglia, N.; Benedetto, M.; Baldan, A.; Borgna-Pignatti, C.; Garani, G. Prevalence and characteristics of positional plagiocephaly in healthy full-term infants at 8–12 weeks of life. Eur. J. Pediatr. 2018, 177, 1547–1554. [Google Scholar] [CrossRef]
- Linz, C.; Kunz, F.; Böhm, H.; Schweitzer, T. Positional Skull Deformities: Etiology, Prevention, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2017, 114, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.; David, L.; Thompson, J. Clinical Classification of Positional Plagiocephaly. J. Craniofac. Surg. 2004, 15, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.F. Deformational plagiocephaly, brachycephaly, and scaphocephaly. Part I: Terminology, diagnosis, and etiopathogenesis. J. Craniofac. Surg. 2011, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Looman, W.S.; Flannery, A.B. Evidence-Based Care of the Child With Deformational Plagiocephaly, Part I: Assessment and Diagnosis. J. Pediatr. Health Care 2012, 26, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.B.K.; Looman, W.S.; Kemper, K. Evidence-based care of the child with deformational plagiocephaly, part II: Management. J. Pediatr. Health Care 2012, 26, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Branch, L.G.; Kesty, K.; Krebs, E.; Wright, L.; Leger, S.; David, L.R. Argenta Clinical Classification of Deformational Plagiocephaly. J. Craniofac. Surg. 2015, 26, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Bruneteau, R.J.; Mulliken, J.B. Frontal Plagiocephaly: Synostotic, Compensational, or Deformational. Plast. Reconstr. Surg. 1992, 89, 21–31. [Google Scholar] [CrossRef]
- Mewes, A.U.J.; Zöllei, L.; Hüppi, P.S.; Als, H.; McAnulty, G.B.; Inder, T.E.; Wells, W.M.; Warfield, S.K. Displacement of brain regions in preterm infants with non-synostotic dolichocephaly investigated by MRI. Neuroimage 2007, 36, 1074–1085. [Google Scholar] [CrossRef]
