Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
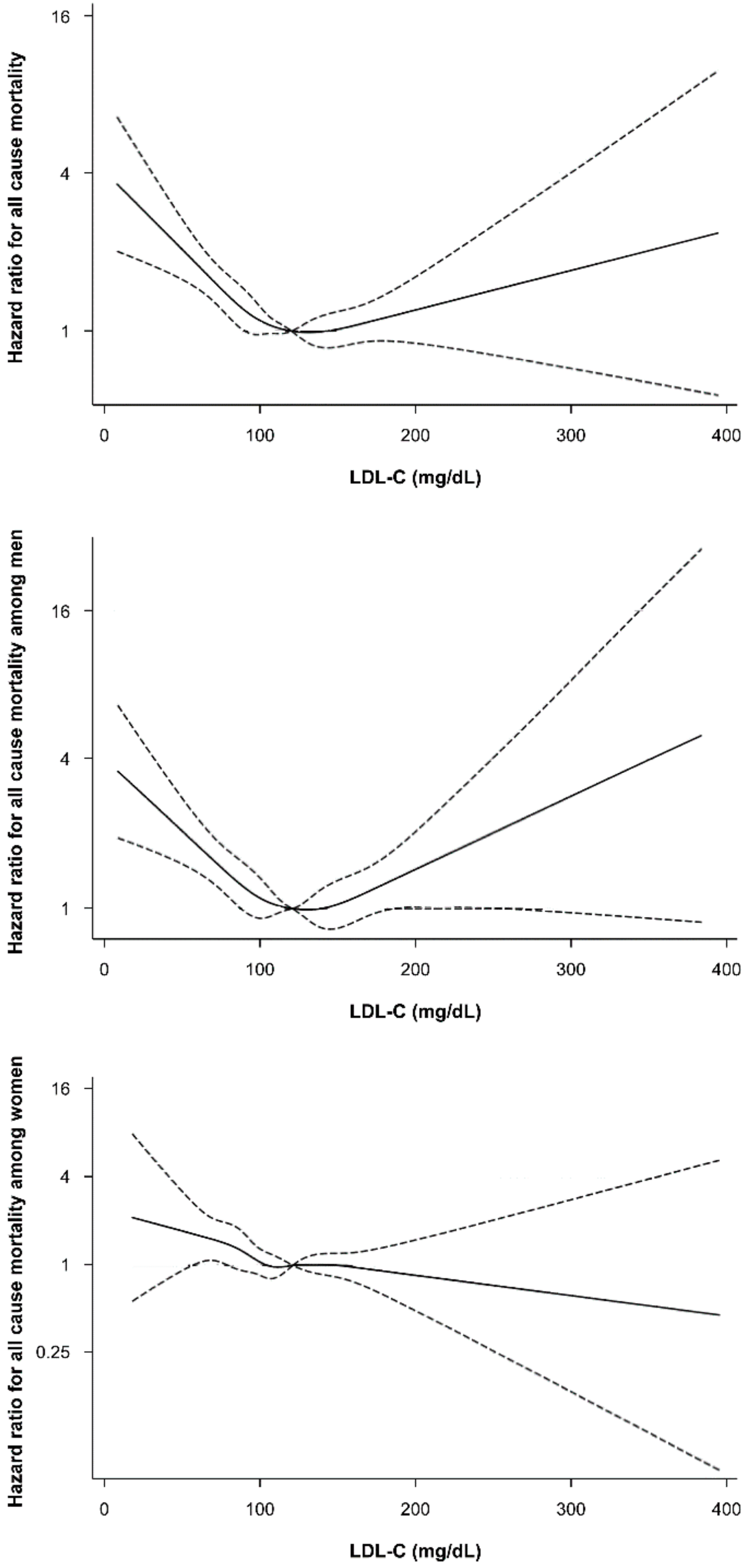
3. Results
3.1. Baseline Characteristics of Participants
3.2. Association between Low-Density Lipoprotein Cholesterol and All-Cause, CVD, and Cancer Mortality in Kangbuk Samsung Health Study
3.3. Association between Low-Density Lipoprotein Cholesterol and All-Cause, CVD, and Cancer Mortality in Korean Genome and Epidemiology Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [PubMed]
- Benn, M.; Tybjaerg-Hansen, A.; Stender, S.; Frikke-Schmidt, R.; Nordestgaard, B.G. Low-density lipoprotein cholesterol and the risk of cancer: A mendelian randomization study. J. Natl. Cancer Inst. 2011, 103, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.; Blackburn, H.; Higgins, M.; Reed, D.; Iso, H.; McMillan, G.; Neaton, J.; Nelson, J.; Potter, J.; Rifkind, B.; et al. Report of the conference on low blood cholesterol: Mortality associations. Circulation 1992, 86, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Thompson, S.G. Low serum cholesterol and the risk of cancer: An analysis of the published prospective studies. Cancer Causes Control 1991, 2, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Linsel-Nitschke, P.; Gotz, A.; Erdmann, J.; Braenne, I.; Braund, P.; Hengstenberg, C.; Stark, K.; Fischer, M.; Schreiber, S.; El Mokhtari, N.E.; et al. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—A Mendelian Randomisation study. PLoS ONE 2008, 3, e2986. [Google Scholar] [CrossRef]
- Neaton, J.D.; Blackburn, H.; Jacobs, D.; Kuller, L.; Lee, D.J.; Sherwin, R.; Shih, J.; Stamler, J.; Wentworth, D. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Blackburn, H.; Keys, A.; Taylor, H.L.; Kannel, W.B.; Paul, O.; Reid, D.D.; Stamler, J. Colon cancer and blood-cholesterol. Lancet 1974, 1, 181–183. [Google Scholar] [CrossRef]
- Sherwin, R.W.; Wentworth, D.N.; Cutler, J.A.; Hulley, S.B.; Kuller, L.H.; Stamler, J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA 1987, 257, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Strasak, A.M.; Pfeiffer, R.M.; Brant, L.J.; Rapp, K.; Hilbe, W.; Oberaigner, W.; Lang, S.; Borena, W.; Concin, H.; Diem, G.; et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: A prospective 19-year follow-up study. Ann. Oncol. 2009, 20, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, T.; Okuyama, H.; Ogushi, Y.; Hama, R. Towards a paradigm shift in cholesterol treatment. A re-examination of the cholesterol issue in Japan. Ann. Nutr. Metab. 2015, 66, 1–116. [Google Scholar] [PubMed]
