Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner
Abstract
1. Introduction
2. Experimental Section
2.1. Histology and Immunochemistry
2.2. Cell Culture
2.3. RT-qPCR Analysis
2.4. Scanning Electron Microscopy (SEM) and FTIR (Fourier Transform Infrared) Spectroscopy
2.5. Proteomic and Bioinformatics Analysis
2.6. Statistics
3. Results
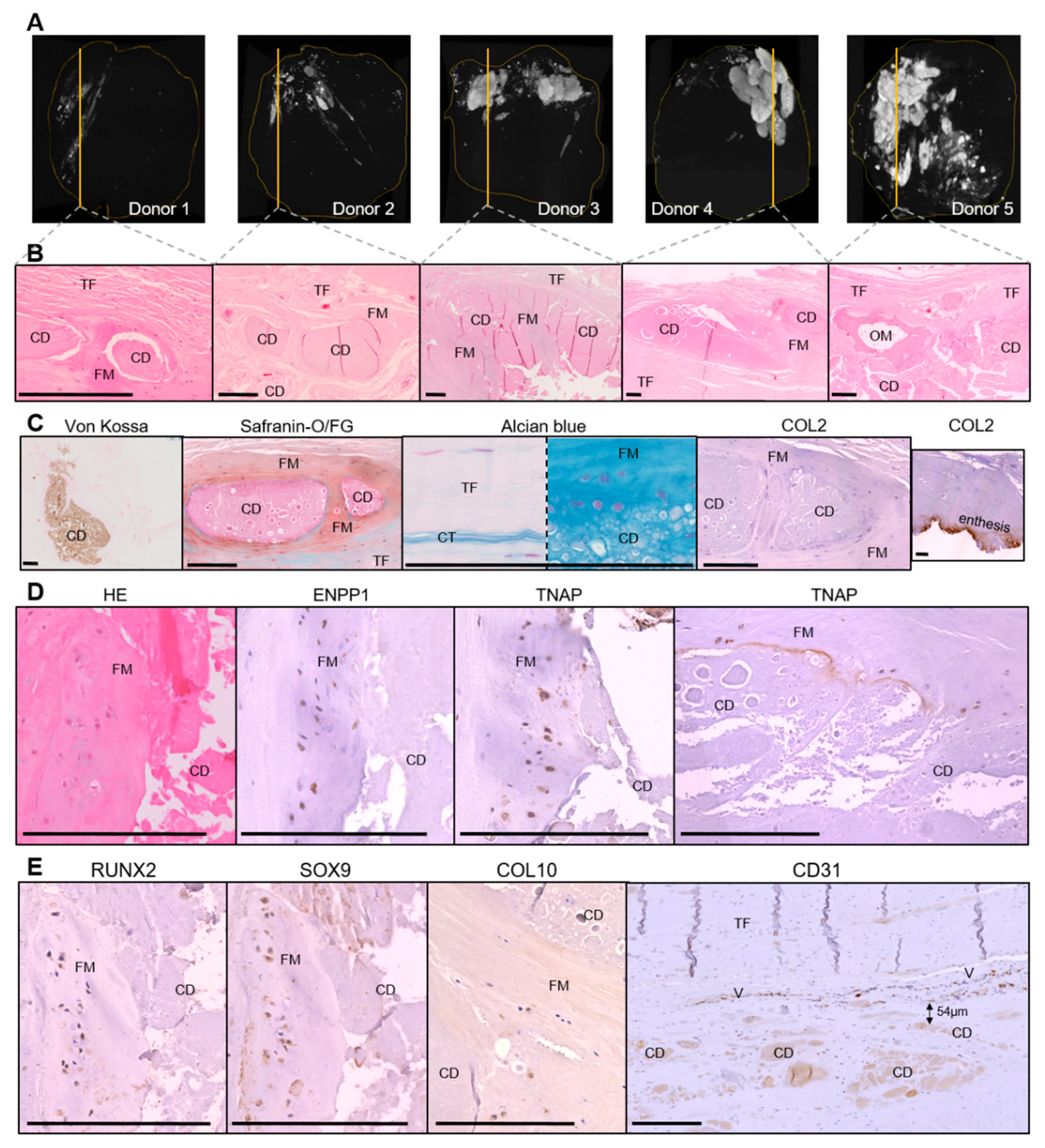
3.1. Histological Characterization of Calcific Tendonitis
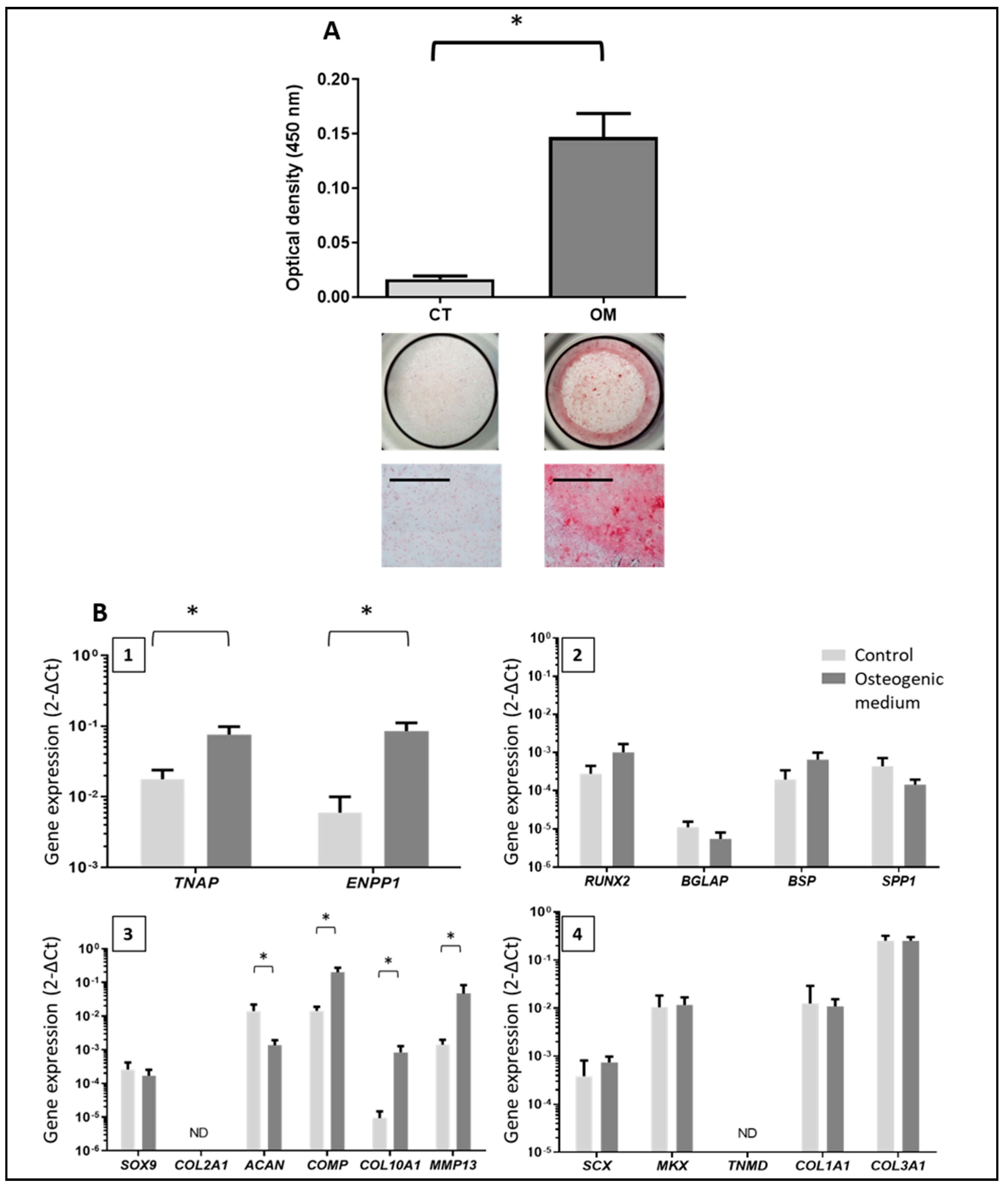
3.2. Characterization of Tendon Cells and Assessment of their Mineralization Ability
3.3. Phenotype of the Mineralizing TLC
3.4. TNAP Involvement in TLC-Induced Mineralization
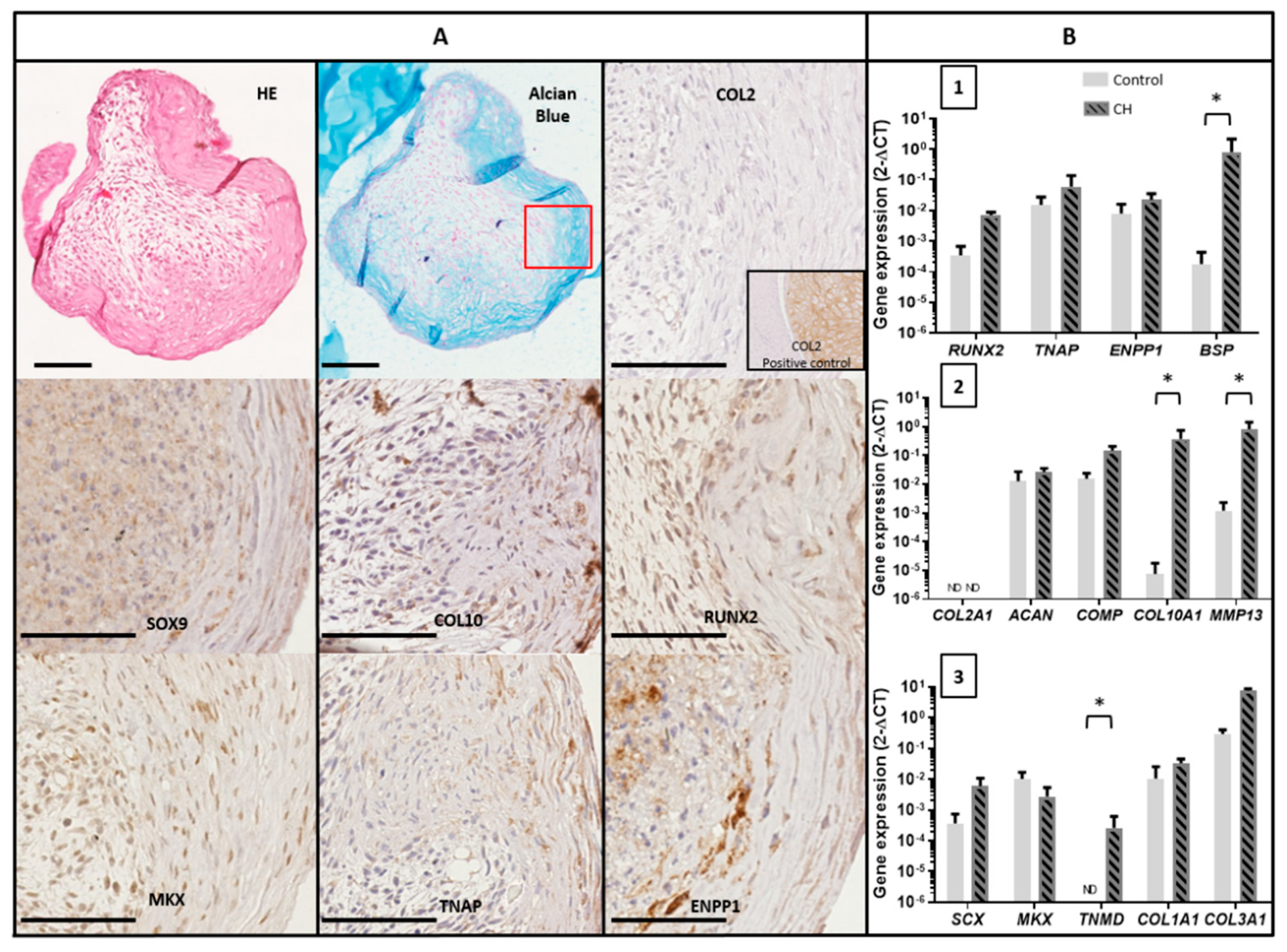
3.5. Mineralization Ability of TLC in 3D Chondrogenic Culture
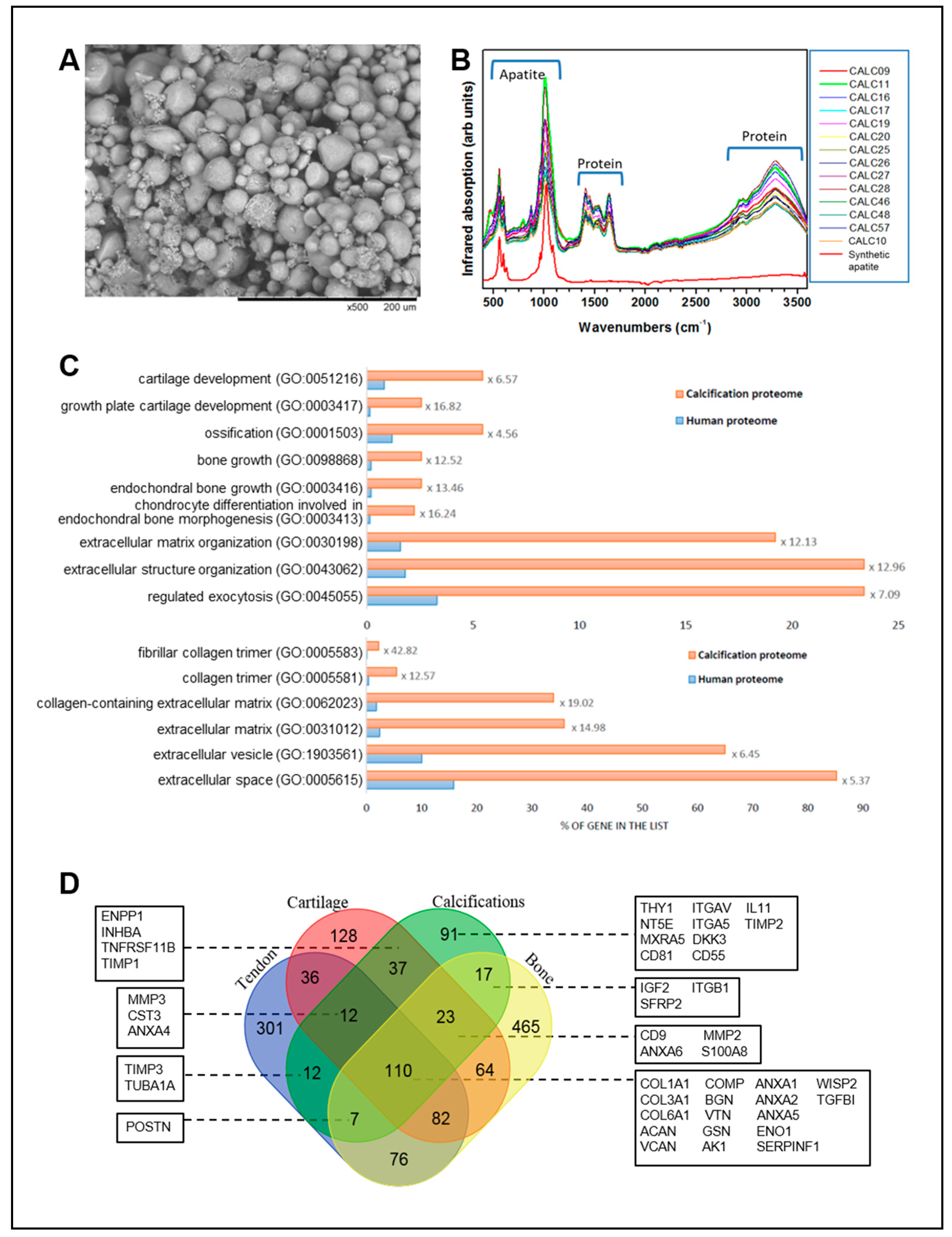
3.6. Composition of Calcium Deposits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darrieutort-Laffite, C.; Blanchard, F.; Le Goff, B. Calcific tendonitis of the rotator cuff: From formation to resorption. Jt. Bone Spine 2018, 85, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch. A Pathol. Anat. Histol. 1975, 366, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Archer, R.S.; Bayley, J.I.; Archer, C.W.; Ali, S.Y. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J. Anat. 1993, 82, 1–11. [Google Scholar]
