A Systematic Review on Materno-Foetal Outcomes in Pregnant Women with IgA Nephropathy: A Case of “Late-Maternal” Preeclampsia?
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
3. Results
3.1. Case Series: Overall Data
3.2. Case Series: Pregnancy Outcomes
3.3. Case Reports
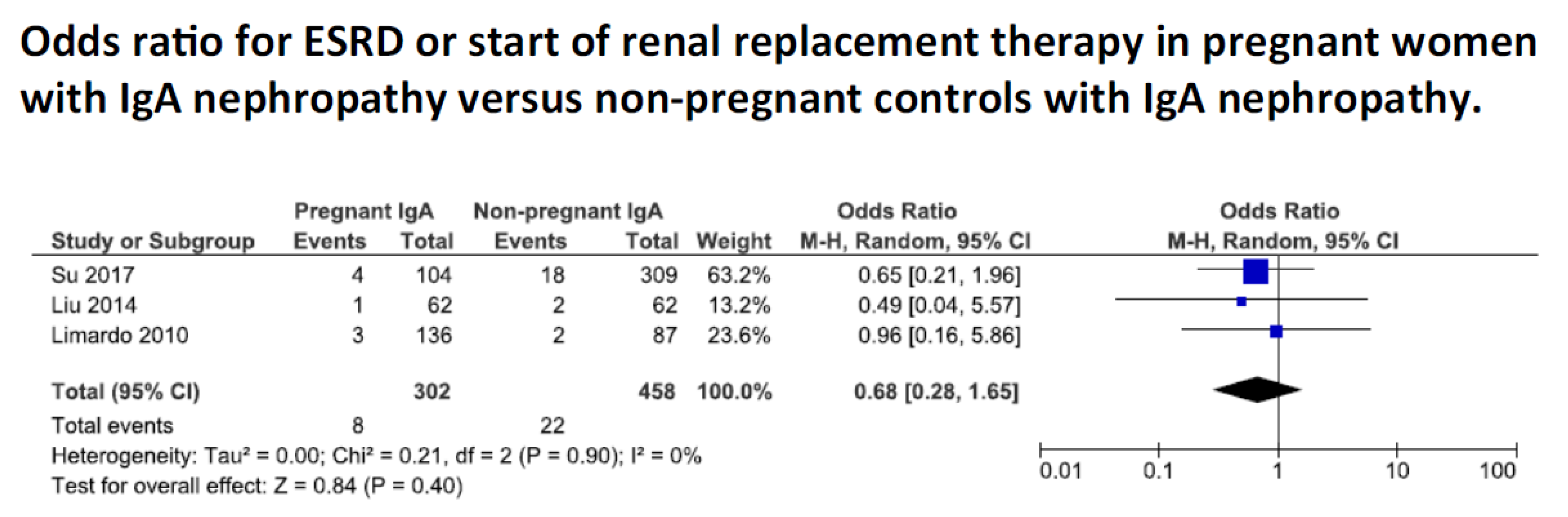
3.4. Meta-Analysis
Kidney Function
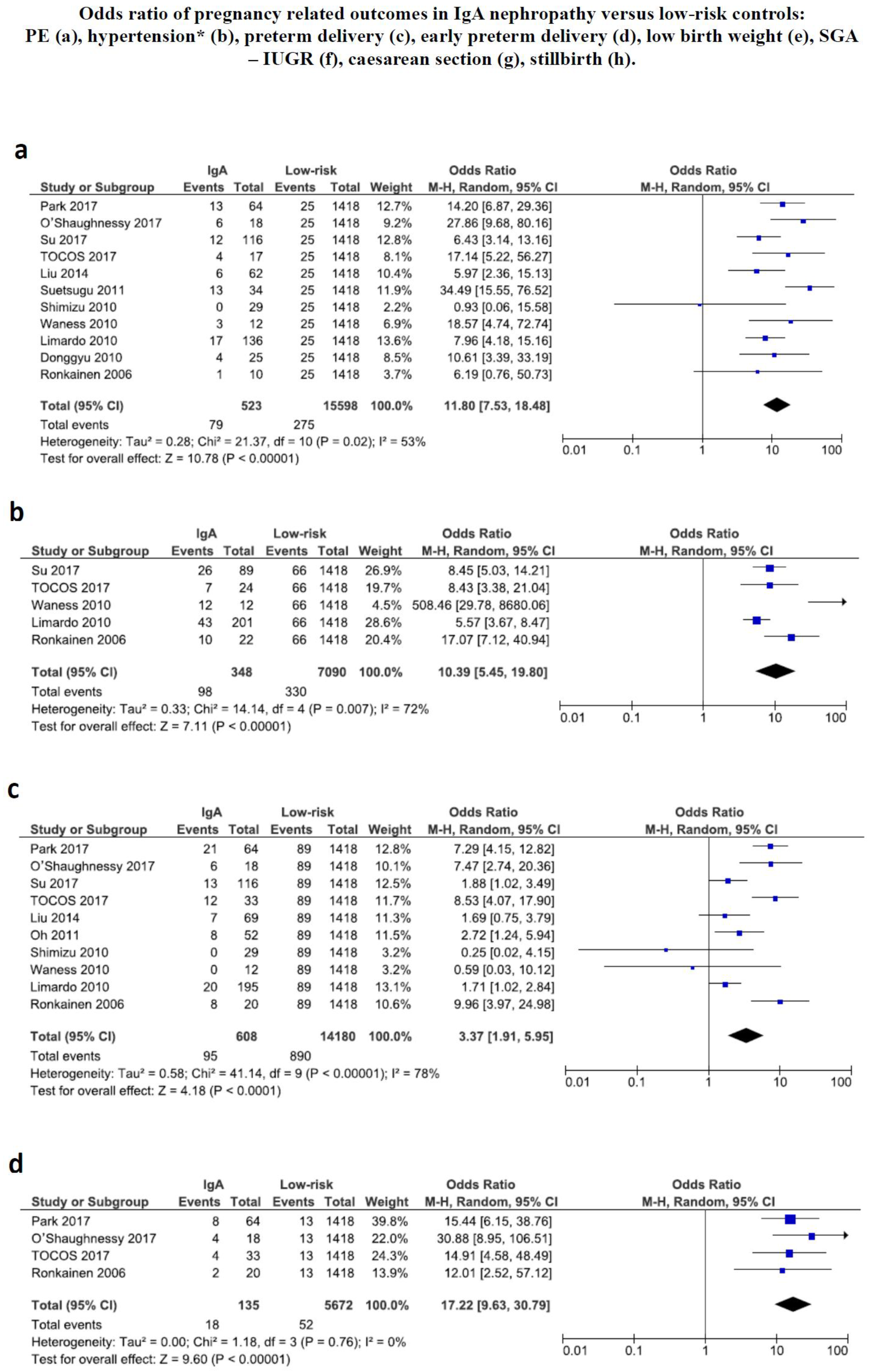
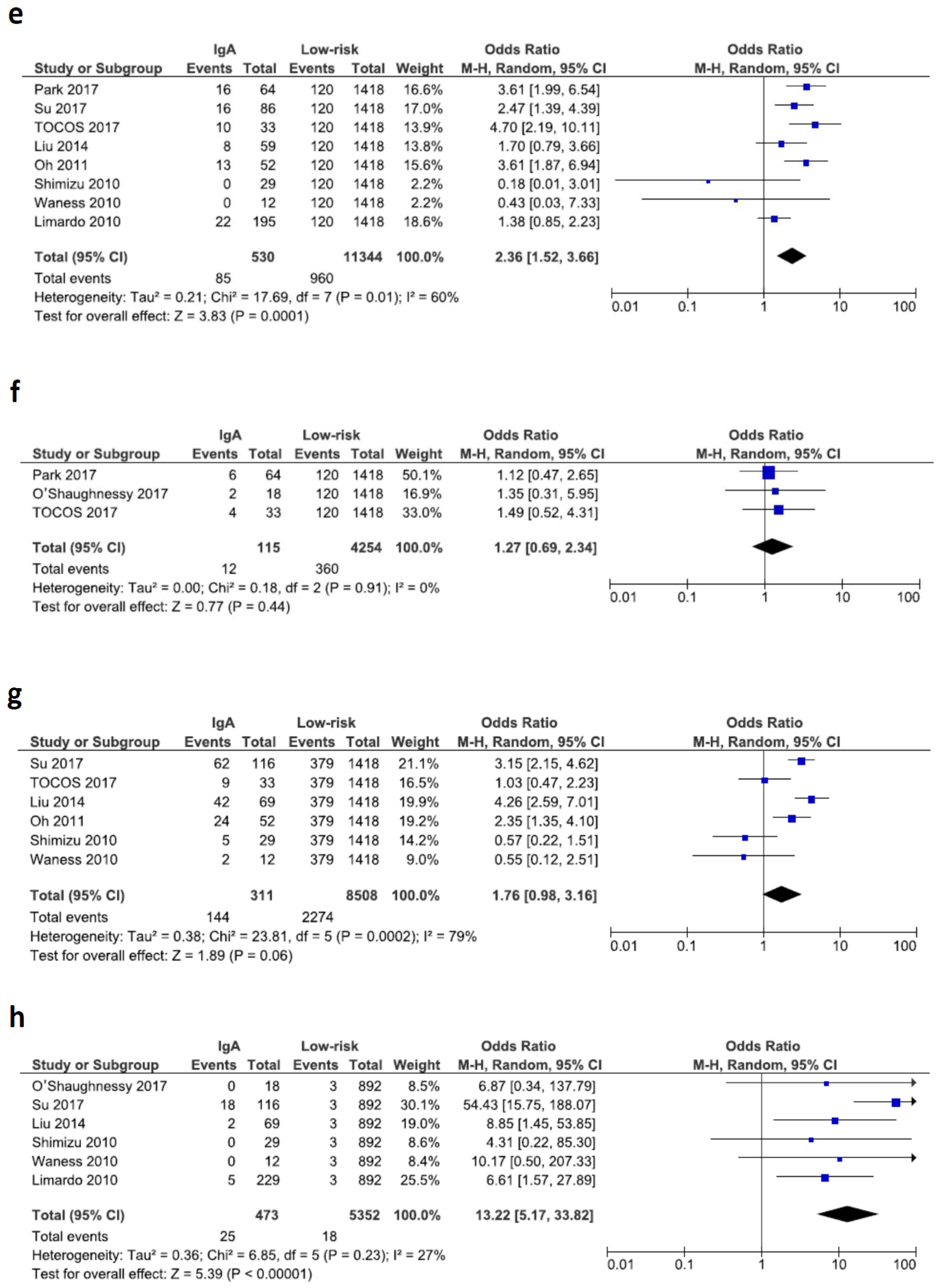
3.5. Pregnancy Related Outcomes
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Floege, J.; Amann, K. Primary glomerulonephritides. Lancet 2016, 387, 2036–2048. [Google Scholar] [CrossRef]
- Kurokawa, M.; Hisano, S.; Ueda, K. Berger disease/Henoch-Schönlein syndrome. J. Pediatr. 1985, 107, 648–649. [Google Scholar] [CrossRef]
- Novak, J.; Renfrow, M.B.; Gharavi, A.G.; Julian, B.A. Pathogenesis of immunoglobulin A nephropathy. Curr. Opin. Nephrol. Hypertens. 2013, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Feehally, J.; Barratt, J. The genetics of IgA nephropathy: An overview from Western countries. Kidney Dis. 2015, 1, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, H. The genetics of IgA nephropathy: An overview from China. Kidney Dis. 2015, 1, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F. Familial IgA nephropathy. J. Nephrol. 1999, 12, 213–219. [Google Scholar] [PubMed]
- Lee, H.; Hwang, J.H.; Paik, J.H.; Ryu, H.J.; Kim, D.K.; Chin, H.J.; Oh, Y.K.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol. 2014, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Tanaka, K.; Iwasaki, C.; Oshima, Y.; Ochi, A.; Kataoka, H.; Itabashi, M.; Takei, T.; Uchida, K.; Nitta, K.; et al. Prognosis in IgA nephropathy: 30-year analysis of 1012 patients at a single center in Japan. PLoS ONE 2014, 9, e91756. [Google Scholar] [CrossRef] [PubMed]
- Tomino, Y. Immunopathological predictors of prognosis in IgA nephropathy. Contrib. Nephrol. 2013, 181, 65–74. [Google Scholar] [PubMed]