- Ravnskov, U.; McCully, K.S. Infections may be causal in the pathogenesis of atherosclerosis. Am. J. Med. Sci. 2012, 344, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Ravnskov, U.; McCully, K.S.; Rosch, P.J. The statin-low cholesterol-cancer conundrum. QJM 2012, 105, 383–388. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort profile: The Korean Genome and Epidemiology Study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Ko, S.H.; Kwon, H.S.; Kim, N.H.; Kim, J.H.; Kim, C.S.; Song, K.H.; Won, J.C.; Kim, D.J.; Choi, S.H.; et al. Prevalence and management of dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab. J. 2013, 37, 433–449. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Greenland, S. Dose-response and trend analysis in epidemiology: Alternatives to categorical analysis. Epidemiology 1995, 6, 356–365. [Google Scholar] [CrossRef]
- Penson, P.E.; Long, D.L.; Howard, G.; Toth, P.P.; Muntner, P.; Howard, V.J.; Safford, M.M.; Jones, S.R.; Martin, S.S.; Mazidi, M.; et al. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. Eur. Heart J. 2018, 39, 3641–3653. [Google Scholar] [CrossRef]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Ravnskov, U.; McCully, K.S. Review and hypothesis: Vulnerable plaque formation from obstruction of Vasa vasorum by homocysteinylated and oxidized lipoprotein aggregates complexed with microbial remnants and LDL autoantibodies. Ann. Clin. Lab. Sci. 2009, 39, 3–16. [Google Scholar]
- Ravnskov, U. High cholesterol may protect against infections and atherosclerosis. QJM 2003, 96, 927–934. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Thorell, A.; Claudel, T.; Jha, P.; Koefeler, H.; Lackner, C.; Hoesel, B.; Fauler, G.; Stojakovic, T.; Einarsson, C.; et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 2015, 62, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | LDL-C (mg/dL) | p for Trend | ||||
|---|---|---|---|---|---|---|---|
| <70 | 70–99 | 100–129 | 130–159 | ≥160 | |||
| Number | 347,971 | 18,298 | 97,660 | 131,879 | 73,614 | 26,520 | |
| Age (years) | 39.5 (9.8) | 37.2 (9.4) | 37.6 (9.0) | 39.6 (9.6) | 41.4 (10.1) | 42.7 (10.5) | <0.001 |
| BMI (kg/m2) | 23.4 (3.1) | 21.8 (3.0) | 23.3 (2.9) | 23.5 (3.0) | 24.5 (3.0) | 25.1 (3.0) | <0.001 |
| Systolic BP (mmHg) | 113.8 (14.4) | 110.2 (14.2) | 110.9 (13.8) | 114.0 (14.2) | 116.6(14.3) | 118.20 (14.7) | <0.001 |
| Diastolic BP (mmHg) | 73.3 (10.1) | 70.6 (10.0) | 71.3 (9.8) | 73.5 (10.0) | 75.4 (10.1) | 76.4 (10.2) | <0.001 |
| Laboratory | |||||||
