Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis
Abstract
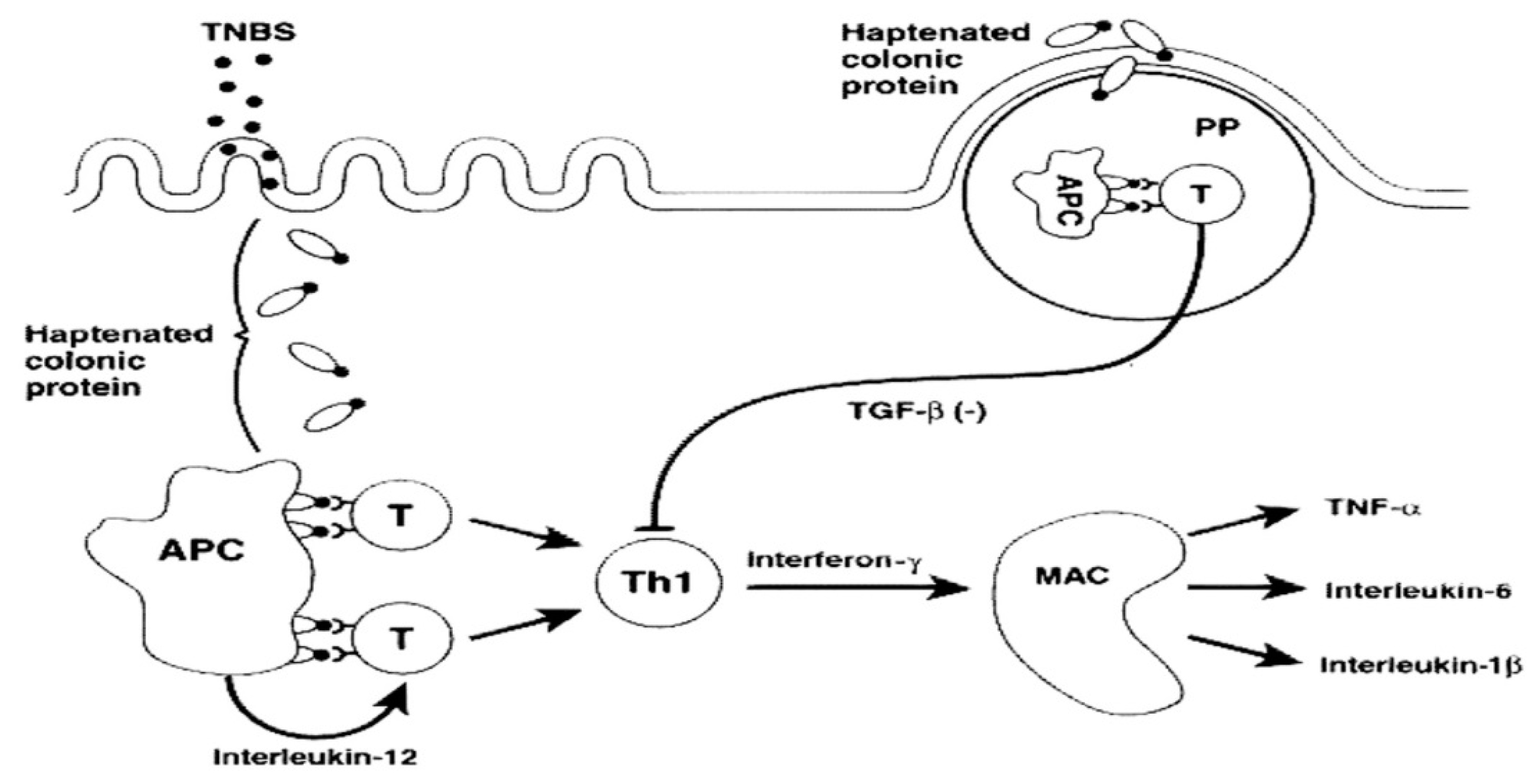
1. Introduction
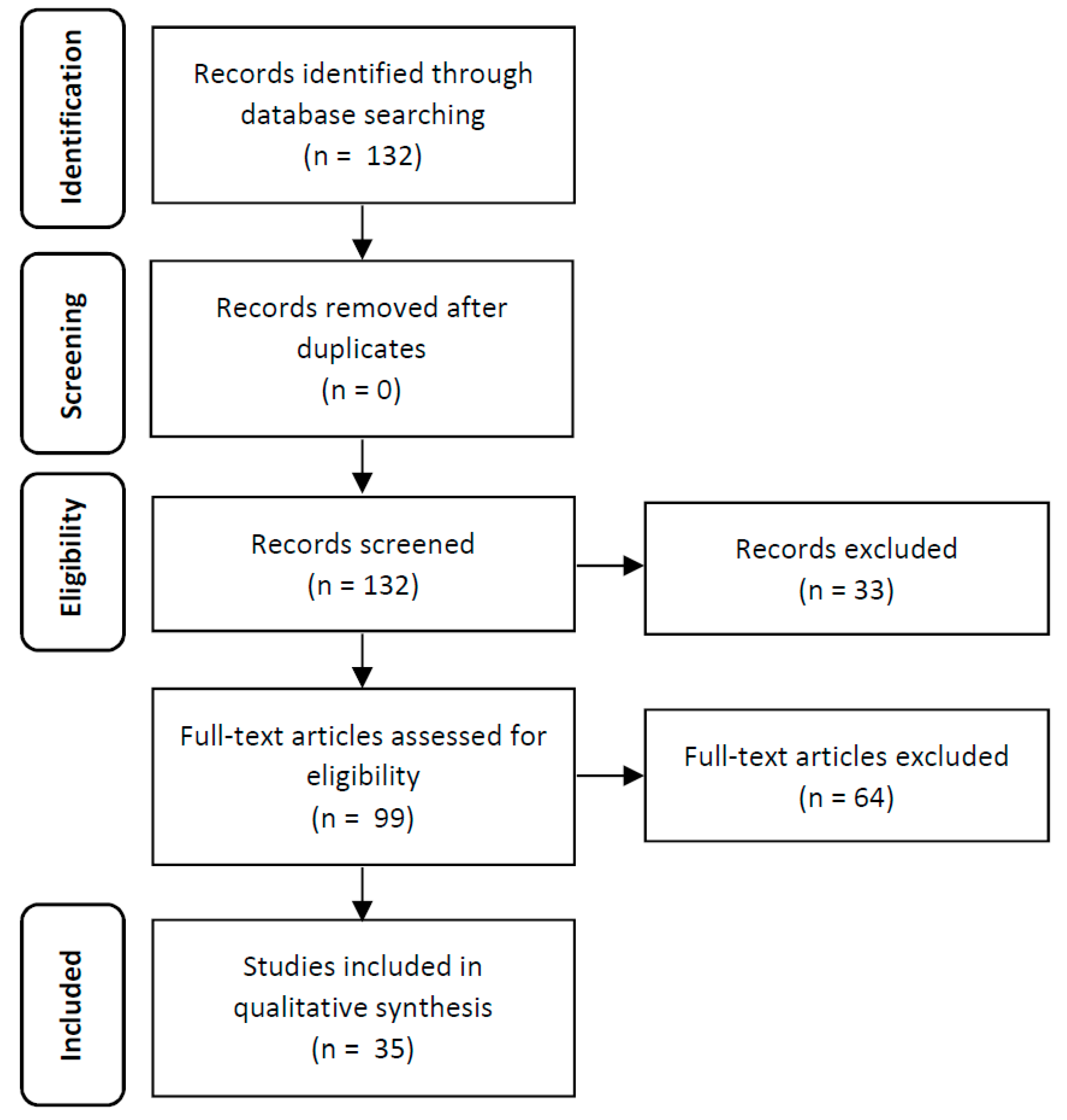
2. Material and Methods
2.1. Search Strategy
2.2. Selection of Studies
2.3. Data Extraction
3. Results
3.1. TNBS-Related Parameters
3.1.1. Number of TNBS Administrations
3.1.2. Dose
3.1.3. Volume
3.1.4. Vehicle
3.1.5. Presensitization
3.2. Mice-Related Parameters
3.2.1. Strain
3.2.2. Gender
3.2.3. Age
3.3. Characterization of the Preclinical Model
3.3.1. Clinical Signs and Symptoms
3.3.2. Biochemical Markers
3.3.3. Macroscopic Evaluation
3.3.4. Histological Evaluation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.; Santiago, M.; Correia, L.; Portela, F.; Ministro, P.; Lago, P.; Trindade, E.; Freitas, A.; Magro, F. Hospitalization trends of the inflammatory bowel disease landscape: A nationwide overview of 16 years. Dig. Liver Dis. 2019, 51, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 2066–2078. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef]
- Mayer, L. Evolving paradigms in the pathogenesis of IBD. J. Gastroenterol. 2010, 45, 9–16. [Google Scholar] [CrossRef]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Love, B.L. Management of patients with inflammatory bowel disease: Current and future treatments. Clin. Pharm. 2017, 9, 3. [Google Scholar] [CrossRef]
- Engel, M.; Neurath, M. New pathophysiological insights and modern treatment of IBD. J. Gastroenterol. 2010, 45, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Majewska-Szczepanik, M.; Góralska, M.; Marciñska, K.; Zemelka-Wiacek, M.; Strzepa, A.; Dorozynska, I.; Szczepanik, M. Epicutaneous immunization with protein antigen TNP-Ig alleviates TNBS-induced colitis in mice. Pharmacol. Rep. 2012, 64, 1497–1504. [Google Scholar] [CrossRef]
- Neurath, M.F. Animal models of inflammatory bowel diseases: Illuminating the pathogenesis of colitis, ileitis and cancer. Dig. Dis. 2012, 30, 91–94. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Motavallian-Naeini, A.; Andalib, S.; Rabbani, M.; Mahzouni, P.; Afsharipour, M.; Minaiyan, M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res. Pharm. Sci. 2012, 7, 159–169. [Google Scholar]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R.M. Anti-inflammatory effect of erythropoietin in the TNBS-induced colitis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 138–145. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Thiadiazolidinone-8 ameliorates inflammation associated with experimental colitis in mice. Pharmacology 2018, 101, 35–42. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Hemin reduces inflammation associated with TNBS-induced colitis. Clin. Exp. Gastroenterol. 2018, 11, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-Inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Lima, A.; Gonçalves, M.M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.; Ferreira, R.B.; et al. Reduction of inflammation and colon injury by a spearmint phenolic extract in experimental bowel disease in mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, B.F.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.; Lichtenberger, L.M. Methods of inducing inflammatory bowel disease in mice. Curr. Protoc. Pharmacol. 2016, 72, 1–42. [Google Scholar] [CrossRef]
