18F–Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers
Abstract
1. Introduction
2. Methods
2.1. Prostate Samples Collection
2.2. 18F–Choline PET/CT Analysis
2.3. MRI-Guided Biopsies
2.4. Histology
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Histological Classification
3.2. Comparison between 18F–Choline Uptake and Gleason Score
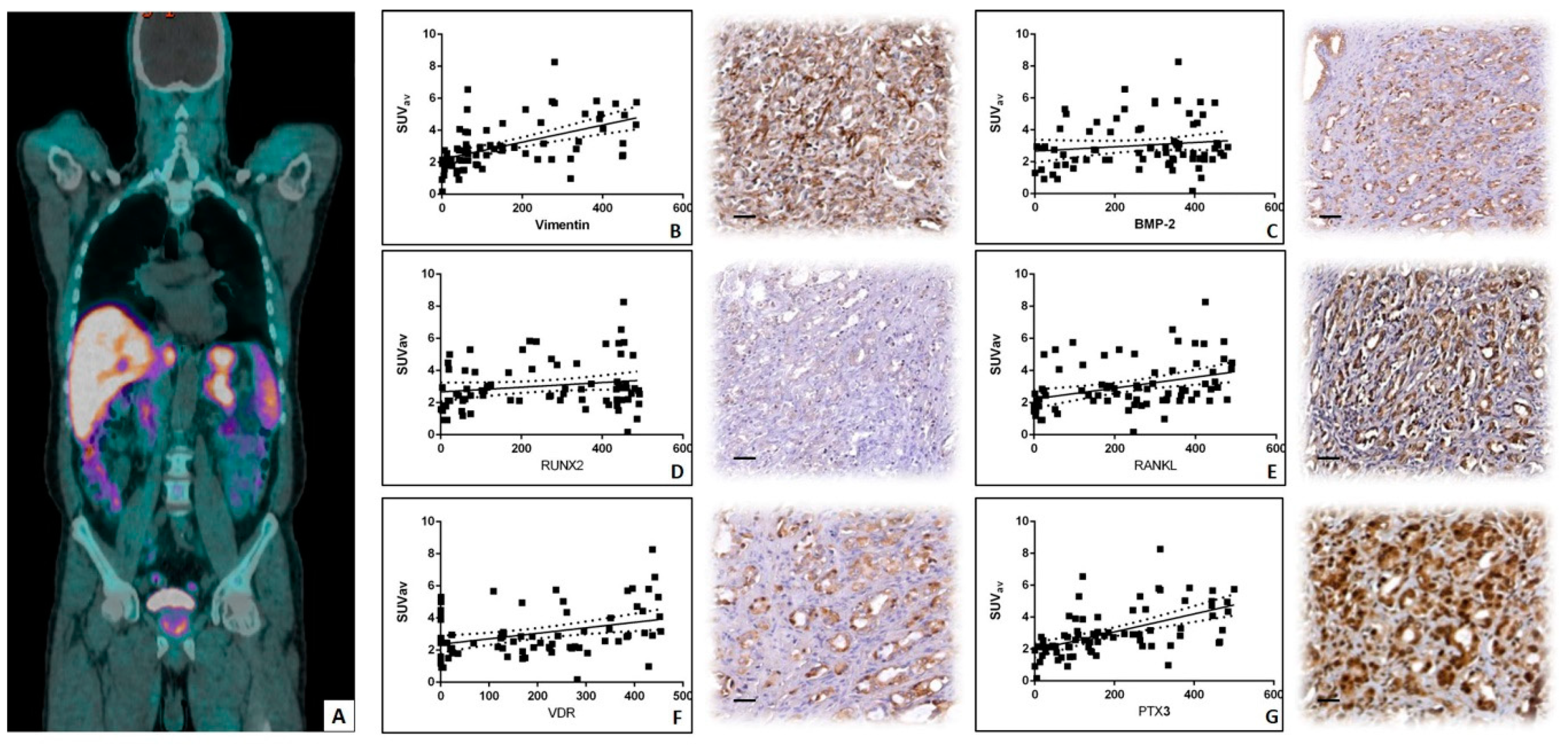
3.3. Linear Regression Analysis between 18F–Choline Uptake and in Situ Biomarkers
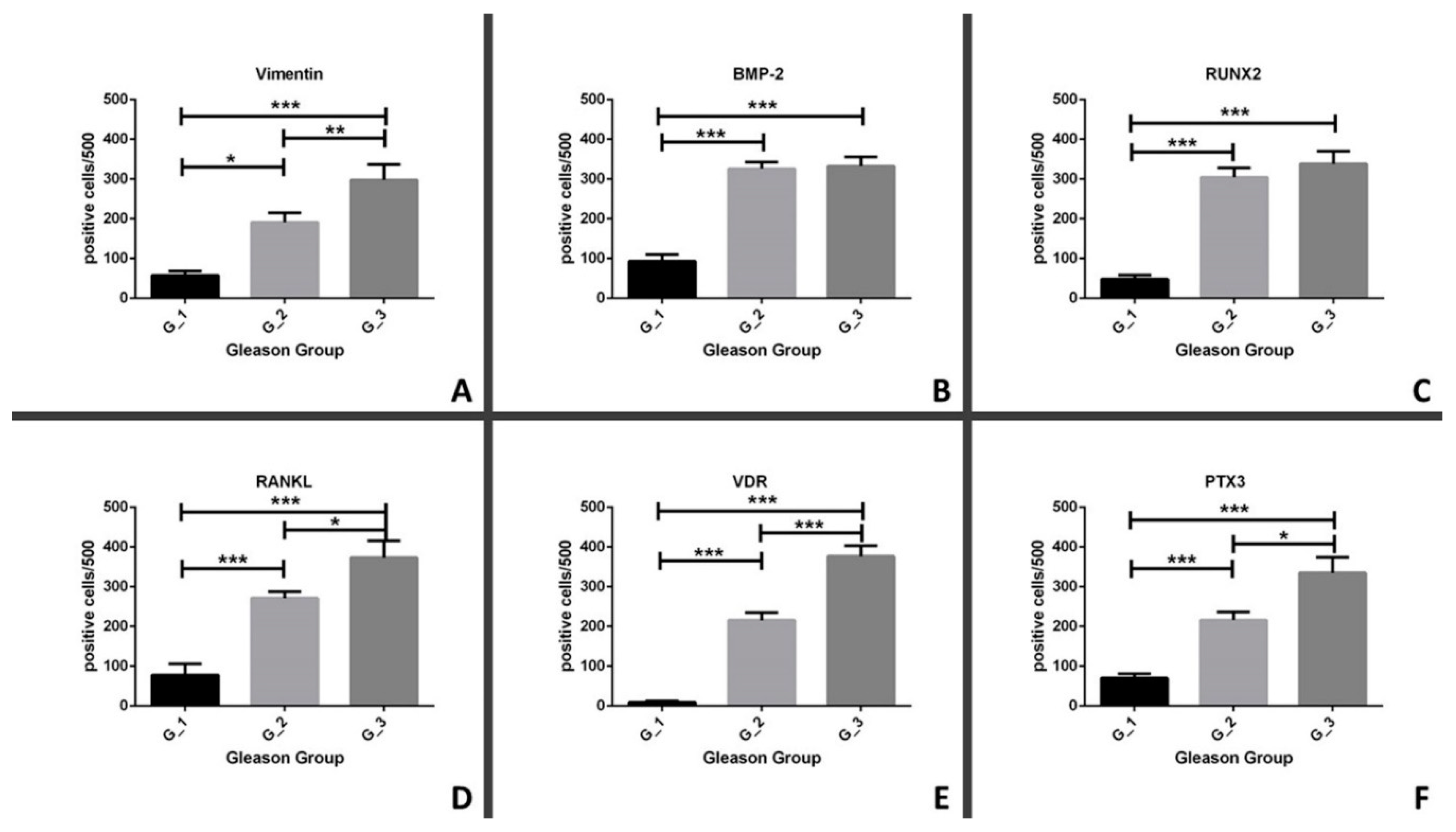
3.4. Comparison between in Situ Biomarkers and Gleason Score
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA A Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Skanjeti, A.; Pelosi, E. Lymph Node Staging with Choline PET/CT in Patients with Prostate Cancer: A Review. ISRN Oncol. 2011, 2011, 219064. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Scattoni, V.; Castellucci, P.; Picchio, M.; Corti, B.; Briganti, A.; Franceschelli, A.; Sanguedolce, F.; Bertaccini, A.; Farsad, M.; et al. 11C-Choline Positron Emission Tomography/Computerized Tomography for Preoperative Lymph-Node Staging in Intermediate-Risk and High-Risk Prostate Cancer: Comparison with Clinical Staging Nomograms. Eur. Urol. 2008, 54, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.; Gallo, G.; Schillaci, O.; Cascini, G.L. Bio-Distribution, Imaging Protocols and Diagnostic Accuracy of PET with Tracers of Lipogenesis in Imaging Prostate Cancer: A Comparison between 11C-Choline, 18FFluoroethylcholine and 18F-Methylcholine. Curr. Pharm. Des. 2015, 21, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.; Chiaravalloti, A.; Cicciò, C.; Gangemi, V.; Gullà, D.; Rocca, F.; Gallo, G.; Cascini, G.L.; Schillaci, O. PET/CT with 18F-choline: Physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients: 18F-choline PET/CT bio-distribution and pitfalls. A southern Italian experience. Nucl. Med. Biol. 2017, 51, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Schillaci, O.; Bonanno, E. Management of oncological patients in the digital era: Anatomic pathology and nuclear medicine teamwork. Future Oncol. 2018, 14, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Mapelli, S.N.; Catapano, C.V.; Carbone, G.M.; Ciuffa, S.; Tavolozza, M.; Schillaci, O.; Mauriello, A.; et al. Prostate Osteoblast-Like Cells: A Reliable Prognostic Marker of Bone Metastasis in Prostate Cancer Patients. Contrast Media Mol. Imaging 2018, 2018, 9840962. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Varone, F.; Ciuffa, S.; Mauriello, A.; Bonanno, E. Calcifications in prostate cancer: An active phenomenon mediated by epithelial cells with osteoblast-phenotype. Microsc. Res. Technol. 2018, 81, 745–748. [Google Scholar] [CrossRef]
- Calabria, F.; Rubello, D.; Schillaci, O. The optimal timing to perform 18F/11C-choline PET/CT in patients with suspicion of relapse of prostate cancer: Trigger PSA versus PSA velocity and PSA doubling time. Int. J. Biol. Markers 2014, 29, e423–e430. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, e19–e297. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2018, 68, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, G.O. The effect of multidisciplinary team care on cancer management. Pan Afr. Med. J. 2011, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, M.; Pitsillides, B.; Al-Alfi, N.; Omran, S.; Al-Jabri, K.; ElShamy, K.; Ghrayeb, I.; Livneh, J.; Daher, M.; Charalambous, H.; et al. Multidisciplinary care team for cancer patients and its implementation in several Middle Eastern countries. Ann. Oncol. 2013, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Duggento, A.; Toschi, N.; Schillaci, O.; Bonanno, E. Novel insights into breast cancer progression and metastasis: A multidisciplinary opportunity to transition from biology to clinical oncology. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 138–148. [Google Scholar] [CrossRef]
- Schillaci, O.; Scimeca, M.; Toschi, N.; Bonfiglio, R.; Urbano, N.; Bonanno, E. Combining Diagnostic Imaging and Pathology for Improving Diagnosis and Prognosis of Cancer. Contrast Media Mol. Imaging 2019, 2019, 9429761. [Google Scholar] [CrossRef]
- Schillaci, O.; Scimeca, M.; Trivigno, D.; Chiaravalloti, A.; Facchetti, S.; Anemona, L.; Bonfiglio, R.; Santeusanio, G.; Tancredi, V.; Bonanno, E.; et al. Prostate cancer and inflammation: A new molecular imaging challenge in the era of personalized medicine. Nucl. Med. Boil. 2019, 66–79. [Google Scholar] [CrossRef]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Schillaci, O.; Bonanno, E. Breast osteoblast-like cells: A new biomarker for the management of breast cancer. Br. J. Cancer 2018, 119, 1129–1132. [Google Scholar] [CrossRef]