| Fasting glucose (mg/dL) | 94.8 (16.5) | 93.2 (16.5) | 92.7 (13.9) | 94.6 (15.6) | 96.6 (18.0) | 99.1 (22.4) | <0.001 |
| Total cholesterol (mg/dL) | 194.6 (34.9) | 144.4 (26.8) | 166.6 (18.7) | 193.6 (17.9) | 222.7 (18.0) | 259.6 (25.1) | <0.001 |
| HDL-C (mg/dL) | 55.8 (13.0) | 58.2 (15.8) | 57.4 (14.0) | 55.3 (12.8) | 54.2 (11.6) | 54.7 (11.2) | <0.001 |
| Triglycerides (mg/dL) | 101 (71–150) | 73 (53–114) | 80 (59–118) | 102 (74–148) | 123 (91–171) | 139 (104–186) | <0.001 |
| Smoking status (%) | |||||||
| Never smoker | 55.5 | 64.4 | 63.5 | 54.9 | 47.4 | 45.2 | <0.001 |
| Former smoker | 16.8 | 12.6 | 13.4 | 17.2 | 20.2 | 20.6 | <0.001 |
| Current smoker | 27.6 | 22.8 | 22.9 | 27.8 | 32.3 | 34.1 | <0.001 |
| Alcohol intake (%) | |||||||
| 0 g/day | 33.6 | 37.7 | 36.8 | 33.1 | 30.3 | 30.7 | <0.001 |
| 10 g/day | 49.7 | 46.7 | 49.1 | 50.4 | 50.3 | 48.7 | <0.001 |
| 20 g/day | 16.6 | 15.4 | 13.9 | 16.4 | 19.3 | 20.5 | <0.001 |
| Regular exercise (%) 1 | 15.7 | 15.2 | 15.4 | 16.1 | 15.9 | 14.6 | 0.969 |
| Hx of Hypertension (%) | 7.6 | 6.3 | 5.6 | 7.3 | 9.8 | 11.6 | <0.001 |
| Hx of Diabetes mellitus (%) | 2.3 | 3.1 | 2.0 | 2.1 | 2.4 | 2.7 | <0.001 |
| Hx of coronary artery disease (%) | 3.6 | 4.6 | 3.6 | 3.4 | 3.6 | 4.0 | 0.159 |
| Diabetes (%) | 3.7 | 4.2 | 2.8 | 3.4 | 4.4 | 6.0 | <0.001 |
| Hypertension (%) | 15.8 | 11.6 | 11.3 | 15.5 | 20.5 | 23.6 | <0.001 |
| Medication for diabetes (%) | 1.6 | 2.5 | 1.4 | 1.5 | 1.6 | 1.6 | 0.029 |
| Medication for hypertension (%) | 5.3 | 5.2 | 4.1 | 5.2 | 6.5 | 7.2 | <0.001 |
| Higher education (%) 2 | 72.3 | 70.3 | 72.5 | 72.7 | 72.5 | 70.2 | 0.194 |
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 347,322) | |||||||
| LDL < 70 | 102,558.00 | 113 | 11 | 2.21 | 2.07 | 1.94 | 1.95 |
| (1.80–2.71) | (1.68–2.54) | (1.54–2.45) | (1.55–2.47) | ||||
| LDL 70–99 | 548,590.40 | 323 | 5.8 | 1.16 | 1.16 | 1.14 | 1.15 |
| (1.01–1.34) | (1.01–1.34) | (0.97–1.34) | (0.98–1.35) | ||||
| LDL 100–129 | 756,557.60 | 488 | 6.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 413,829.30 | 312 | 7.5 | 0.96 | 0.96 | 0.97 | 0.95 |
| (0.83–1.10) | (0.83–1.10) | (0.82–1.13) | (0.81–1.12) | ||||
| LDL ≥ 160 | 139,579.80 | 143 | 10.2 | 1.16 | 1.19 | 1.18 | 1.15 |
| (0.96–1.40) | (0.99–1.44) | (0.96–1.46) | (0.93–1.42) | ||||
| p for trend | 0.001 | 0.001 | 0.003 | 0.001 | |||
| Men (n = 199,195) | |||||||
| LDL < 70 | 43,175.10 | 89 | 20.6 | 2.37 | 2.21 | 2.06 | 2.07 |
| (1.87–3.00) | (1.75–2.80) | (1.58–2.69) | (1.58–2.70) | ||||
| LDL 70–99 | 261,503.70 | 221 | 8.5 | 1.17 | 1.15 | 1.11 | 1.12 |