- Qin, H.Y.; Wu, J.C.; Tong, X.D.; Sung, J.J.; Xu, H.X.; Bian, Z.X. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J. Gastroenterol. 2011, 46, 164–174. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E. Animal models of IBD: Linkage to human disease. Curr. Opin. Pharmacol. 2010, 10, 578–587. [Google Scholar] [CrossRef]
- Jurjus, A.R.; Khoury, N.N.; Reimund, J.M. Animal models of inflammatory bowel disease. J. Pharmacol. Toxicol. Methods. 2004, 50, 81–92. [Google Scholar] [CrossRef]
- Hibi, T.; Ogata, H.; Sakuraba, A. Animal models of inflammatory bowel disease. J. Gastroenterol. 2002, 37, 409–417. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. (Lond.) 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bramhall, M.; Flórez-Vargas, O.; Stevens, R.; Brass, A.; Cruickshank, S. Quality of methods reporting in animal models of colitis. Inflamm. Bowel Dis. 2015, 21, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and change of intestinal microflora. Jpn. J. Gastroenterol. 1985, 82, 1327–1336. [Google Scholar]
- Okayasu, I.; Hatekeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Moschetta, A. Exploration of inflammatory bowel disease in mice: Chemically induced murine models of inflammatory bowel disease (IBD). Curr. Protoc. Mouse Biol. 2017, 7, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef]
- Scheiffele, F.; Fuss, I.J. Induction of TNBS colitis in mice. Curr. Protoc. Immunol. 2001, 15, 1–14. [Google Scholar] [CrossRef]
- ALJahdali, N.; Gadonna-Widehem, P.; Delayre-Orthez, C.; Marier, D.; Garnier, B.; Carbonero, F.; Anton, P.M. Repeated oral exposure to n ε-carboxymethyllysine, a Maillard reaction product, alleviates gut microbiota dysbiosis in colitic mice. Dig. Dis. Sci. 2017, 62, 3370–3384. [Google Scholar] [CrossRef]
- Strober, W.; Ludviksson, B.R.; Fuss, I.J. The pathogenesis of mucosal inflammation in murine models of Inflammatory Bowel Disease and Crohn Disease. Ann. Intern. Med. 1998, 128, 848–856. [Google Scholar] [CrossRef]
- Aharoni, R.; Kayhan, B.; Arnon, R. Therapeutic effect of the immunomodulator glatiramer acetate on trinitrobenzene sulfonic acid-induced experimental colitis. Inflamm. Bowel Dis. 2005, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Siczek, K.; Zatorski, H.; Chmielowiec-Kornzeniowska, A.; Kordek, R.; Tymczyna, L.; Fichna, J. Evaluation of anti-inflammatory effect of silver-coated glass beads in mice with experimentally induced colitis as a new type of treatment in inflammatory bowel disease. Pharmacol. Rep. 2017, 69, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, T.; Ullrich, S.; Schulz, S.; Chamaillard, M.; Schaltenberg, N.; Rath, E.; Goebel, U.; Sartor, B.; Prager, M.; Buining, C.; et al. Colonic expression of the peptide transporter PEPT1 is downregulated during intestinal inflammation and is not required for NOD2-dependent immune activation. Inflamm. Bowel Dis. 2014, 20, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Duchmann, R.; Schmitt, E.; Knolle, P.; zum Buschenfelde, K.M.; Neurath, M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur. J. Immunol. 