- Ifflaender, S.; Rüdiger, M.; Konstantelos, D.; Wahls, K.; Burkhardt, W. Prevalence of head deformities in preterm infants at term equivalent age. Early Hum. Dev. 2013, 89, 1041–1047. [Google Scholar] [CrossRef]
- Geerdink, J.J.; Hopkins, B.; Hoeksma, J.B. The development of head position preference in preterm infants beyond term age. Dev. Psychobiol. 1994, 27, 153–168. [Google Scholar] [CrossRef]
- Chan, J.S.; Kelley, M.L.; Khan, J. Predictors of postnatal head molding in very low birth weight infants. Neonatal Netw. 1995, 14, 47–51. [Google Scholar] [PubMed]
- Lennartsson, F.; Nordin, P. Nonsynostotic plagiocephaly: A child health care intervention in Skaraborg, Sweden. BMC Pediatr. 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Mandrusiak, A.; Watter, P.; Gavranich, J.; Johnston, L.M. Impact of Parent Practices of Infant Positioning on Head Orientation Profile and Development of Positional Plagiocephaly in Healthy Term Infants. Phys. Occup. Ther. Pediatr. 2018, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vuollo, V.; Holmström, L.; Aarnivala, H.; Harila, V.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A.M. Analyzing infant head flatness and asymmetry using kernel density estimation of directional surface data from a craniofacial 3D model. Stat. Med. 2016, 35, 4891–4904. [Google Scholar] [CrossRef] [PubMed]
- Aarnivala, H.; Vuollo, V.; Heikkinen, T.; Harila, V.; Holmström, L.; Pirttiniemi, P.; Valkama, A.M. Accuracy of measurements used to quantify cranial asymmetry in deformational plagiocephaly. J. Craniomaxillofac. Surg. 2017, 45, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, P.; Kunz, F.; Schweitzer, T.; Wachter, B.; Böhm, H.; Waßmuth, N.; Linz, C. Cranial growth in infants—A longitudinal three-dimensional analysis of the first months of life. J. Cranio-Maxillofac. Surg. 2018, 46, 987–993. [Google Scholar] [CrossRef]