- Speed, C.A.; Hazleman, B.L. Calcific tendinitis of the shoulder. N. Engl. J. Med. 1999, 340, 1582–1584. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.D.; Chowaniec, D.; McCarthy, M.B.; Beitzel, K.; Cote, M.P.; McKinnon, W.; Arciero, R. In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: Multiple passages result in changes in routine cell markers. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Schmidmaier, G.; Wildemann, B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur. Cell Mater. 2012, 24, 74–89. [Google Scholar] [CrossRef]
- De Mos, M.; Koevoet, W.J.; Jahr, H.; Verstegen, M.M.; Heijboer, M.P.; Kops, N.; van Leeuwen, J.P.; Weinans, H.; Verhaar, J.A.; van Osch, G.J. Intrinsic differentiation potential of adolescent human tendon tissue: An in vitro cell differentiation study. BMC Musculoskelet. Disord. 2007, 8, 16. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Boutet, M.A.; Najm, A.; Bart, G.; Brion, R.; Touchais, S.; Trichet, V.; Layrolle, P.; Gabay, C.; Palmer, G.; Blanchard, F.; et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann. Rheum. Dis. 2017, 76, 1304–1312. [Google Scholar] [CrossRef]
- Guihard, P.; Boutet, M.A.; Brounais-Le Royer, B.; Gamblin, A.L.; Amiaud, J.; Renaud, A.; Berreur, M.; Rédini, F.; Heymann, D.; Layrolle, P.; et al. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am. J. Pathol. 2015, 185, 765–775. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Guihard, P.; Brounais, B.; Riet, A.; Charrier, C.; Battaglia, S.; Gouin, F.; Ponsolle, S.; Bot, R.L.; Richards, C.D.; et al. Direct anti-cancer effect of oncostatin M on chondrosarcoma. Int. J. Cancer 2011, 128, 1822–1835. [Google Scholar] [CrossRef]