1996, 26, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Philippe, D.; Heupel, E.; Blum-Sperisen, S.; Riedel, C.U. Treatment with bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int. J. Food Microbiol. 2011, 149, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Millan, B.; Guardia, R.D.; Roca-Ho, H.; García-Herrero, C.M.; Lavoie, J.R.; Rosu-Myles, M.; Gonzalez-Rey, E.; O’Valle, F.; Criado, G.; Delgado, M.; et al. Therapeutic effect of the imunomodulatory drug lenalidomide, but not pomalidomide, in experimental models of rheumatoid arthritis and inflammatory bowel disease. Exp. Mol. Med. 2017, 49, e290. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Bortolotto, V.C.; Poetini, M.R.; Araujo, S.M.; de Paula, M.T.; Roman, S.S.; Spiazzi, C.; Cibin, F.W.S.; Rodrigues, O.E.D.; Jesse, C.R.; et al. Anti-inflammatory and anti-oxidant effects of p-Chloro-Phenyl-Selenoesterol on TNBS-induced inflammatory bowel disease in mice. J. Cell Biochem. 2017, 118, 709–717. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, T.; Shen, Y.; Zhou, X.; Chen, T.; Xu, Q. Si-Ni-San. A traditional chinese prescription, and its active ingredient glycyrrhizin ameliorate experimental colitis through regulating cytokine balance. Int. Immunopharmacol. 2009, 9, 1437–1443. [Google Scholar] [CrossRef]
- Neurath, M.F. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Fernandez-Martin, A.; Chorny, A.; Delgado, M. Therapeutic effect of urocortin and adrenomedullin in a murine model of crohn’s disease. Gut 2006, 55, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Distrutti, E.; Renga, B.; Cipriani, S.; Palladino, G.; Booth, C.; Tudor, G.; Guse, J.; Hahn, U.; Burnet, M.; et al. Development of non-antibiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. Eur. J. Pharmacol. 2011, 665, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, R.; Huang, X.; Cheng, S.; Huang, M.; Yue, H.; Wang, X.; Zou, Y.; Lu, A.; Liu, D. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J. Gastroenterol. 2016, 22, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Heuvelman, D.M.; Masferrer, J.L.; Benson, E.L. Pharmacologic evaluation of sulfasalazine, FTY720, and anti-IL-12/23p40 in a TNBS-induced crohn’s disease model. Dig. Dis. Sci. 2011, 56, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lin, L.; Lin, Y.; Zheng, C. Exogenous carcinoembryonic antigen-related cell adhesion molecule 1 suppresses 2,4,6-Trinitrobenzene sulfonic acid-induced ulcerative colitis in mice. J. Surg. Res. 2015, 195, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Lehr, H.; Endres, S.; Schnurr, M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: Influence of genetic and environmental factors. Dig. Dis. 2012, 30, 82–90. [Google Scholar] [CrossRef]