- Saari, A.; Sankilampi, U.; Hannila, M.; Kiviniemi, V.; Kesseli, K.; Dunkel, L. New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann. Med. 2011, 43, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Collett, B.R.; Heike, C.L.; Atmosukarto, I.; Starr, J.R.; Cunningham, M.L.; Speltz, M.L. Longitudinal, three-dimensional analysis of head shape in children with and without deformational plagiocephaly or brachycephaly. J. Pediatr. 2012, 160, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, B.L.; Hutchison, L.A.D.; Thompson, J.M.D.; Mitchell, E.A. Plagiocephaly and Brachycephaly in the First Two Years of Life: A Prospective Cohort Study. Pediatrics 2004, 114, 970–980. [Google Scholar] [CrossRef]
- Boere-Boonekamp, M.M.; van der Linden-Kuiper, L.T. Positional Preference: Prevalence in Infants and Follow-Up After Two Years. Pediatrics 2001, 107, 339–343. [Google Scholar] [CrossRef]
- Leung, A.Y.F.; Mandrusiak, A.; Watter, P.; Gavranich, J.; Johnston, L.M. Clinical assessment of head orientation profile development and its relationship with positional plagiocephaly in healthy term infants—A prospective study. Early Hum. Dev. 2016, 96, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nuysink, J.; van Haastert, I.C.; Eijsermans, M.J.C.; Koopman-Esseboom, C.; van der Net, J.; de Vries, L.S.; Helders, P.J.M. Prevalence and predictors of idiopathic asymmetry in infants born preterm. Early Hum. Dev. 2012, 88, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Nuysink, J.; Eijsermans, M.J.C.; van Haastert, I.C.; Koopman-Esseboom, C.; Helders, P.J.M.; de Vries, L.S.; van der Net, J. Clinical course of asymmetric motor performance and deformational plagiocephaly in very preterm infants. J. Pediatr. 2013, 163, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Dunsirn, S.; Smyser, C.; Liao, S.; Inder, T.; Pineda, R. Defining the nature and implications of head turn preference in the preterm infant. Early Hum. Dev. 2016, 96, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ifflaender, S.; Rüdiger, M.; Konstantelos, D.; Lange, U.; Burkhardt, W. Individual course of cranial symmetry and proportion in preterm infants up to 6 months of corrected age. Early Hum. Dev. 2014, 90, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Roby, B.B.; Finkelstein, M.; Tibesar, R.J.; Sidman, J.D. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol. Head Neck Surg. 2012, 146, 823–828. [Google Scholar] [CrossRef] [PubMed]