- Kawamura, T.; Joh, K.; Okonogi, H.; Koike, K.; Utsunomiya, Y.; Miyazaki, Y.; Matsushima, M.; Yoshimura, M.; Horikoshi, S.; Suzuki, Y.; et al. Study Group Special IgA Nephropathy. A histologic classification of IgA nephropathy for predicting long-term prognosis: Emphasis on end-stage renal disease. J. Nephrol. 2013, 26, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Reily, C.; Bian, Q.; Neprasova, M.; Novak, J.; Tesar, V. Markers for the progression of IgA nephropathy. J. Nephrol. 2016, 29, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.N.; Troyanov, S.; Scholey, J.W.; Cattran, D.C. Toronto Glomerulonephritis Registry. Remission of week improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Usui, J.; Yamagata, K.; Kai, H.; Outeki, T.; Yamamoto, S.; Muro, K.; Hirayama, A.; Yoh, K.; Tomida, C.; Hirayama, K.; et al. Heterogeneity of prognosis in adult IgA nephropathy, especially with mild week or mild histological features. Intern. Med. 2001, 40, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Lofaro, D.; Camilla, R.R.; Bellur, S.; Cattran, D.; Cook, H.T.; Roberts, I.S.; Peruzzi, L.; Amore, A.; Emma, F.; et al. Risk factors for progression in children and young adults with IgA nephropathy: An analysis of 261 cases from the VALIGA European cohort. Pediatr. Nephrol. 2017, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pesce, F.; Diciolla, M.; Binetti, G.; Naso, D.; Ostuni, V.C.; Di Noia, T.; Vågane, A.M.; Bjørneklett, R.; Suzuki, H.; Tomino, Y.; et al. Clinical decision support system for end-stage kidney disease risk estimation in IgA nephropathy patients. Nephrol. Dial. Transplant. 2016, 31, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sarcina, C.; Tinelli, C.; Ferrario, F.; Pani, A.; De Silvestri, A.; Scaini, P.; Del Vecchio, L.; Alberghini, E.; Buzzi, L.; Baragetti, I.; et al. Changes in week and side effects of corticosteroids alone or in combination with azathioprine at different stages of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R. Is a legacy effect possible in IgA nephropathy? Nephrol. Dial. Transplant. 2013, 28, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Maxia, S.; Lepori, N.; Tuveri, M.; Massidda, M.; Marchi, C.; Mura, S.; et al. Risk of adverse pregnancy outcomes in women with CKD. J. Am. Soc. Nephrol. 2015, 26, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Lightstone, L. Pre-pregnancy counseling for women with chronic kidney disease. J. Nephrol. 