The Error of Estimated GFR in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Measured GFR
2.3. Estimated GFR by Formulas
2.4. Biochemistry
2.5. Statistical Analysis
2.5.1. Agreement between eGFR and mGFR
2.5.2. Error in the Classification of CKD Stages
2.5.3. Accuracy of Estimated GFR with Measured GFR
3. Results
3.1. Patients
3.2. Agreement between Measured GFR and Estimated GFR
3.2.1. Creatinine Based Formulas
3.2.2. Cystatin-C Based Formulas
3.2.3. Creatinine and Cystatin-C Based Formulas
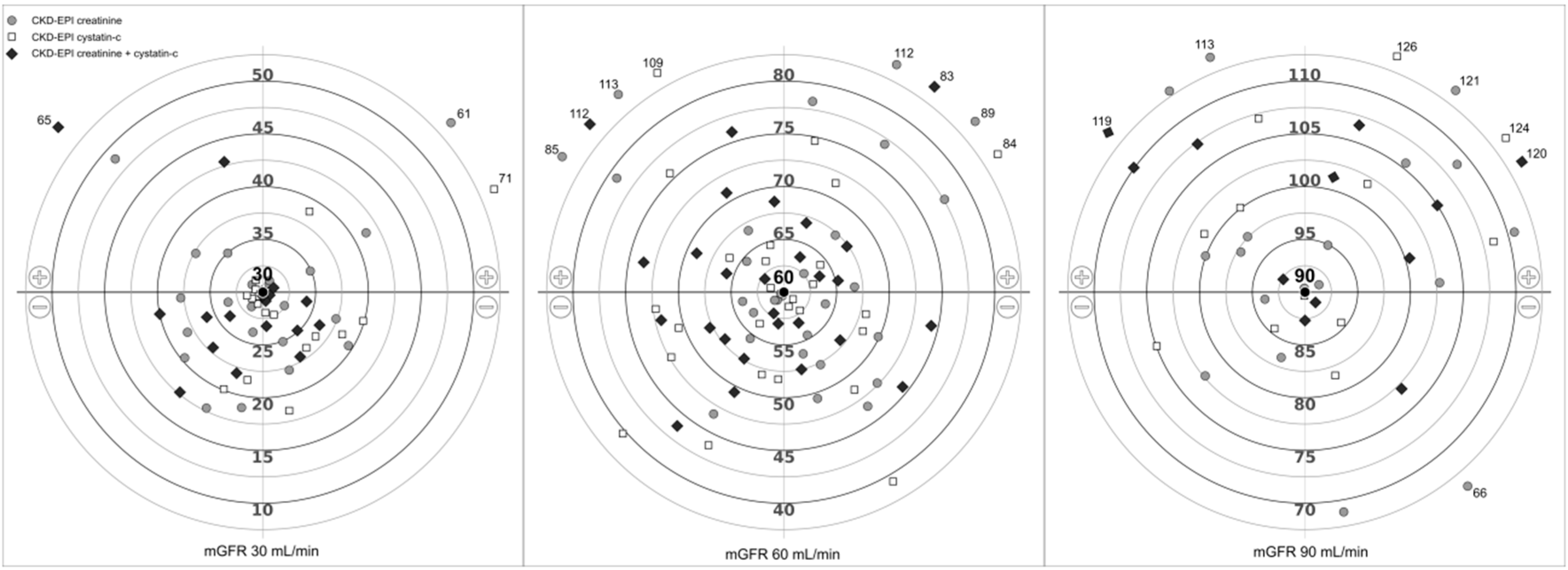
3.3. Subgroups of GFR
3.4. The Error in the CKD Classification
3.5. Hyperfiltering Patients
3.6. Examples of Under or Overestimation of GFR
3.7. Accuracy of Estimated GFR with Measured GFR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Gregg, E. Global economic burden of diabetes and its implications. Lancet Diabetes Endocrinol. 2017, 5, 404–405. [Google Scholar] [CrossRef]
- Schaubel, D.E.; Morrison, H.I.; Desmeules, M.; Parsons, D.A.; Fenton, S.S. End-stage renal disease in Canada: Prevalence projections to 2005. CMAJ 1999, 160, 1557–1563. [Google Scholar] [PubMed]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Perkins, B.A.; Nelson, R.G.; Ostrander, B.E.; Blouch, K.L.; Krolewski, A.S.; Myers, B.D.; Warram, J.H. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J. Am. Soc. Nephrol. 2005, 16, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Rossing, K.; Gaede, P.; Pedersen, O.; Parving, H.H. Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care 2006, 29, 1024–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rigalleau, V.; Lasseur, C.; Perlemoine, C.; Barthe, N.; Raffaitin, C.; de La Faille, R.; Combe, C.; Gin, H. A simplified Cockcroft-Gault formula to improve the prediction of the glomerular filtration rate in diabetic patients. Diabetes Metab. 2006, 32, 56–62. [Google Scholar] [CrossRef]
- Fontseré, N.; Salinas, I.; Bonal, J.; Bayés, B.; Riba, J.; Torres, F.; Rios, J.; Sanmartí, A.; Romero, R. Are prediction equations for glomerular filtration rate useful for the long-term monitoring of type 2 diabetic patients? Nephrol. Dial. Transplant. 2006, 21, 2152–2158. [Google Scholar] [CrossRef]
- Beauvieux, M.C.; Le Moigne, F.; Lasseur, C.; Raffaitin, C.; Perlemoine, C.; Barthe, N.; Chauveau, P.; Combe, C.; Gin, H.; Rigalleau, V. New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care 2007, 30, 1988–1994. [Google Scholar] [CrossRef][Green Version]