- John, D.S.; Mulliken, J.B.; Kaban, L.B.; Padwa, B.L. Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. J. Oral Maxillofac. Surg. 2002, 60, 873–877. [Google Scholar] [CrossRef]
- Smartt, J.; James, M.; Elliott, R.M.; Reid, R.R.; Bartlett, S.P. Analysis of Differences in the Cranial Base and Facial Skeleton of Patients with Lambdoid Synostosis and Deformational Plagiocephaly. Plast. Reconstr. Surg. 2011, 127, 303–312. [Google Scholar] [CrossRef]
- Moon, I.Y.; Lim, S.Y.; Oh, K.S. Analysis of Facial Asymmetry in Deformational Plagiocephaly Using Three-Dimensional Computed Tomographic Review. Arch. Craniofac. Surg. 2014, 15, 109–116. [Google Scholar] [CrossRef]
- Meyer-Marcotty, P.; Bohm, H.; Linz, C.; Kochel, J.; Stellzig-Eisenhauer, A.; Schweitzer, T. Three-dimensional analysis of cranial growth from 6 to 12 months of age. Eur. J. Orthod. 2014, 36, 489–496. [Google Scholar] [CrossRef]
- Schaaf, H.; Pons-Kuehnemann, J.; Malik, C.Y.; Streckbein, P.; Preuss, M.; Howaldt, H.P.; Wilbrand, J.F. Accuracy of Three-Dimensional Photogrammetric Images in Non-Synostotic Cranial Deformities. Neuropediatrics 2010, 41, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, G.B.; Naidoo, S.D.; Nguyen, D.C.; Patel, K.B.; Woo, A.S. Comparison of Direct and Digital Measures of Cranial Vault Asymmetry for Assessment of Plagiocephaly. J. Craniofac. Surg. 2015, 26, 1900–1903. [Google Scholar] [CrossRef]
- Bialocerkowski, A.E.; Vladusic, S.L.; Wei Ng, C. Prevalence, risk factors, and natural history of positional plagiocephaly: A systematic review. Dev. Med. Child Neurol. 2008, 50, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wilbrand, J.; Schmidtberg, K.; Bierther, U.; Streckbein, P.; Pons-Kuehnemann, J.; Christophis, P.; Hahn, A.; Schaaf, H.; Howaldt, H. Clinical classification of infant nonsynostotic cranial deformity. J. Pediatr. 