2012, 25, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Brown, M.A.; Winkelmayer, W.C.; Craig, J.C.; Jesudason, S. Perspectives on pregnancy in women with CKD: A semistructured interview Study. Am. J. Kidney Dis. 2015, 66, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.; Fassio, F.; Rolfo, A.; Giuffrida, D.; Pani, A.; Gaglioti, P.; Todros, T.; et al. Pregnancy in chronic kidney disease: Questions and answers in a changing panorama. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Zheng, J.; Liu, X.; Yan, T. A systematic review and meta-analysis of kidney and pregnancy outcomes in IgA nephropathy. Am. J. Nephrol. 2016, 44, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Atai, E.; Chan, M.; Phoon, R.K.; Scott, C.; Toussaint, N.D.; Turner, G.L.; Usherwood, T.; Wiggins, K.J.; KHA-CARI. KHA-CARI guideline: Early chronic kidney disease: Detection, prevention and management. Nephrology 2013, 18, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Moroni, G.; Giannattasio, M.; Gregorini, G.; Giacchino, F.; Attini, R.; Loi, V.; et al. A best practice position statement on pregnancy in chronic kidney disease: The Italian Study Group on Kidney and Pregnancy. J. Nephrol. 2016, 29, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Odutayo, A.; Bramham, K.; Hladunewich, M.A. Pregnancy and glomerular disease: A systematic review of the literature with management guidelines. Clin. J. Am. Soc. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoo, K.D.; Park, J.S.; Hong, J.S.; Baek, S.; Park, S.K.; Chin, H.J.; Na, K.Y.; Choi, Y.; Kim, D.K.; et al. Pregnancy in women with immunoglobulin A nephropathy: Are obstetrical complications associated with renal prognosis? Nephrol. Dial. Transplant. 2017, 33, 459–465. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.M.; Jobson, M.A.; Sims, K.; Liberty, A.L.; Nachman, P.H.; Pendergraft, W.F. pregnancy outcomes in patients with glomerular disease attending a single Academic Center in North Carolina. Am. J. Nephrol. 