- Urbano, N.; Scimeca, M.; Bonanno, E.; Schillaci, O. Nuclear medicine and anatomic pathology in personalized medicine: A challenging alliance. Pers. Med. 2018, 15, 457–459. [Google Scholar] [CrossRef]
- Evangelista, L.; Cimitan, M.; Hodolic, M.; Baseric, T.; Fettich, J.; Borsatti, E. The ability of 18F-choline PET/CT to identify local recurrence of prostate cancer. Abdom. Imaging 2015, 40, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.I.; Reichel, K.; Krönig, M.; Wetterauer, U.; Schultze-Seemann, W.; Meyer, P.T.; Vach, W. Detection Rate of 18F-Choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for Prostate Cancer Lymph Node Metastases with Direct Link from PET to Histopathology: Dependence on the Size of Tumor Deposits in Lymph Nodes. J. Nucl. Med. 2019, 60, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Marzola, M.C.; Chondrogiannis, S.; Ferretti, A.; Grassetto, G.; Rampin, L.; Massaro, A.; Castellucci, P.; Picchio, M.; Al-Nahhas, A.; Colletti, P.M.; et al. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: Correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin. Nucl. Med. 2013, 38, e26–e32. [Google Scholar] [CrossRef] [PubMed]
- Cimitan, M.; Evangelista, L.; Hodolič, M.; Mariani, G.; Baseric, T.; Bodanza, V.; Saladini, G.; Volterrani, D.; Cervino, A.R.; Gregianin, M.; et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: Experience with 1,000 patients. J. Nucl. Med. 2015, 56, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Giovacchini, G.; Picchio, M.; Coradeschi, E.; Bettinardi, V.; Gianolli, L.; Scattoni, V.; Cozzarini, C.; Di Muzio, N.; Rigatti, P.; Fazio, F.; et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Roehl, K.A.; Han, M.; Ramos, C.G.; Antenor, J.A.V.; Catalona, W.J. Cancer Progression And Survival Rates Following Anatomical Radical Retropubic Prostatectomy In 3,478 Consecutive Patients: Long-Term Results. J. Urol. 2004, 172, 910–914. [Google Scholar] [CrossRef]
- Schwartz, E.; Albertsen, P. Nomograms for clinically localized disease. Part III: Watchful waiting. Semin. Urol. Oncol. 2002, 20, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, P.; Fuccio, C.; Rubello, D.; Schiavina, R.; Santi, I.; Nanni, C.; Allegri, V.; Montini, G.C.; Ambrosini, V.; Boschi, S.; et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 55–63. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Ciuffa, S.; Tancredi, V.; Tarantino, U.; Albonici, L.; et al. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland Biol. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lin, H.-H.; Tang, M.-J.; Wang, Y.-K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. Genome Ed. 2018, 1095, 101–110. [Google Scholar]