2012, 161, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, B.L.; Hutchison, L.A.D.; Thompson, J.M.D.; Mitchell, E.A. Quantification of Plagiocephaly and Brachycephaly in Infants Using a Digital Photographic Technique. Cleft Palate Craniofac. J. 2005, 42, 539–547. [Google Scholar] [CrossRef] [PubMed]
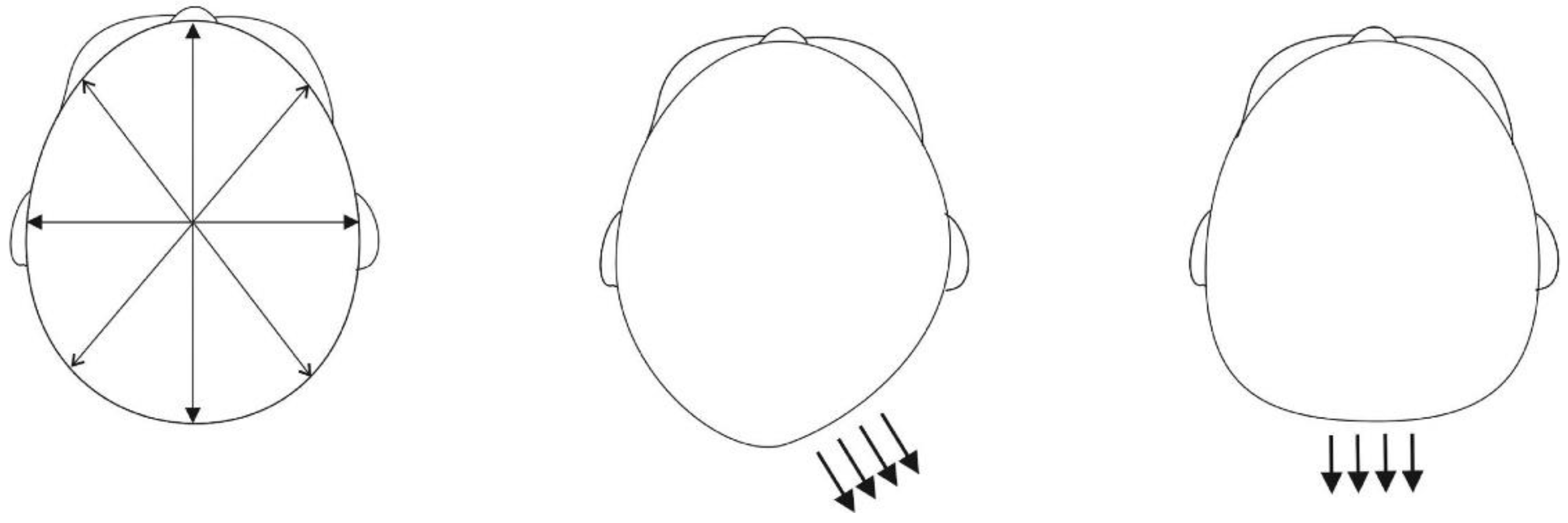
| Characteristic | Preterm | Term | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Mean (SD) | Median | Range | p | |
| Maternal Age, years | 31.3 (5.7) | 31.5 | 26.8–35.0 | 30.9 (5.4) | 30.5 | 26.8–36.0 | 0.779 1 |
| Gestational Age, weeks | 32.7 (3.2) | 33.3 | 31.1–35.3 | 40.0 (1.2) | 40.0 | 39.1–41.0 | 0.000 2 |
| Birth: | |||||||
| Weight, g | 2006.5 (712.3) | 1887.5 | 1460.0–2645.0 | 3528.5 (361.7) | 3470.0 | 3215.0–3745.0 | 0.000 2 |
| Length, cm | 42.0 (5.0) | 42.5 | 38.8–46.3 | 50.5 (1.8) | 50.25 | 49.0–52.0 | 0.000 1 |
| Head Circumference, cm | 30.6 (3.2) | 31.5 | 28.9–32.6 | 34.9 (1.3) | 34.5 | 34.0–35.6 | 0.000 2 |
| T1: | |||||||
| Weight, kg | 5.9 (1.0) | 5.93 | 5.1–6.3 | 6.4 (9.7) | 6.4 | 5.7–7.9 | 0.035 1 |
| Length, cm | 59.2 (2.9) | 58.6 | 57.4–61.0 | 61.8 (3.3) | 62.25 | 59.3–64.6 | 0.001 1 |
| Head Circumference, cm | 41.1 (1.8) | 40.85 | 39.5–42.6 | 41.7 (1.7) | 41.45 | 40.7–43.0 | 0.145 1 |
| T2: | |||||||
| Weight, kg | 7.9 (1.1) | 7.8 | 7.1–8.8 | 8.0 (1.0) | 8.1 | 7.3–8.5 | 0.739 1 |
| Length, cm | 67.2 (2.7) | 67.0 | 65.7–68.2 | 67.9 (2.6) | 67.9 | 65.6–70.2 | 0.263 1 |
| Head Circumference, cm | 44.4 (1.4) | 44.2 | 43.6–45.4 | 44.5 (1.6) | 44.5 | 43.4–45.8 | 0.876 1 |
| T3: | |||||||
| Weight, kg | 9.9 (1.4) | 9.77 | 8.8–10.5 | 9.9 (1.2) | 9.9 | 9.0–10.4 | 0.971 1 |
| Length, cm | 75.4 (2.9) | 75.1 | 73.5–77.2 | 76.9 (4.2) | 76.5 | 74.0–78.5 | 0.170 2 |
| Head Circumference, cm | 47.6 (1.1) | 47.6 | 46.8–48.2 | 47.4 (1.5) | 47.3 | 46.3–48.6 | 0.634 1 |
| T4: | |||||||