2017, 45, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lv, J.; Liu, Y.; Wang, J.; Ma, X.; Shi, S.; Liu, L.; Zhang, H. Pregnancy and kidney outcomes in patients with IgA nephropathy: A cohort study. Am. J. Kidney Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Attini, R.; Cabiddu, G.; Kooij, I.; Fassio, F.; Gerbino, M.; Maxia, S.; Biolcati, M.; Versino, E.; Todros, T.; et al. Maternal-foetal outcomes in pregnant women with glomerulonephritides. Are all glomerulonephritides alike in pregnancy? J. Autoimmun. 2017, 79, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Lv, J.; Shi, S.; Liu, L.; Chen, Y.; Zhang, H. Risk factors for pregnancy outcomes in patients with IgA nephropathy: A matched cohort study. Am. J. Kidney Dis. 2014, 64, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Han, S.H.; Yoo, D.E.; Kim, S.J.; Park, J.T.; Kim, J.K.; Yoo, T.H.; Kang, S.W.; Choi, K.H. Reduced pre-pregnancy week is associated with improving postnatal maternal renal outcomes in IgA nephropathy women. Clin. Nephrol. 2011, 76, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, Y.; Tokudome, G.; Sugano, N.; Yoshizawa, T.; Endo, S.; Hara, Y.; Takane, K.; Kuriyama, S.; Hosoya, T. Study on the predictors for superimposed preeclampsia in patients with IgA nephropathy. Nihon Jinzo Gakkai Shi 2011, 53, 1139–1149. [Google Scholar] [PubMed]
- Shimizu, A.; Takei, T.; Moriyama, T.; Itabashi, M.; Uchida, K.; Nitta, K. Effect of kidney disease stage on pregnancy and delivery outcomes among patients with immunoglobulin A nephropathy. Am. J. Nephrol. 2010, 32, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Waness, A.; Al Sayyari, A.; Salih, S.B.; Al Shohaib, S. Increased risk of hypertension, week and preeclampsia in pregnant Saudi females with IgA nephropathy. Hypertens. Pregnancy 2010, 29, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Limardo, M.; Imbasciati, E.; Ravani, P.; Surian, M.; Torres, D.; Gregorini, G.; Magistroni, R.; Casellato, D.; Gammaro, L.; Pozzi, C.; et al. Pregnancy and progression of IgA nephropathy: Results of an Italian multicenter study. Am. J. Kidney Dis. 2010, 56, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Donggyu, J.; Jisun, W.; Sekyoung, C.; Jaeun, S.; Inyang, P.; Jongchul, S. IgA nephropathy in pregnancy. J. Matern.-Fetal Neonatal Med. 2010, 23 (Suppl. 1), 205. [Google Scholar]