- Pucci, L.; Triscornia, S.; Lucchesi, D.; Fotino, C.; Pellegrini, G.; Pardini, E.; Miccoli, R.; Del Prato, S.; Penno, G. Cystatin C and estimates of renal function: Searching for a better measure of kidney function in diabetic patients. Clin. Chem. 2007, 53, 480–488. [Google Scholar] [CrossRef]
- Li, H.X.; Xu, G.B.; Wang, X.J.; Zhang, X.C.; Yang, J.M. Diagnostic accuracy of various glomerular filtration rates estimating equations in patients with chronic kidney disease and diabetes. Chin. Med. J. (Engl.) 2010, 123, 745–751. [Google Scholar]
- Iliadis, F.; Didangelos, T.; Ntemka, A.; Makedou, A.; Moralidis, E.; Gotzamani-Psarakou, A.; Kouloukourgiotou, T.; Grekas, D. Glomerular filtration rate estimation in patients with type 2 diabetes: Creatinine- or cystatin C-based equations? Diabetologia 2011, 54, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, F.; Ruggenenti, P.; Porrini, E.; Motterlini, N.; Cannata, A.; Carrara, F.; Jiménez Sosa, A.; Cella, C.; Ferrari, S.; Stucchi, N.; et al. GFR Study Investigators. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int. 2013, 84, 164–173. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, R.J.; Ekinci, E.I.; Premaratne, E.; Lu, Z.X.; Seah, J.M.; Li, Y.; Boston, R.; Ward, G.M.; Jerums, G. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol. 2015, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Porrini, E.; Ruggenenti, P.; Luis-Lima, S.; Carrara, F.; Jiménez, A.; de Vries, A.P.J.; Torres, A.; Gaspari, F.; Remuzzi, G. Estimated GFR: Time for a critical appraisal. Nat. Rev. Nephrol. 2019, 15, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Luis-Lima, S.; Marrero-Miranda, D.; González-Rinne, A.; Torres, A.; González-Posada, J.M.; Rodríguez, A.; Salido, E.; Aldea-Perona, A.; Gaspari, F.; Carrara, F.; et al. Estimated Glomerular Filtration Rate in Renal Transplantation: The Nephrologist in the Mist. Transplantation 2015, 99, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Luis-Lima, S.; Gaspari, F.; Negrín-Mena, N.; Carrara, F.; Díaz-Martín, L.; Jiménez-Sosa, A.; González-Rinne, F.; Torres, A.; Porrini, E. Iohexol plasma clearance simplified by dried blood spot testing. Nephrol. Dial. Transpl. 2018, 33, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–313. [Google Scholar]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Lin, L.; Hedayat, A.S.; Wu, W. A Comparative Model for Continuous and Categorical Data. In Statistical Tools for Measuring Agreement, 1st ed.; Springer: New York, NY, USA, 2012; pp. 111–137. [Google Scholar]