| Weight, kg, | 14.4 (1.8) | 14.05 | 13.0–15.6 | 14.6 (1.6) | 14.75 | 13.7–15.1 | 0.397 2 |
| Length, cm | 94.4 (3.8) | 94.2 | 91.2–95.5 | 95.5 (4.5) | 95.85 | 93.0–98.4 | 0.277 1 |
| Head Circumference, cm | 50.8 (1.2) | 51.0 | 49.7–51.9 | 51.1 (1.5) | 50.9 | 50.0–52.4 | 0.442 1 |
| Parameters at T1–T4 (PT/T, Numbers) | Preterm | Term | Mann-Whitney | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Mean (SD) | Median | Range | p | |
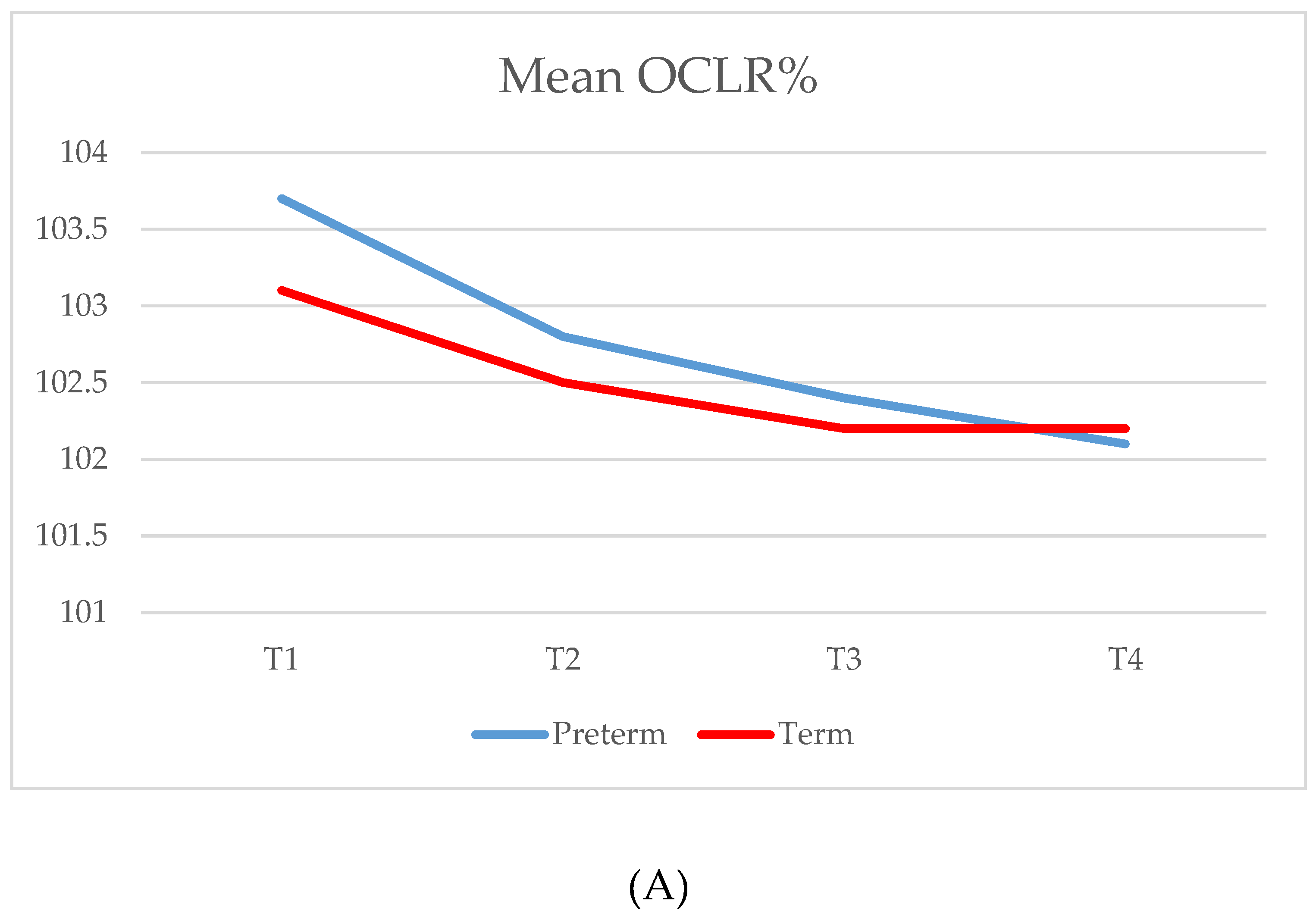
| OCLR % | |||||||
| T1 (34/34) | 103.7 (2.8) | 103.1 | 101.1–105.8 | 103.1 (2.3) | 102.5 | 101.1–104.6 | 0.425 |
| T2 (32/34) | 102.8 (2.3) | 102.2 | 101.2–103.7 | 102.5 (2.3) | 101.6 | 100.7–104.0 | 0.330 |
| T3 (30/34) | 102.4 (2.0) | 101.8 | 100.9–103.5 | 102.2 (1.9) | 101.6 | 100.7–103.3 | 0.778 |
| T4 (32/33) | 102.1 (1.4) | 101.7 | 101.0–103.0 | 102.2 (1.7) | 101.9 | 100.9–103.3 | 0.958 |
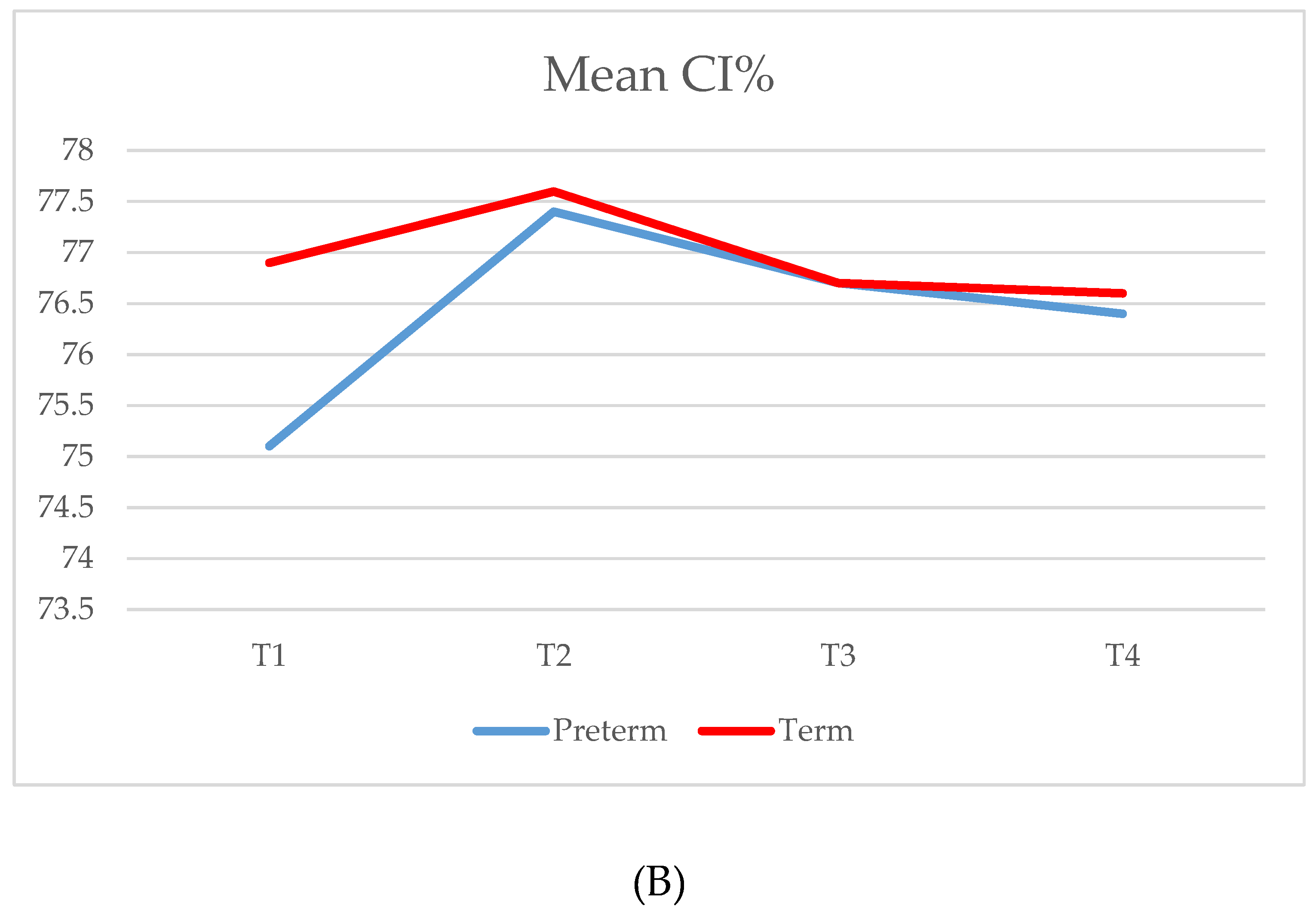
| CI % | |||||||
| T1 (34/34) | 75.1 (5.4) | 73.2 | 71.4–78.7 | 76.9 (3.5) | 77.1 | 73.6–79.3 | 0.057 |
| T2 (32/34) | 77.4 (6.1) | 75.5 | 73.1–80.5 | 77.6 (3.8) | 77.4 | 75.5–80.7 | 0.293 |
| T3 (30/34) | 76.7 (5.7) | 75.6 | 72.8–80.0 | 76.7 (3.3) | 76.7 | 75.1–78.5 | 0.484 |
| T4 (32/33) | 76.4 (4.6) | 76.0 | 73.2–78.9 | 76.6 (3.1) | 76.9 | 73.9–78.0 | 0.529 |
| wAS | |||||||
| T1 (34/34) | 30.7 (36.1) | 19.5 | 3.8–45.4 | 32.7 (67.5) | 14.2 | 7.6–31.6 | 0.990 |
| T2 (32/34) | 19.5 (24.7) | 8.6 | 2.9–24.3 | 23.9 (36.1) | 9.1 | 2.8–28.4 | 0.797 |
| T3 (30/34) | 15.0 (15.1) | 9.3 | 4.2–21.0 | 22.1 (32.7) | 10.2 | 3.5–29.5 | 0.788 |
| T4 (32/33) | 17.6 (21.7) | 11.3 | 3.1–24.9 | 29.0 (34.8) | 15.6 | 6.5–34.0 | 0.160 |
| FS | |||||||
| T1 (34/34) | 0.212 (0.019) | 0.211 | 0.198–0.225 | 0.203 (0.015) | 0.199 | 0.194–0.21 | 0.030 |
| T2 (32/34) | 0.216 (0.022) | 0.218 | 0.196–0.229 | 0.201 (0.011) | 0.200 | 0.194–0.209 | 0.003 |
| T3 (30/34) | 0.206 (0.018) | 0.204 | 0.191–0.218 | 0.196 (0.01) | 0.194 | 0.188–0.202 | 0.037 |
| T4 (32/33) | 0.208 (0.016) | 0.205 | 0.195–0.224 | 0.201 (0.011) | 0.200 | 0.195–0.204 | 0.081 |