- Bennink, R.J.; van Montfrans, C.; de Jonge, W.J.; Bruin, K.; van Deventer, S.J.; te Velde, A.A. Imaging of intestinal lymphocyte homing by means of pinhole SPECT in a TNBS colitis mouse model. Nucl. Med. Biol. 2004, 31, 93–101. [Google Scholar] [CrossRef]
- Keates, A.C.; Castagliuolo, I.; Cruickshank, W.W.; Qiu, B.; Arseneau, K.O.; Brazer, W.; Kelly, C.P. Interleukin 16 is up-regulated in crohn’s disease and participates in TNBS colitis in mice. Gastroenterology 2000, 119, 972–982. [Google Scholar] [CrossRef]
- Dohi, T.; Fujihashi, K.; Rennert, P.D.; Iwatani, K.; Kiyono, H.; McGhee, J.R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2–type responses. J. Exp. Med. 1999, 189, 1169–1180. [Google Scholar] [CrossRef]
- Maines, L.W.; Fitzpatrick, L.R.; Green, C.L.; Zhuang, Y.; Smith, C.D. Efficacy of a novel sphingosine kinase inhibitor in experimental crohn’s disease. Inflammopharmacology 2010, 18, 73–85. [Google Scholar] [CrossRef]
- Abad, C.; Martinez, C.; Juarranz, M.G.; Arranz, A.; Leceta, J.; Delgado, M.; Gomariz, R.P. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of crohn’s disease. Gastroenterology 2003, 124, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, X.; Wang, X.; Chen, X.; Song, C.; Du, Y.; Su, J.; Yaseen, S.A.; Yang, P. Chemokine CXCL11 links microbial stimuli to intestinal inflammation: Microbes and IL-17 production. Clin. Exp. Immunol. 2011, 164, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Beagley, K.W.; Sharmanov, A.T.; Fujihashi, K.; Kiyono, H.; Tennyson, G.S.; Cong, Y.; Black, C.A.; Ridwan, B.W.; McGhee, J.R. Hapten-induced model of murine inflammatory bowel disease: Mucosa immune responses and protection by tolerance. J. Immunol. 1996, 157, 2174–2185. [Google Scholar] [PubMed]
- Cromer, W.E.; Ganta, C.V.; Patel, M.; Traylor, J.; Kevil, C.G.; Alexander, J.S.; Mathis, J.M. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: Protective effects of a VEGF164b therapy. J. Transl. Med. 2013, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.; Peucelle, V.; Boutillier, D.; Breton, J.; Kuylle, S.; Pot, B.; Holowacz, S.; Grangette, C. New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Benef. Microbes 2018, 9, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, C.; Zheng, X.; Zhu, X.; Yan, S.; Wang, H.; Fu, R.; Fan, H.; Chen, Y. Insight into the pharmacokinetic behavior of tanshinone IIA in the treatment of crohn’s disease: Comparative data for tanshinone IIA and Its two glucuronidated metabolites in normal and recurrent colitis models after oral administration. Xenobiotica 2017, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Talero, E.; Rodríguez-Luna, A.; García-Mauriño, S.; Motilva, V. Anti-inflammatory effects of an oxylipin-containing lyophilised biomass from a microalga in a murine recurrent colitis model. Br. J. Nutr. 2017, 116, 2044–2052. [Google Scholar] [CrossRef]