- Lin, L.; Hedayat, A.S.; Sinha, B.; Yang, M. Statistical methods in assessing agreement. J. Am. Stat. Assoc. 2002, 97, 257. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [PubMed]
- Jones, J.D.; Burnett, P.C. Creatinine metabolism in humans with decreased renal function: Creatinine deficit. Clin. Chem. 1974, 20, 1204–1212. [Google Scholar] [PubMed]
- Tomlinson, B.E.; Walton, J.N.; Rebeiz, J.J. The effects of ageing and of cachexia upon skeletal muscle. A histopathological study. J. Neurol. Sci. 1969, 9, 321–346. [Google Scholar] [CrossRef]
- Bleiler, R.E.; Schedl, H.P. Creatinine excretion: Variability and relationships to diet and body size. J. Lab. Clin. Med. 1962, 59, 945–955. [Google Scholar] [PubMed]
- Knight, E.L.; Verhave, J.C.; Spiegelman, D.; Hillege, H.L.; de Zeeuw, D.; Curhan, G.C.; de Jong, P.E. Factors influencing serum cystatin-C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004, 65, 1416–1421. [Google Scholar] [CrossRef]
| N | 475 |
|---|---|
| Age (years) | 63.1 ± 11.2 |
| Gender (male-%) | 333 (70.1) |
| Clinical condition (n—%) | |
| With renal disease | 290 (61.1) |
| Diabetic nephropathy | 120 (25.3) |
| Kidney transplantation | 112 (23.6) |
| Nephroangioesclerosis | 33 (6.9) |
| Glomerulonephritis | 11 (2.3) |
| Acquired Dominant Polycystic Kidney Disease | 8 (1.7) |
| Intertitial nephritis | 6 (1.3) |
| Without renal disease | 185 (38.9) |
| Heart failure | 34 (7.2) |
| Cirrhosis | 33 (6.9) |
| Liver transplantation | 12 (2.5) |
| Other | 106 (22.3) |
| measured GFR mean ± SD (mL/min) | 57.3 ± 36.3 |
| measured GFR range (mL/min) | 8.5–180.6 |
| CKD stages (n—%) | |
| 1 (>90 mL/min) | 86 (18.1) |
| 2 (60–90 mL/min) | 94 (19.8) |
| 3 (30–60 mL/min) | 156 (32.8) |
| 4 (15–30 mL/min) | 116 (24.4) |
| 5 (<15 mL/min) | 23 (4.9) |
| Height (m) | 1.68 ± 0.09 |
| Weight (kg) | 85.2 ± 18.4 |
| Body Mass Index (kg/m2) | 30.2 ± 5.6 |
| Body Surface Area (m2) | 1.94 ± 0.22 |
| Serum Creatinine (mg/dL) | 1.82 ± 1.15 |
| Serum Cystatin-C (g/dL) | 1.84 ± 0.96 |
| 24 h creatinine clearance median (IQR) (mL/min) | 41.8 (40.4) |
| 24-h proteinuria median (IQR) (mg/24 h) | 427.7 (1440.1) |
| Formula | CCC | TDI | CP | Formula | CCC | TDI | CP |
|---|---|---|---|---|---|---|---|
| Creatinine-based-formulas | |||||||
| Effersøe | 0.91 (0.89) | 61 (65) | 25 (24) | aMDRD | 0.92 (0.91) | 59 (63) | 27 (25) |
| Edward-White | 0.85 (0.83) | 82 (88) | 20 (19) | Wright | 0.88 (0.87) | 70 (75) | 22 (21) |
| Jelliffe-1 | 0.87 (0.85) | 88 (95) | 19 (18) | MCQ | 0.88 (0.87) | 77 (82) | 22 (20) |