| Timepoints | Severity | Preterm | Term |
|---|---|---|---|
| n = 34 | n = 34 | ||
| T1 Plagiocephaly | Total | 13 (38.2%) | 11 (32.4%) |
| Mild | 10 (29.4%) | 10 (29.4%) | |
| Moderate | 3 (8.8%) | 1 (2.9%) | |
| Severe | 0 | 0 | |
| T2 Plagiocephaly | Total | 7 (21.9%) | 8 (23.5%) |
| Mild | 5 (15.6%) | 7 (20.6%) | |
| Moderate | 2 (6.3%) | 1 (2.9%) | |
| Severe | 0 | 0 | |
| T3 Plagiocephaly | Total | 6 (20.0%) | 6 (17.6%) |
| Mild | 6 (20.0%) | 6 (17.6%) | |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 | |
| T4 Plagiocephaly | Total | 4 (12.5%) | 5 (15.2%) |
| Mild | 4 (12.5%) | 5 (15.2%) | |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 |
| Parameters at Timepoints | Kruskal-Wallis | Post-Hoc Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1–T4 | n | VPT | MPT | LPT | MPT-VPT | LPT-VPT | LPT-MPT | ||||
| Median | Range | Median | Range | Median | Range | p | p | p | p | ||
| OCLR % | |||||||||||
| T1 | 10/13/11 | 102.3 | 101.1–104.2 | 102.1 | 101.0–105.3 | 105.8 | 102.5–108.7 | 0.106 | – | - | - |
| T2 | 8/13/11 | 102.0 | 101.3–104.6 | 101.7 | 100.7–103.1 | 103.5 | 101.3–106.7 | 0.262 | – | - | - |
| T3 | 8/13/9 | 102.0 | 101.2–104.0 | 101.2 | 100.3–102.0 | 102.9 | 102.1–106.3 | 0.032 | 0.511 | 0.878 | 0.028 |
| T4 | 10/12/10 | 101.6 | 101.1–102.9 | 101.1 | 100.7–102.5 | 102.6 | 101.7–104.7 | 0.046 | 1.000 | 0.199 | 0.051 |
| CI % | |||||||||||
| T1 | 10/13/11 | 70.0 | 68.4–73.1 | 73.0 | 72.5–77.3 | 78.4 | 75.9–79.2 | 0.003 | 0.115 | 0.002 | 0.404 |
| T2 | 8/13/11 | 72.5 | 70.8–79.1 | 75.4 | 73.5–79.0 | 78.8 | 75.3–83.8 | 0.052 | – | - | - |
| T3 | 8/13/9 | 73.1 | 70.3–77.1 | 75.6 | 72.5–79.0 | 79.3 | 75.1–81.9 | 0.061 | – | - | - |
| T4 | 10/12/10 | 73.4 | 71.0–76.6 | 75.6 | 73.2–80.3 | 77.9 | 76.0–80.6 | 0.039 | 0.582 | 0.032 | 0.517 |
| wAS | |||||||||||
| T1 | 10/13/11 | 13.9 | 3.6–44.3 | 8.8 | 3.4–30.4 | 43.9 | 8.0–88.2 | 0.076 | – | - | - |
| T2 | 8/13/11 | 7.7 | 3.7–14.1 | 8.8 | 2.7–17.5 | 15.6 | 2.6–63.9 | 0.649 | – | - | - |