| (0.99–1.38) | (0.97–1.36) | (0.92–1.34) | (0.92–1.34) | ||||
| LDL 100–129 | 462,384.70 | 351 | 7.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 290,047.80 | 231 | 8 | 1 | 0.99 | 0.98 | 0.97 |
| (0.85–1.18) | (0.84–1.17) | (0.81–1.18) | (0.80–1.17) | ||||
| LDL ≥ 160 | 99,505.80 | 99 | 9.9 | 1.27 | 1.24 | 1.29 | 1.25 |
| (1.01–1.58) | (0.99–1.55) | (1.01–1.65) | (0.98–1.61) | ||||
| p for trend | 0.001 | 0.001 | 0.032 | 0.016 | |||
| Women (n = 148,127) | |||||||
| LDL < 70 | 59,382.90 | 24 | 4 | 1.6 | 1.54 | 1.53 | 1.56 |
| (1.04–2.48) | (0.99–2.39) | (0.94–2.51) | (0.95–2.55) | ||||
| LDL 70–99 | 287,086.70 | 102 | 3.5 | 1.2 | 1.15 | 1.2 | 1.21 |
| (0.91–1.52) | (0.89–1.50) | (0.90–1.59) | (0.91–1.62) | ||||
| LDL 100–129 | 294,173.00 | 137 | 4.7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 123,781.60 | 81 | 6.5 | 0.9 | 0.91 | 0.97 | 0.96 |
| (0.68–1.19) | (0.69–1.20) | (0.72–1.30) | (0.71–1.29) | ||||
| LDL ≥ 160 | 40,074.00 | 44 | 11 | 1.16 | 1.15 | 1.01 | 0.98 |
| (0.82–1.63) | (0.82–1.63) | (0.68–1.50) | (0.66–1.47) | ||||
| p for trend | 0.12 | 0.172 | 0.103 | 0.07 | |||
| p for interaction by gender | 0.507 | 0.608 | 0.487 | 0.495 | |||
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 347,322) | |||||||
| LDL < 70 | 102,558.00 | 14 | 1.3 | 1.99 | 1.83 | 2.03 | 2.02 |
| (1.12–3.55) | (1.02–3.27) | (1.12–3.67) | (1.11–3.64) | ||||
| LDL 70–99 | 548,590.40 | 34 | 0.6 | 0.9 | 0.91 | 0.93 | 0.92 |
| (0.59–1.36) | (0.60–1.38) | (0.59–1.45) | (0.59–1.43) | ||||
| LDL 100–129 | 756,557.60 | 67 | 0.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 413,829.30 | 53 | 1.2 | 1.17 | 1.15 | 1.17 | 1.19 |
| (0.81–1.68) | (0.80–1.66) | (0.79–1.73) | (0.80–1.75) | ||||
| LDL ≥ 160 | 139,579.80 | 20 | 1.4 | 1.16 | 1.16 | 1.05 | 1.08 |
| (0.70–1.92) | (0.70–1.92) | (0.60–1.84) | (0.61–1.89) | ||||
| p for trend | 0.957 | 0.93 | 0.678 | 0.764 | |||
| Men (n = 199,195) | |||||||
| LDL < 70 | 43,175.10 | 10 | 2 | 1.91 | 1.77 | 2.01 | 1.99 |
| (0.97–3.77) | (0.89–3.52) | (0.99–4.04) | (0.99–4.02) | ||||
| LDL 70–99 | 261,503.70 | 25 | 0.9 | 0.94 | 0.94 | 1.01 | 1 |
| (0.58–1.53) | (0.58–1.53) | (0.60–1.69) | (0.59–1.68) | ||||
| LDL 100–129 | 462,384.70 | 49 | 1.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 290,047.80 | 35 | 1.2 | 1.09 | 1.05 | 1.07 | 1.08 |
| (0.71–1.68) | (0.68–1.62) | (0.66–1.72) | (0.67–1.75) | ||||
| LDL ≥ 160 | 99,505.80 | 15 | 1.6 | 1.37 | 1.27 | 1.2 | 1.23 |
| (0.77–2.45) | (0.71–2.28) | (0.63–2.29) | (0.64–2.37) | ||||