- Scimeca, M.; Giannini, E.; Antonacci, C.; Pistolese, C.A.; Spagnoli, L.G.; Bonanno, E. Microcalcifications in breast cancer: An active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Kawaguchi, H.; Jinno, S.; Hoshi, K.; Itaka, K.; Takato, T.; Nakamura, K.; Okayama, H. Bone Morphogenetic Protein 2-Induced Osteoblast Differentiation Requires Smad-Mediated Down-Regulation of Cdk6. Mol. Cell. Boil. 2004, 24, 6560–6568. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.F.; Jenkinson, A.; Pohl, K.; O’Brien, F.J.; Morgan, M.P. Osteomimicry of Mammary Adenocarcinoma Cells In Vitro; Increased Expression of Bone Matrix Proteins and Proliferation within a 3D Collagen Environment. PLoS ONE 2012, 7, e41679. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Anderson, P.H.; Wijenayaka, A.R.; Barratt, K.R.; Triliana, R.; Stapledon, C.J.M.; Zhou, H.; Findlay, D.M.; Morris, H.A.; Atkins, G.J. Both ligand and VDR expression levels critically determine the effect of 1α,25-dihydroxyvitamin-D3 on osteoblast differentiation. J. Steroid Biochem. Mol. Biol. 2018, 177, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.M.; Kwak, S.C.; Yoon, K.-H.; Kim, J.-Y.; Lee, M.S. Role of ANTXR1 in the regulation of RANKL-induced osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 2019, 510, 296–302. [Google Scholar] [CrossRef]
- Scimeca, M.; Salustri, A.; Bonanno, E.; Nardozi, D.; Rao, C.; Piccirilli, E.; Feola, M.; Tancredi, V.; Rinaldi, A.; Iolascon, G.; et al. Impairment of PTX3 expression in osteoblasts: A key element for osteoporosis. Cell Death Dis. 2017, 8, e3125. [Google Scholar] [CrossRef][Green Version]
- de Ridder, J.J. The uptake of choline by rat liver mitochondria. Biochim. Biophys. Acta 1976, 449, 236–244. [Google Scholar] [CrossRef]
- An, J.H.; Yang, J.Y.; Ahn, B.Y.; Cho, S.W.; Jung, J.Y.; Cho, H.Y.; Cho, Y.M.; Kim, S.W.; Park, K.S.; Kim, S.Y.; et al. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone 2010, 47, 140–150. [Google Scholar] [CrossRef]

| Antibody | Characteristics | Dilution | Retrieval |
|---|---|---|---|