- Le Goff, B.; Soltner, E.; Charrier, C.; Maugars, Y.; Rédini, F.; Heymann, D.; Berthelot, J.M. A combination of methotrexate and zoledronic acid prevents bone erosions and systemic bone mass loss in collagen induced arthritis. Arthritis Res. Ther. 2009, 11, R185. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Klatte, F.; Strobel, C.; Schmidmaier, G.; Greiner, S.; Scheibel, M.; Wildemann, B. Characterization of tendon cell cultures of the human rotator cuff. Eur. Cell Mater. 2010, 20, 84–97. [Google Scholar] [CrossRef]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Renaud, A.; Guilloton, F.; Mebarki, M.; Trichet, V.; Sensebé, L.; Deschaseaux, F.; Chevallier, N.; Layrolle, P. Inferior in vivo osteogenesis and superior angiogenesis of human adipose tissue: A comparison with bone marrow-derived stromal stem cells cultured in xeno-free conditions. Stem Cells Transl. Med. 2017, 6, 2160–2172. [Google Scholar] [CrossRef]
- Boutet, M.A.; Bart, G.; Penhoat, M.; Amiaud, J.; Brulin, B.; Charrier, C.; Morel, F.; Lecron, J.C.; Rolli-Derkinderen, M.; Bourreille, A.; et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 2016, 184, 159–173. [Google Scholar] [CrossRef]
- Salvetti, A.; Couté, Y.; Epstein, A.; Arata, L.; Kraut, A.; Navratil, V.; Bouvet, P.; Greco, A. Nuclear functions of nucleolin through global proteomics and interactomic approaches. J. Proteome Res. 2016, 15, 1659–1669. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Hashimoto, S.; Ochs, R.L.; Rosen, F.; Quach, J.; McCabe, G.; Solan, J.; Seegmiller, J.E.; Terkeltaub, R.; Lotz, M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc. Natl. Acad. Sci. USA 1998, 95, 3094–3099. [Google Scholar] [CrossRef]