| p for trend | 0.889 | 0.983 | 0.612 | 0.674 | |||
| Women (n = 148,127) | |||||||
| LDL < 70 | 290,047.80 | 4 | 0.6 | 2.47 | 2.35 | 2.44 | 2.41 |
| (0.83–7.34) | (0.79–7.02) | (0.81–7.35) | (0.80–7.29) | ||||
| LDL 70–99 | 287,086.70 | 9 | 0.3 | 0.91 | 0.87 | 0.82 | 0.81 |
| (0.41–2.03) | (0.39–1.94) | (0.35–1.91) | (0.35–1.90) | ||||
| LDL 100–129 | 294,173.00 | 18 | 0.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 123,781.60 | 18 | 1.5 | 1.34 | 1.37 | 1.35 | 1.36 |
| (0.70–2.59) | (0.71–2.65) | (0.68–2.66) | (0.69–2.68) | ||||
| LDL ≥ 160 | 40,074.00 | 5 | 1.2 | 0.85 | 0.87 | 0.74 | 0.75 |
| (0.31–2.30) | (0.32–2.35) | (0.25–2.21) | (0.25–2.27) | ||||
| p for trend | 0.747 | 0.888 | 0.751 | 0.798 | |||
| p for interaction by gender | 0.808 | 0.798 | 0.76 | 0.759 | |||
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 347,322) | |||||||
| LDL < 70 | 102,558.00 | 50 | 4.8 | 2.01 | 1.95 | 2.05 | 2.06 |
| (1.48–2.73) | (1.43–2.65) | (1.45–2.88) | (1.46–2.90) | ||||
| LDL 70–99 | 548,590.40 | 159 | 2.8 | 1.17 | 1.19 | 1.2 | 1.21 |
| (0.96–1.44) | (0.98–1.46) | (0.96–1.51) | (0.97–1.52) | ||||
| LDL 100–129 | 756,557.60 | 238 | 3.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 413,829.30 | 150 | 3.6 | 0.94 | 0.93 | 0.97 | 0.96 |
| (0.77–1.16) | (0.76–1.14) | (0.77–1.22) | (0.76–1.20) | ||||
| LDL ≥ 160 | 139,579.80 | 78 | 5.5 | 1.3 | 1.3 | 1.31 | 1.27 |
| (1.00–1.68) | (1.01–1.69) | (0.98–1.74) | (0.95–1.70) | ||||
| p for trend | 0.033 | 0.03 | 0.058 | 0.033 | |||
| Men (n = 199,195) | |||||||
| LDL < 70 | 43,175.10 | 43 | 9.9 | 2.41 | 2.33 | 2.42 | 2.42 |
| (1.72–3.38) | (1.66–3.26) | (1.66–3.53) | (1.66–3.53) | ||||
| LDL 70–99 | 261,503.70 | 111 | 4.2 | 1.24 | 1.24 | 1.19 | 1.19 |
| (0.98–1.58) | (0.97–1.57) | (0.91–1.56) | (0.91–1.57) | ||||
| LDL 100–129 | 462,384.70 | 166 | 3.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 290,047.80 | 109 | 3.8 | 1 | 0.98 | 1 | 0.99 |
| (0.78–1.27) | (0.77–1.25) | (0.76–1.31) | (0.76–1.30) | ||||
| LDL ≥ 160 | 99,505.80 | 53 | 5.3 | 1.43 | 1.38 | 1.37 | 1.36 |
| (1.05–1.95) | (1.01–1.88) | (0.97–1.95) | (0.96–1.93) | ||||
| p for trend | 0.025 | 0.02 | 0.06 | 0.047 | |||
| Women (n = 148,127) | |||||||
| LDL < 70 | 290,047.80 | 7 | 1.1 | 0.83 | 0.86 | 0.97 | 0.99 |
| (0.38–1.81) | (0.39–1.87) | (0.42–2.27) | (0.43–2.32) | ||||
| LDL 70–99 | 287,086.70 | 48 | 1.6 | 1 | 1.03 | 1.17 | 1.19 |
| (0.69–1.44) | (0.71–1.49) | (0.78–1.75) | (0.79–1.79) | ||||
| LDL 100–129 | 294,173.00 | 72 | 2.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 123,781.60 | 41 | 3.3 | 0.91 | 0.89 | 0.98 | 0.97 |