| anti-vimentin | mouse monoclonal clone V9; Ventana, Tucson, AZ, USA | Pre-diluted | EDTA citrate pH 7.8 |
| anti-BMP2 | rabbit monoclonal clone N/A; Novus Biologicals, Littleton, CO, USA | 1:250 | Citrate pH 6.0 |
| anti-PTX3 | rat monoclonal clone MNB1; AbCam, Cambridge, UK | 1:100 | Citrate pH 6.0 |
| anti-RUNX2 | Mouse monoclonal; cloneEPR14334AbCam, Cambridge, UK | 1:100 | Citrate pH 6.0 |
| anti-RANKL | rabbit monoclonal clone 12A668; AbCam, Cambridge, UK | 1:100 | EDTA citrate pH 7.8 |
| anti-VDR | rabbit polyclonal clone NBP1-19478; Novus Biologicals, Littleton, CO, USA | 1:100 | Citrate pH 6.0 |
| G_1 (n = 29) | G_2 (n = 26) | G_3 (n = 24) | P Value | |
|---|---|---|---|---|
| Age | 75.80 ± 2.52 | 76.75 ± 1.06 | 72.00 ± 2.58 | G_1 v.s. G_2, p = 0.5236; G_1 v.s. G_3, p = 0.0971; G_2 v.s. G_3, p = 0.0594 |
| PSA (ng/mL) [14] | 13.23 ± 1.26 | 9.26 ± 2.36 | 13.63 ± 1.20 | G_1 v.s. G_2, p = 0.049 *; G_1 v.s. G_3, p = 0.8746; G_2 v.s. G_3, p = 0.0551 |
| cT/pT | ||||
| T1–T2 | 15 (51.7%) | 13 (50%) | 9 (37.5%) | / |
| T3–T4 | 13 (44.8%) | 11 (42.3%) | 15 (62.5%) | / |
| unknown | 1 (3.5%) | 2 (7.7%) | / | / |
| cN/pN | ||||
| N0 | 20 (68.9%) | 12 (46.2%) | 10 (41.6%) | / |
| N1 | 9 (31.1%) | 12 (53.8%) | 14 (58.4%) | / |
| c/M/pM | ||||
| M0 | 26 (89.6%) | 18 (69.2%) | 17 (70.8%) | / |
| M1 | 3 (11.4%) | 8 (30.8%) | 7 (29.2%) | / |
| bone lesions | 2 (66.6%) | 5 (62.5%) | 5 (71.4%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbano, N.; Scimeca, M.; Crocco, A.; Mauriello, A.; Bonanno, E.; Schillaci, O. 18F–Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers. J. Clin. Med. 2019, 8, 1657. https://doi.org/10.3390/jcm8101657
Urbano N, Scimeca M, Crocco A, Mauriello A, Bonanno E, Schillaci O. 18F–Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers. Journal of Clinical Medicine. 2019; 8(10):1657. https://doi.org/10.3390/jcm8101657
Chicago/Turabian StyleUrbano, Nicoletta, Manuel Scimeca, Antonio Crocco, Alessandro Mauriello, Elena Bonanno, and Orazio Schillaci. 2019. "18F–Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers" Journal of Clinical Medicine 8, no. 10: 1657. https://doi.org/10.3390/jcm8101657
APA StyleUrbano, N., Scimeca, M., Crocco, A., Mauriello, A., Bonanno, E., & Schillaci, O. (2019). 18F–Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers. Journal of Clinical Medicine, 8(10), 1657. https://doi.org/10.3390/jcm8101657