- Monderer, D.; Luseau, A.; Bellec, A.; David, E.; Ponsolle, S.; Saiagh, S.; Bercegeay, S.; Piloquet, P.; Denis, M.G.; Lodé, L.; et al. New chondrosarcoma cell lines and mouse models to study the link between chondrogenesis and chemoresistance. Lab. Investig. 2013, 93, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Little, D.; Thompson, J.W.; Dubois, L.G.; Ruch, D.S.; Moseley, M.A.; Guilak, F. Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS ONE 2014, 9, e96526. [Google Scholar] [CrossRef] [PubMed]
- Kharaz, Y.A.; Tew, S.R.; Peffers, M.; Canty-Laird, E.G.; Comerford, E. Proteomic differences between native and tissue-engineered tendon and ligament. Proteomics 2016, 16, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Ashraf Kharaz, Y.; Zamboulis, D.; Sanders, K.; Comerford, E.; Clegg, P.; Peffers, M. Comparison between chaotropic and detergent-based sample preparation workflow in tendon for mass spectrometry analysis. Proteomics 2017, 17, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P. Human Bone Paleoproteomics utilizing the single-pot, solid-phase-enhanced sample preparation method to maximize detected proteins and reduce humics. J. Proteome Res. 2018, 17, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Kuntz, L.A.; Kunold, E.; Schock, J.; Müller, K.W.; Grabmayr, H.; Stolberg-Stolberg, J.; Pfeiffer, F.; Sieber, S.A.; Burgkart, R.; et al. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017, 16, 664–670. [Google Scholar] [CrossRef]