| (0.62–1.34) | (0.61–1.31) | (0.64–1.49) | (0.63–1.48) | ||||
| LDL ≥ 160 | 40,074.00 | 25 | 6.2 | 1.34 | 1.28 | 1.27 | 1.22 |
| (0.85–2.12) | (0.81–2.04) | (0.75–2.13) | (0.73–2.06) | ||||
| p for trend | 0.418 | 0.624 | 0.94 | 0.91 | |||
| p for interaction by gender | 0.271 | 0.305 | 0.448 | 0.46 | |||
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 182,943) | |||||||
| LDL < 70 | 68,149 | 207 | 30.4 | 2.16 | 2.15 | 1.82 | 1.81 |
| (1.85–2.51) | (1.71–2.70) | (1.45–2.29) | (1.44–2.28) | ||||
| LDL 70–99 | 357,358 | 565 | 15.8 | 1.28 | 1.45 | 1.35 | 1.35 |
| (1.15–1.43) | (1.23–1.70) | (1.15–1.59) | (1.15–1.59) | ||||
| LDL 100–129 | 597,261 | 800 | 13.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 388,906 | 520 | 13.4 | 0.88 | 1.03 | 1.06 | 1.06 |
| (0.79–0.98) | (0.87–1.22) | (0.89–1.25) | (0.90–1.25) | ||||
| LDL ≥ 160 | 157,296 | 246 | 15.6 | 0.94 | 1.05 | 1.08 | 1.08 |
| (0.81–1.08) | (0.84–1.31) | (0.86–1.35) | (0.87–1.36) | ||||
| p for trend | <0.0001 | <0.0001 | <0.0001 | 0.0001 | |||
| Men (n = 63,318) | |||||||
| LDL < 70 | 33,865 | 174 | 51.4 | 2.06 | 2.17 | 1.83 | 1.83 |
| (1.73–2.45) | (1.69–2.79) | (1.42–2.36) | (1.42–2.36) | ||||
| LDL 70–99 | 133,332 | 405 | 30.4 | 1.31 | 1.46 | 1.37 | 1.37 |
| (1.14–1.49) | (1.21–1.76) | (1.13–1.65) | (1.13–1.65) | ||||
| LDL 100–129 | 210,838 | 487 | 23.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 124,980 | 258 | 20.6 | 0.9 | 0.97 | 0.98 | 0.98 |
| (0.78–1.05) | (0.78–1.21) | (0.79–1.22) | (0.79–1.22) | ||||
| LDL ≥ 160 | 41,542 | 114 | 27.4 | 1.2 | 1.09 | 1.11 | 1.11 |
| (0.98–1.47) | (0.80–1.49) | (0.81–1.52) | (0.81–1.52) | ||||
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Women (n = 119,625) | |||||||
| LDL < 70 | 34,284 | 33 | 9.6 | 1.29 | 1.48 | 1.3 | 1.3 |
| (0.90–1.85) | (0.83–2.64) | (0.73–2.33) | (0.73–2.32) | ||||
| LDL 70–99 | 224,026 | 160 | 7.1 | 1.06 | 1.31 | 1.23 | 1.23 |
| (0.87–1.28) | (0.96–1.78) | (0.91–1.68) | (0.90–1.68) | ||||
| LDL 100–129 | 386,423 | 313 | 8.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 263,926 | 262 | 9.9 | 0.96 | 1.24 | 1.28 | 1.28 |
| (0.81–1.13) | (0.95–1.63) | (0.98–1.68) | (0.98–1.68) | ||||
| LDL ≥ 160 | 115,755 | 132 | 11.4 | 0.97 | 1.21 | 1.25 | 1.26 |
| (0.79–1.19) | (0.86–1.69) | (0.89–1.75) | (0.90–1.76) | ||||
| p for trend | 0.1964 | 0.8682 | 0.6045 | 0.5733 | |||
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 180,804) | |||||||
| LDL < 70 | 66,931 | 14 | 2.1 | 2 | 2.53 | 2 | 1.93 |