- Hiraishi, K.; Kurahara, L.H.; Sumiyoshi, M.; Hu, Y.P.; Koga, K.; Onitsuka, M.; Kojima, D.; Yue, L.; Takedatsu, H.; Jian, Y.W.; et al. Daikenchuto (Da-Jian-Zhong-Tang) ameliorates intestinal fibrosis by activating myofibroblast transient receptor potential ankyrin 1 channel. World J. Gastroenterol. 2018, 24, 4036–4053. [Google Scholar] [CrossRef]
- Lian, L.; Huang, Q.; Zhang, L.; Qin, H.; He, X.; He, X.; Ke, J.; Xie, M.; Lan, P. Anti-fibrogenic potential of mesenchymal stromal cells in treating fibrosis in crohn’s disease. Dig. Dis. Sci. 2018, 63, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, X.; Liu, C.; Su, L.; Xia, Z.; Li, X.; Li, Y.; Li, L.; Yan, T.; Feng, Q.; et al. Dysbiosis of the fecal microbiota in the TNBS-induced crohn’s disease mouse model. Appl. Microbiol. Biotechnol. 2016, 100, 4485–4494. [Google Scholar] [CrossRef]
- Kuemmerle, J.F. Murine trinitrobenzoic acid-induced colitis as a model of crohn’s disease. Methods Mol. Biol. 2016, 1422, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lv, Y.; Li, S.; Li, Q.; Zhang, Q.; Zhang, X.; Hao, Z. Recombinant adeno-associated virus carrying thymosin β4 suppresses experimental colitis in mice. World J. Gastroenterol. 2017, 23, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lu, C.; Chen, J.; Cui, G.; Wang, L.; Yu, T.; Yang, Y.; Wu, W.; Ding, Y.; Li, L.; et al. High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget 2017, 8, 70–82. [Google Scholar] [CrossRef]
- Klopcic, B.; Appelbee, A.; Raye, W.; Lloyd, F.; Jooste, J.C.I.; Forrest, C.H.; Lawrance, I.C. Indomethacin and retinoic acid modify mouse intestinal inflammation and fibrosis: A role for SPARC. Dig. Dis. Sci. 2008, 53, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Popp, V.; Gerlach, K.; Mott, S.; Turowska, A.; Garn, H.; Atreya, R.; Lehr, H.; Ho, I.; Renz, H.; Weigmann, B.; et al. Rectal delivery of a DNAzyme that specifically blocks the transcription factor GATA3 and reduces colitis in mice. Gastroenterology 2017, 152, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Tomassello, G.; Sinagra, E.; Raimondo, D.; Palumbro, V.D.; Puleio, R.; Cottone, M.; Damiani, P.; Traina, G.; Abruzzo, A.; Damiani, F.; et al. Validation of a modified model of TNBS-induced colitis in rats. How to induce a chemical colitis in rats. Acta Biomed. 2015, 86, 92–96. [Google Scholar]
- Yang, X.L.; Guo, T.K.; Wang, Y.H.; Gao, M.T.; Qin, H.; Wu, Y.J. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch. Pharm Res. 2012, 35, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Cheon, G.J.; Cui, Y.; Yeon, D.S.; Kwon, S.C.; Park, B.G. Mechanisms of motility change on trinitrobenzenesulfonic acid-induced coloni inflammation in mice. Korean J. Physiol Pharmacol. 2012, 16, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Zhong, F.; Gebhart, G.; Bielefeldt, K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Zimmermann, K.; Filipovic, M.R.; Izydorczyk, I.; Eberhardt, M.; Kichko, T.I.; Mueller-Tribbensse, S.M.; et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; pp. 105–133. [Google Scholar]