| Mawer | 0.88 (0.87) | 74 (79) | 21 (20) | Sobh | 0.85 (0.83) | 87 (93) | 18 (17) |
| Jelliffe-2 | 0.91 (0.90) | 57 (61) | 27 (26) | Virga | 0.88 (0.87) | 72 (77) | 21 (20) |
| Cockcroft-Gault | 0.89 (0.87) | 70 (75) | 22 (21) | CHUQ | 0.84 (0.82) | 96 (103) | 18 (17) |
| Björnsson | 0.87 (0.85) | 80 (85) | 19 (18) | CKD-EPI-cr | 0.92 (0.91) | 58 (62) | 27 (25) |
| Mogensen | 0.76 (0.73) | 144 (156) | 13 (12) | Lund-Malmö (LBM) | 0.85 (0.83) | 91 (97) | 18 (17) |
| Hull | 0.88 (0.87) | 76 (81) | 21 (20) | Lund-Malmö | 0.91 (0.90) | 59 (63) | 26 (25) |
| Gates | 0.91 (0.89) | 66 (70) | 24 (23) | Lund-1 | 0.91 (0.90) | 53 (56) | 29 (27) |
| Walser | 0.90 (0.89) | 64 (68) | 25 (23) | Lund-2 (LBM) | 0.81 (0.77) | 107 (114) | 14 (13) |
| Davis Chandler | 0.89 (0.87) | 68 (73) | 24 (22) | Lund-Malmö (Rv) | 0.92 (0.91) | 55 (59) | 28 (26) |
| Baracskay | 0.82 (0.80) | 79 (85) | 21 (20) | Lund-Malmö (RvLBM) | 0.88 (0.86) | 74 (79) | 21 (20) |
| Martin | 0.83 (0.81) | 96 (102) | 14 (13) | FAS-cr | 0.83 (0.81) | 93 (98) | 15 (13) |
| Cystatin-C-based | |||||||
| Le Bricon | 0.82 (0.80) | 81 (87) | 19 (17) | Jonsson | 0.92 (0.91) | 59 (64) | 26 (25) |
| Tan | 0.92 (0.90) | 54 (58) | 28 (27) | Stevens-1 | 0.93 (0.92) | 49 (53) | 31 (29) |
| Hoek | 0.92 (0.91) | 51 (55) | 29 (28) | Stevens-2 | 0.93 (0.92) | 47 (50) | 32 (30) |
| Larsson | 0.93 (0.92) | 49 (53) | 30 (29) | Tidman | 0.91 (0.90) | 61 (66) | 26 (24) |
| Perkins | 0.74 (0.71) | 119 (126) | 8 (7) | Grubb-2009 | 0.86 (0.84) | 98 (107) | 18 (17) |
| Orebro | 0.86 (0.84) | 96 (104) | 18 (17) | Hojs | 0.88 (0.86) | 70 (74) | 21 (20) |
| Grubb-2005 | 0.88 (0.87) | 85 (92) | 20 (19) | Grubb-2014 (CAPA) | 0.93 (0.92) | 52 (56) | 29 (27) |
| Rule-cy | 0.91 (0.90) | 60 (65) | 26 (24) | CKD-EPI-cy | 0.93 (0.92) | 51 (55) | 30 (28) |
| MacIsaac | 0.89 (0.88) | 62 (66) | 24 (23) | FAS-cy | 0.82 (0.80) | 86 (92) | 17 (15) |
| Arnal-Dade | 0.93 (0.92) | 53 (57) | 29 (27) | 24 h-CrCl | 0.81 (0.77) | 85 (94) | 20 (18) |
| Creatinine-cystatin-C-based-formulas | |||||||
| Ma | 0.93 (0.91) | 54 (58) | 27 (26) | CKD-EPI-cr-cy | 0.94 (0.94) | 45 (49) | 32 (31) |
| Stevens | 0.95 (0.94) | 44 (47) | 33 (32) | FAS-cr-cy | 0.85 (0.83) | 78 (83) | 16 (14) |
| Creatinine | Cystatin-C | Creatinine and Cystatin-C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | mGFR | CG | aMDRD | MCQ | CKD-EPI | Rule | MacIsaac | CKD-EPI | Ma | Stevens | CKD-EPI |
| 1 | 17 | 13 * | 11 * | 11 * | 10 * | 13 * | 20 | 13 * | 13 * | 12 * | 11 * |