| (1.12–3.58) | (1.07–6.01) | (0.84–4.78) | (0.81–4.61) | ||||
| LDL 70–99 | 353,873 | 39 | 1.1 | 1.21 | 2.07 | 1.88 | 1.86 |
| (0.81–1.81) | (1.14–3.74) | (1.04–3.42) | (1.03–3.37) | ||||
| LDL 100–129 | 592,339 | 59 | 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 385,834 | 53 | 1.4 | 1.21 | 1.7 | 1.77 | 1.82 |
| (0.83–1.75) | (0.94–3.07) | (0.98–3.20) | (1.01–3.31) | ||||
| LDL ≥ 160 | 155,906 | 34 | 2.2 | 1.74 | 2.65 | 2.83 | 3 |
| (1.14–2.65) | (1.37–5.14) | (1.46–5.49) | (1.54–5.81) | ||||
| p for trend | 0.2492 | 0.7319 | 0.3009 | 0.2016 | |||
| Men (n = 62,001) | |||||||
| LDL < 70 | 32,840 | 11 | 3.3 | 2.25 | 3.91 | 3.25 | 3.15 |
| (1.12–4.50) | (1.51–10.01) | (1.24–8.47) | (1.21–8.21) | ||||
| LDL 70–99 | 130,856 | 30 | 2.3 | 1.64 | 2.78 | 2.6 | 2.55 |
| (0.99–2.74) | (1.32–5.84) | (1.23–5.47) | (1.21–5.38) | ||||
| LDL 100–129 | 207,799 | 29 | 1.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 123,520 | 31 | 2.5 | 1.82 | 2.25 | 2.35 | 2.4 |
| (2.00–3.02) | (1.04–4.91) | (1.08–5.13) | (1.10–5.22) | ||||
| LDL ≥ 160 | 40,912 | 20 | 4.9 | 3.57 | 3.46 | 3.7 | 3.85 |
| (2.02–6.31) | (1.39–8.63) | (1.49–9.24) | (1.54–9.60) | ||||
| p for trend | 0.0164 | 0.9761 | 0.6235 | 0.5223 | |||
| Women (n = 118,803) | |||||||
| LDL < 70 | 34,091 | 3 | 0.9 | 1.21 | NA * | NA * | NA * |
| (0.37–3.96) | |||||||
| LDL 70–99 | 223,017 | 9 | 0.4 | 0.63 | 1.11 | 0.97 | 0.97 |
| (0.30–1.32) | (0.37–3.31) | (0.33–2.92) | (0.32–2.90) | ||||
| LDL 100–129 | 384,541 | 30 | 0.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 262,314 | 22 | 0.8 | 0.83 | 1.12 | 1.21 | 1.24 |
| (0.48–1.43) | (0.45–2.83) | (0.48–3.05) | (0.49–3.13) | ||||
| LDL ≥ 160 | 114,994 | 14 | 1.2 | 1.04 | 2 | 2.18 | 2.27 |
| (0.55–1.95) | (0.77–5.20) | (0.84–5.67) | (0.87–5.93) | ||||
| p for trend | 0.53 | 0.3287 | 0.1649 | 0.1363 | |||
| LDL-C (mg/dL) | Person-Years | Number of Events | Mortality Rate (10,000 Person-Year) | Age-Adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Total (n = 182,428) | |||||||
| LDL < 70 | 67,784 | 145 | 21.4 | 1.92 | 1.31 | 1.24 | 1.24 |
| (1.60–2.29) | (1.01–1.72) | (0.95–1.62) | (0.95–1.63) | ||||
| LDL 70–99 | 356,508 | 449 | 12.6 | 1.26 | 1.07 | 1.04 | 1.04 |
| (1.12–1.43) | (0.90–1.26) | (0.88–1.23) | (0.88–1.23) | ||||
| LDL 100–129 | 596,054 | 640 | 10.7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 388,202 | 421 | 10.8 | 0.9 | 0.97 | 0.98 | 0.97 |
| (0.80–1.02) | (0.82–1.14) | (0.83–1.15) | (0.83–1.15) | ||||
| LDL ≥ 160 | 156,791 | 168 | 10.7 | 0.82 | 0.89 | 0.9 | 0.9 |