- Borm, M.E.A.; Bouma, G. Animal models of inflammatory bowel disease. Drug Discov. Today Dis. Models 2004, 1, 467–477. [Google Scholar] [CrossRef]
- Padua, D.; Vu, J.P.; Germano, P.M.; Pisegna, J.R. The role of neuropeptides in mouse models of colitis. J. Mol. Neurosci. 2016, 59, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Holst, I.; Kleinschmidt, S.; Nolte, I.; Hewicker-Trautwein, M. Expression of inducible nitric oxide, nitrotyrosine and manganese superoxide dismutase in dogs with inflammatory bowel disease. J. Comp. Pathol. 2012, 146, 76. [Google Scholar] [CrossRef]
- Mansfield, K.G.; Lin, K.C.; Xia, D.; Newman, J.V.; Schauer, D.B.; MacKey, J.; Lackner, A.A.; Carville, A. Enteropathogenic Escherichia coli and ulcerative colitis in cotton-top tamarins (Saguinus oedipus). J. Infect. Dis. 2001, 184, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Devoss, J.; Diehl, L. Murine models of inflammatory bowel disease (IBD): Challenges of modeling human disease. Toxicol. Pathol. 2014, 42, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Di Paola, R.; Patel, N.S.; Genovese, T.; Muià, C.; De Sarro, A.; Thiemermann, C. Erythropoietin reduces the development of experimental inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2004, 311, 1272–1280. [Google Scholar] [CrossRef]
- Corica, D.; Romano, C. Renal involvement in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 10, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, K.; Kapsoritakis, A.; Eleftheriadis, T.; Stefanidis, I.; Potamianos, S. Renal manifestations and complications of inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1034–1045. [Google Scholar] [CrossRef]
- Rojas-Feria, M.; Castro, M.; Suárez, E.; Ampuero, J.; Romero-Gómez, M. Hepatobiliary manifestations in inflammatory bowel disease: The gut, the drugs and the liver. World J. Gastroenterol. 2013, 19, 7327–7340. [Google Scholar] [CrossRef]
- Carty, E.; De Branbander, M.; Feakins, R.M.; Rampton, D.S. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 2000, 46, 487–492. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Moschen, A.; Sonnweber, T.; Theurl, M.; Theurl, I.; Taub, N.; Jamnig, C.; Neurather, D.; Huber, L.A.; et al. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-kB-inducible immune pathways. Immunity 2011, 34, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Coquerelle, C.; Oldenhove, G.; Acolty, V.; Denoeud, J.; Vansanten, G.; Verdebout, J.M.; Mellor, A.; Bluestone, J.A.; Moser, M. Anti- CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDOdependent anti-inflammatory properties in a mouse model of colitis. Gut 2009, 58, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, S.; Jiang, H.; Chen, T.; Wu, W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand. J. Gastroenterol. 2010, 45, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.V.K.; Kandhare, A.D.; Bhise, S.D. Anti-inflammatory effect of daucus carota root on experimental colitis in rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 337–343. [Google Scholar]