- Ronkainen, J.; Ala-Houhala, M.; Autio-Harmainen, H.; Jahnukainen, T.; Koskimies, O.; Merenmies, J.; Mustonen, J.; Örmälä, T.; Turtinen, J.; Nuutinen, M.; et al. Long-term outcome 19 years after childhood IgA nephritis: A retrospective cohort study. Pediatr. Nephrol. 2006, 21, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Nuutinen, M.; Koskimies, O. The adult kidney 24 years after childhood Henoch-Schonlein purpura: A retrospective cohort study. Lancet 2002, 360, 666–670. [Google Scholar] [CrossRef]
- Kaul, A.; Pradhan, M.; Bhaduaria, D.; Prasad, N.; Gupta, A.; Sharma, R. Nephrotic syndrome in pregnancy maternal and foetal outcome. Nephrology 2016, 21 (Suppl. 2), 237–238. [Google Scholar]
- Lim, D.; Smith, M.P. Lost to follow up. J. Gen. Intern. Med. 2016, 31 (Suppl. 1), S665–S666. [Google Scholar]
- Sun, L.X.; Ye, W.L.; Wen, Y.B.; Li, X.M. Postpartum atypical hemolytic uremic syndrome: An unusual and severe complication associated with IgA nephropathy. Chin. Med. Sci. J. 2015, 30, 189–192. [Google Scholar] [CrossRef]
- Nagai, K.; Kishi, J.; Morizumi, S.; Minakuchi, J.; Bando, Y.; Nishioka, Y.; Doi, T. Henoch-Schonlein purpura nephritis occurring postpartum in a patient with anti-PL-7 anti-synthetase syndrome. Mod. Rheumatol. 2015, 27, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Bastacky, S. IGA nephropathy presenting during pregnancy. Am. J. Kidney Dis. 2015, 65, A54. [Google Scholar]
- Zand, L.; Williams, A.; Babovic-Vuksanovic, D.; Nwoko, R.; Cornell, L.; Garovic, V. The Case|Renal dysfunction in a pregnant patient with IgA nephropathy. Kidney Int. 2014, 85, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, T.; Spaanderman, M.; Beerenhout, C.; Perschel, F.H.; Verlohren, S.; Schalkwijk, C.G.; van der Sande, F.M.; Kooman, J.P.; Hladunewich, M. Antiangiogenic factors and maternal hemodynamics during intensive hemodialysis in pregnancy. Hemodial. Int. 2013, 17, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Hou, S. A woman with GN presenting during pregnancy. Clin. J. Am. Soc. Nephrol. 