| 2 | 17 | 26 | 24 | 22 | 23 | 23 | 35 | 25 | 28 | 25 | 23 |
| 3 | 27 | 22 | 16 | 15 | 15 | 25 | 37 | 27 | 24 | 21 | 20 |
| 4 | 28 | 34 | 31 | 33 | 33 | 30 | 43 | 35 | 38 | 34 | 33 |
| 5 | 48 | 57 | 56 | 63 | 55 | 41 | 57 | 44 | 59 | 52 | 48 |
| 6 | 49 | 36 | 29 * | 27 * | 28 * | 47 | 65 | 53 | 44 | 38 | 38 |
| 7 | 67 | 41 * | 47 * | 50 * | 44 * | 43 * | 59 * | 45 * | 54 * | 48 * | 44 * |
| 8 | 68 | 142 | 82 | 110 | 91 | 47 * | 65 | 57 * | 79 | 72 | 70 |
| 9 | 99 | 80 * | 65 * | 81 * | 68 * | 108 | 127 | 127 | 103 | 88 * | 94 |
| 10 | 97 | 131 | 133 | 95 | 105 | 78 * | 95 | 80 * | 123 | 109 | 94 |
| 11 | 116 | 154 | 159 | 131 | 124 | 117 | 132 | 125 | 172 | 152 | 129 |
| 12 | 118 | 95 | 106 | 120 | 100 | 96 | 113 | 108 | 125 | 109 | 106 |
| 13 | 150 | 299 | 206 | 182 | 171 | 130 | 151 | 159 | 209 | 188 | 167 |
| 14 | 151 | 201 | 115 | 133 | 133 | 137 | 154 | 141 | 153 | 134 | 139 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luis-Lima, S.; Higueras Linares, T.; Henríquez-Gómez, L.; Alonso-Pescoso, R.; Jimenez, A.; López-Hijazo, A.M.; Negrín-Mena, N.; Martín, C.; Sánchez-Gallego, M.; Galindo-Hernández, S.J.; et al. The Error of Estimated GFR in Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 1543. https://doi.org/10.3390/jcm8101543
Luis-Lima S, Higueras Linares T, Henríquez-Gómez L, Alonso-Pescoso R, Jimenez A, López-Hijazo AM, Negrín-Mena N, Martín C, Sánchez-Gallego M, Galindo-Hernández SJ, et al. The Error of Estimated GFR in Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2019; 8(10):1543. https://doi.org/10.3390/jcm8101543
Chicago/Turabian StyleLuis-Lima, Sergio, Tomás Higueras Linares, Laura Henríquez-Gómez, Raquel Alonso-Pescoso, Angeles Jimenez, Asunción María López-Hijazo, Natalia Negrín-Mena, Candelaria Martín, Macarena Sánchez-Gallego, Sara Judith Galindo-Hernández, and et al. 2019. "The Error of Estimated GFR in Type 2 Diabetes Mellitus" Journal of Clinical Medicine 8, no. 10: 1543. https://doi.org/10.3390/jcm8101543
APA StyleLuis-Lima, S., Higueras Linares, T., Henríquez-Gómez, L., Alonso-Pescoso, R., Jimenez, A., López-Hijazo, A. M., Negrín-Mena, N., Martín, C., Sánchez-Gallego, M., Galindo-Hernández, S. J., Socas Fernández del Castillo, R., Castilla-Marrero, M., Domínguez-Coello, S., Vilchez de León, V., Valcárcel-Lopez, R., Insausti-Garmendia, N., Escamilla, B., Estupiñán, S., Delgado-Mallén, P., ... Porrini, E. (2019). The Error of Estimated GFR in Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 8(10), 1543. https://doi.org/10.3390/jcm8101543