- Fuss, I.J.; Neurath, M.; Borivant, M.; Klein, J.S.; de la Motte, C.; Strong, S.A.; Fiocchi, C.; Strober, W. Disparate CD4+ lamina propria lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells maniest increased secretion of IL-5. J. Immunol. 1996, 157, 1261–1270. [Google Scholar] [PubMed]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.; Matsuse, R. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.G.; Niphadkar, P.V.; Phadke, A.S. Protective effect of aqueous extract of Bombax malabaricum DC on experimental models of inflammatory bowel disease in rats and mice. Indian J. Exp. Biol. 2011, 49, 343–351. [Google Scholar] [PubMed]
- Mooiweer, E.; Fidder, H.H.; Siersema, P.D.; Laheij, R.J.; Oldenburg, B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm. Bowel Dis. 2014, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Czul, F.; Levy, C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1655–1667. [Google Scholar] [CrossRef]
- Nayak, S.B.; George, B.M.; Mishra, S. Abnormal length and position of the sigmoid colon and its clinical significance. Kathmandu Univ. Med. J. 2012, 10, 94–97. [Google Scholar] [CrossRef]
- Marion-Latellier, R.; Bohn, P.; Modzelewski, R.; Vera, P.; Aziz, M.; Guerin, C.; Savoye, G.; Savoye-Collet, C. SPECT-computed tomography in rats with TNBS-induced colitis: A first step toward functional imaging. World J. Gastroenterol. 2017, 23, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Loeuillard, E.; Marion-Larellier, R.; Nicol, L.; Mulder, P.; Guerin, C.; Bôle-Feysot, C.; Aziz, M.; Déchelotte, P.; Vera, P.; et al. Magnetic resonance colonography for fibrosis assessment in rats with chronic colitis. PLoS ONE 2014, 9, e100921. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.; Nguen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.; Gilda, S.; Sharma, S.; Jagtap, S.; Paradkar, A.; Mahadik, K.; Ranjekar, P.; Harsulkar, A. Rectal gel application of Withania somnifera root extract expounds anti inflammatory and mucorestorative activity in TNBS-induced inflammatory bowel disease. BMC Complement. Altern. Med. 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Neurath, M.F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014, 7, 6–19. [Google Scholar] [CrossRef] [PubMed]
| TNBS-Related Parameters | Mice-Related Parameters | Characterization of the Preclinical Model | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Administrations | Dose (mg/mouse*) | Volume (μL) | Vehicle (%Ethanol) | Pre-Sensitization | Strain | Gender | Age (Week) | ||
| (WEEKLY) | |||||||||
| 1 | 2.8 | 100 | _ | No | Balb/c, SJL/J, Transgenic | Female | 8–12 | CSS; BM; M; H | [41] |
| 2.2 | 30 | Balb/c | Male | _ | BM; M; H | [42] | |||