- Mason, K.E.; Anex, D.; Grey, T.; Hart, B.; Parker, G. Protein-based forensic identification using genetically variant peptides in human bone. Forensic Sci. Int. 2018, 288, 89–96. [Google Scholar] [CrossRef]
- Cleland, T.P. Solid digestion of demineralized bone as a method to access potentially insoluble proteins and post-translational modifications. J. Proteome Res. 2018, 17, 536–542. [Google Scholar] [CrossRef]
- Önnerfjord, P.; Khabut, A.; Reinholt, F.P.; Svensson, O.; Heinegård, D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J. Biol. Chem. 2012, 287, 18913–18924. [Google Scholar] [CrossRef]
- Folkesson, E.; Turkiewicz, A.; Englund, M.; Önnerfjord, P. Differential protein expression in human knee articular cartilage and medial meniscus using two different proteomic methods: A pilot analysis. BMC Musculoskelet. Disord. 2018, 19, 416. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, N.; Tombran-Tink, J.; Niyibizi, C. Pigment epithelium-derived factor enhances differentiation and mineral deposition of human mesenchymal stem cells. Stem Cells 2013, 31, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Ridolo, E.; Fassio, A.; Gatti, D.; Montagni, M.; Caminati, M.; Martignago, I.; Incorvaia, C.; Senna, G. Periostin: The bone and beyond. Eur. J. Intern. Med. 2017, 38, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Loehr, J.W. Calcific Tendinopathy of the Rotator Cuff: Pathogenesis, Diagnosis, and Management. J. Am. Acad. Orthop. Surg. 1997, 5, 183–191. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J.R. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004, 233, 1–45. [Google Scholar] [PubMed]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; von der Mark, H.; et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 2010, 137, 901–911. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Hartwig, J.; Schmidmaier, G.; Wildemann, B. Characteristics and stimulation potential with BMP-2 and BMP-7 of tenocyte-like cells isolated from the rotator cuff of female donors. PLoS ONE 2013, 8, e67209. [Google Scholar] [CrossRef]
- Wu, C.W.; Tchetina, E.V.; Mwale, F.; Hasty, K.; Pidoux, I.; Reiner, A.; Chen, J.; Van Wart, H.E.; Poole, A.R. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J. Bone Miner. Res. 2002, 17, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Ruschke, K.; Meier, C.; Ullah, M.; Krebs, A.C.; Silberreis, K.; Kohl, B.; Knaus, P.; Jagielski, M.; Arens, S.; Schulze-Tanzil, G. Bone morphogenetic protein 2/SMAD signalling in human ligamentocytes of degenerated and aged anterior cruciate ligaments. Osteoarthr. Cartil. 2016, 24, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Edwards, R.W.; O’Hara, B.J.; Maltenfort, M.G.; Parks, S.M.; Steplewski, A.; Osterman, A.L.; Shapiro, I.M. Tendinosis develops from age- and oxygen tension-dependent modulation of Rac1 activity. Aging Cell 2019, 18, e12934. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sheen, C.R.; Kuss, P.; Narisawa, S.; Yadav, M.C.; Nigro, J.; Wang, W.; Chhea, T.N.; Sergienko, E.A.; Kapoor, K.; Jackson, M.R.; et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J. Bone Miner. Res. 2015, 30, 824–836. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darrieutort-Laffite, C.; Arnolfo, P.; Garraud, T.; Adrait, A.; Couté, Y.; Louarn, G.; Trichet, V.; Layrolle, P.; Le Goff, B.; Blanchard, F. Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner. J. Clin. Med. 2019, 8, 1544. https://doi.org/10.3390/jcm8101544
Darrieutort-Laffite C, Arnolfo P, Garraud T, Adrait A, Couté Y, Louarn G, Trichet V, Layrolle P, Le Goff B, Blanchard F. Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner. Journal of Clinical Medicine. 2019; 8(10):1544. https://doi.org/10.3390/jcm8101544
Chicago/Turabian StyleDarrieutort-Laffite, Christelle, Paul Arnolfo, Thomas Garraud, Annie Adrait, Yohann Couté, Guy Louarn, Valérie Trichet, Pierre Layrolle, Benoit Le Goff, and Frédéric Blanchard. 2019. "Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner" Journal of Clinical Medicine 8, no. 10: 1544. https://doi.org/10.3390/jcm8101544
APA StyleDarrieutort-Laffite, C., Arnolfo, P., Garraud, T., Adrait, A., Couté, Y., Louarn, G., Trichet, V., Layrolle, P., Le Goff, B., & Blanchard, F. (2019). Rotator Cuff Tenocytes Differentiate into Hypertrophic Chondrocyte-Like Cells to Produce Calcium Deposits in an Alkaline Phosphatase-Dependent Manner. Journal of Clinical Medicine, 8(10), 1544. https://doi.org/10.3390/jcm8101544