| (0.69–0.97) | (0.71–1.13) | (0.71–1.14) | (0.71–1.13) | ||||
| p for trend | <0.0001 | <0.0001 | 0.0022 | 0.0021 | |||
| Men (n = 62,993) | |||||||
| LDL < 70 | 33,550 | 119 | 35.5 | 1.82 | 1.41 | 1.34 | 1.34 |
| (1.48–2.24) | (1.05–1.91) | (0.99–1.82) | (0.99–1.82) | ||||
| LDL 70–99 | 132,685 | 310 | 23.4 | 1.29 | 1.09 | 1.06 | 1.06 |
| (1.11–1.50) | (0.88–1.34) | (0.86–1.31) | (0.86–1.31) | ||||
| LDL 100–129 | 210,008 | 379 | 18 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 124,852 | 239 | 19.1 | 1.07 | 1.14 | 1.14 | 1.14 |
| (0.91–1.26) | (0.92–1.40) | (0.93–1.41) | (0.92–1.41) | ||||
| LDL ≥ 160 | 41,233 | 66 | 16 | 0.9 | 0.87 | 0.88 | 0.87 |
| (0.70–1.17) | (0.62–1.23) | (0.62–1.24) | (0.62–1.24) | ||||
| p for trend | <0.0001 | 0.0949 | 0.1915 | 0.1837 | |||
| Women (n = 119,435) | |||||||
| LDL < 70 | 34,234 | 26 | 7.6 | 1.22 | 0.96 | 0.9 | 0.9 |
| (0.82–1.82) | (0.52–1.77) | (0.48–1.66) | (0.49–1.67) | ||||
| LDL 70–99 | 223,823 | 139 | 6.2 | 1.04 | 1 | 0.98 | 0.98 |
| (0.85–1.28) | (0.75–1.33) | (0.73–1.30) | (0.74–1.30) | ||||
| LDL 100–129 | 386,046 | 261 | 6.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| LDL 130–159 | 263,350 | 182 | 6.9 | 0.86 | 0.82 | 0.83 | 0.833 |
| (0.71–1.04) | (0.63–1.07) | (0.64–1.09) | (0.64–1.09) | ||||
| LDL ≥ 160 | 115,558 | 102 | 8.8 | 0.99 | 0.98 | 1 | 0.99 |
| (0.79–1.25) | (0.71–1.35) | (0.72–1.37) | (0.72–1.37) | ||||
| p for trend | 0.0994 | 0.4654 | 0.6899 | 0.6713 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, K.-C.; Huh, J.H.; Ryu, S.; Lee, J.-Y.; Scorletti, E.; Byrne, C.D.; Kim, J.Y.; Hyun, D.S.; Ko, S.-B. Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users. J. Clin. Med. 2019, 8, 1571. https://doi.org/10.3390/jcm8101571
Sung K-C, Huh JH, Ryu S, Lee J-Y, Scorletti E, Byrne CD, Kim JY, Hyun DS, Ko S-B. Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users. Journal of Clinical Medicine. 2019; 8(10):1571. https://doi.org/10.3390/jcm8101571
Chicago/Turabian StyleSung, Ki-Chul, Ji Hye Huh, Seungho Ryu, Jong-Young Lee, Eleonora Scorletti, Christopher D Byrne, Jang Young Kim, Dae Sung Hyun, and Sang-Baek Ko. 2019. "Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users" Journal of Clinical Medicine 8, no. 10: 1571. https://doi.org/10.3390/jcm8101571
APA StyleSung, K.-C., Huh, J. H., Ryu, S., Lee, J.-Y., Scorletti, E., Byrne, C. D., Kim, J. Y., Hyun, D. S., & Ko, S.-B. (2019). Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users. Journal of Clinical Medicine, 8(10), 1571. https://doi.org/10.3390/jcm8101571