| 2.5 | - | 50 | C57BL/6 | Female | 12 | H | [43] | ||
| 0.3 | Balb/c, SJL/J, C3H/HeJ | _ | 8–16 | CSS; BM | [44] | ||||
| 2.4 | 40 | C57BL/6 | Male | 5 | CSS; BM; M; H | [45] | |||
| 1.7 | 100 | Bagg Albino/c | 7 | CSS; M; H | [46] | ||||
| 1.1 | Swiis | Female | 8–10 | CSS; BM; M; H | [47] | ||||
| Balb/c | 5 | [48] | |||||||
| 0.3 | C57BL/6 | 8–10 | [49] | ||||||
| Balb/c, SJL/J | 8–16 | [50] | |||||||
| 1.7 | _ | 6–8 | [51] | ||||||
| 0.3 | 150 | Balb/c | 8–10 | [52] | |||||
| 2.0 | 300 | C57BL/6 | Male | 9–12 | BM; M; H | [53] | |||
| 2.8 | _ | 80 | Balb/c | Female | 7 | CSS; M | [54] | ||
| 0.3 | 100 | 50 | Yes | 6–8 | CSS; BM; M; H | [55] | |||
| 2 | 1.1–1.1 | _ | 40 | No | Transgenic | _ | 8–16 | CSS; H | [56] |
| 0.3–1.1 | Balb/c | _ | CSS; BM; H | [57] | |||||
| 5.0–2.0 or 3.0–2.0 | 100 | C57BL/6, Balb/c | Male | CSS; BM; M | [58] | ||||
| 0.7 | _ | 50 | Balb/c | _ | 10–16 | CSS; BM; H | [59] | ||
| 0.8–0.8 | 100 | C57BL/6 | Male | 6–8 | BM; M; H | [60] | |||
| 1.7–0.9 | 120 | Balb/c | CSS; BM; H | [61] | |||||
| 1.4–1.4 | 150 | C57BL/6 | _ | [62] | |||||
| 1.1–0.6 | 500 | Yes | C57BL/6, Balb/c, Transgenic | Male, Female | 8–16 | [63] | |||
| 3 | 1.1–1.1–1.1 | 100 | 10 | No | Balb/c | _ | _ | CSS; BM; M; H | [64] |
| 0.5–0.7–1.6 | _ | 50 | Balb/c ByJ | Female | 7–8 | BM; M; H | [65] | ||
| 0.4–0.9–1.2 | 48 | Yes | Balb/c | Male | 8–10 | CSS; BM; H | [66] | ||
| 0.4–0.6–1.4 | 50 | Balb/c | Female | 8 | CSS; BM; M; H | [67] | |||
| 4 or more | 0.3–0.3–0.4–0.4–0.6–0.6 | 50 | 30 | No | Wild-type and Trangenic | _ | 8–9 | H | [68] |
| 0.9–0.9–1.1–1.1–1.4–1.4–1.4 | 100 | 45 | Balb/c | Male | 4–5 | CSS; M | [69] | ||
| 0.6–0.6–1.1–1.1–2.2–2.2 | 50–100 | 50 | 6 | CSS | [70] | ||||
| 0.9 to 1.4 or 2.2 to 3.4 | 100 | Balb/c and C57BL/6 | Male, Female | 6–8 | CSS; M; H | [71] | |||
| 1–1–2–2–3–3 | 200 | Balb/c | Male | 6 | BM; M; H | [72] | |||
| 1.1–1.4–1.4–1.4 | _ | 55 | C57BL/6 | 6 | CSS; BM; M | [73] | |||
| 0.3–0.3–0.4–0.4–0.6–0.7–0.7–0.7 | 45 | Yes | CD-1 outbred | Female | 12 | CSS; M; H | [74] | ||
| 0.6–0.6–0.6–0.6–1.4–1.4–1.4–1.4 | 50 | Transgenic | _ | 6–12 | CSS; BM; H | [75] | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, I.; Pinto, R.; Mateus, V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. J. Clin. Med. 2019, 8, 1574. https://doi.org/10.3390/jcm8101574
Silva I, Pinto R, Mateus V. Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. Journal of Clinical Medicine. 2019; 8(10):1574. https://doi.org/10.3390/jcm8101574
Chicago/Turabian StyleSilva, Inês, Rui Pinto, and Vanessa Mateus. 2019. "Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis" Journal of Clinical Medicine 8, no. 10: 1574. https://doi.org/10.3390/jcm8101574
APA StyleSilva, I., Pinto, R., & Mateus, V. (2019). Preclinical Study in Vivo for New Pharmacological Approaches in Inflammatory Bowel Disease: A Systematic Review of Chronic Model of TNBS-Induced Colitis. Journal of Clinical Medicine, 8(10), 1574. https://doi.org/10.3390/jcm8101574