| T3 | 8/13/9 | 9.0 | 5.4–17.7 | 9.4 | 2.5–15.0 | 10.5 | 5.2–46.2 | 0.477 | – | - | - |
| T4 | 10/12/10 | 4.6 | 2.4–13.8 | 4.8 | 3.1–15.3 | 24.2 | 16.6–45.1 | 0.014 | 1.000 | 0.020 | 0.057 |
| FS | |||||||||||
| T1 | 10/13/11 | 0.218 | 0.209–0.227 | 0.200 | 0.194–0.21 | 0.216 | 0.196–0.228 | 0.042 | 0.056 | 1.000 | 0.190 |
| T2 | 8/13/11 | 0.225 | 0.209–0.231 | 0.204 | 0.192–0.222 | 0.219 | 0.193–0.229 | 0.227 | – | - | - |
| T3 | 8/13/9 | 0.202 | 0.193–0.218 | 0.205 | 0.188–0.230 | 0.204 | 0.193–0.214 | 0.99 | – | - | - |
| T4 | 10/12/10 | 0.206 | 0.201–0.212 | 0.195 | 0.187–0.226 | 0.215 | 0.199–0.225 | 0.428 | – | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Launonen, A.M.; Aarnivala, H.; Kyteas, P.; Vuollo, V.; Heikkinen, T.; Kau, C.H.; Pirttiniemi, P.; Harila, V.; Valkama, A.M. A 3D Follow-Up Study of Cranial Asymmetry from Early Infancy to Toddler Age after Preterm versus Term Birth. J. Clin. Med. 2019, 8, 1665. https://doi.org/10.3390/jcm8101665
Launonen AM, Aarnivala H, Kyteas P, Vuollo V, Heikkinen T, Kau CH, Pirttiniemi P, Harila V, Valkama AM. A 3D Follow-Up Study of Cranial Asymmetry from Early Infancy to Toddler Age after Preterm versus Term Birth. Journal of Clinical Medicine. 2019; 8(10):1665. https://doi.org/10.3390/jcm8101665
Chicago/Turabian StyleLaunonen, Anniina M., Henri Aarnivala, Panagiotis Kyteas, Ville Vuollo, Tuomo Heikkinen, Chung H. Kau, Pertti Pirttiniemi, Virpi Harila, and A. Marita Valkama. 2019. "A 3D Follow-Up Study of Cranial Asymmetry from Early Infancy to Toddler Age after Preterm versus Term Birth" Journal of Clinical Medicine 8, no. 10: 1665. https://doi.org/10.3390/jcm8101665
APA StyleLaunonen, A. M., Aarnivala, H., Kyteas, P., Vuollo, V., Heikkinen, T., Kau, C. H., Pirttiniemi, P., Harila, V., & Valkama, A. M. (2019). A 3D Follow-Up Study of Cranial Asymmetry from Early Infancy to Toddler Age after Preterm versus Term Birth. Journal of Clinical Medicine, 8(10), 1665. https://doi.org/10.3390/jcm8101665