2013, 8, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Giofre, F.; Pugliese, C.; Alati, G.; Messina, A.; Tramontana, D. Three successive pregnancies in a patient with chronic renal disease progressing from chronic renal dysfunction through to institution of dialysis during pregnancy and then on to maintenance dialysis. Nephrol. Dial. Transplant. 2007, 22, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Tanno, Y.; Yamamoto, H.; Yamamoto, I.; Yaginuma, T.; Mitome, J.; Kawamura, Y.; Miyazaki, Y.; Yokoyama, K.; Utsunomiya, Y.; Yamaguchi, Y.; et al. Recurrence of Henoch-Schönlein purpura nephritis superimposed on severe pre-eclampsia in a kidney transplant patient. Clin. Transplant. 2007, 21 (Suppl. 18), 36–39. [Google Scholar] [CrossRef]
- Barquero-Romero, J.; Chaves-Alvarez, A.J.; Catalina-Fernandez, I.; Lopez-Cortezon, C. Schonlein-Henoch purpure in a pregnant patient. Med. Clin. 2006, 127, 276–277. [Google Scholar] [CrossRef]
- Koizumi, M.; Hagino, D.; Fukuyama, C.; Abe, K.; Inoue, K.; Arai, Y.; Takechi, K. Schonlein-Henoch purpura during pregnancy: Case report and review of the literature. J. Obstet. Gynaecol. Res. 2004, 30, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Cusi, D.; Taglietti, M.V.; Liccardo, A. Pregnancy during nephropathy. Giornale Italiano di Nefrologia 2003, 20, 516–524. [Google Scholar] [PubMed]
- Amir, A.R.; Sheikh, S.S. ANCA-associated crescentic IgA glomerulonephritis in pregnancy. J. Nephrol. 2002, 15, 716–719. [Google Scholar] [PubMed]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Lisonkova, S.; Sabr, Y.; Mayer, C.; Young, C.; Skoll, A.; Joseph, K.S. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet. Gynecol. 2014, 124, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cheng, W. Comparison of indications of pregnancy termination and prognosis of mothers and neonates in early- and late-onset preeclampsia. Hypertens. Pregnancy 2016, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in pathogenesis, definitions, and guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Moroni, G.; Spotti, D.; Giacchino, F.; Attini, R.; Limardo, M.; et al. A best practice position statement on the role of the nephrologist in the prevention and follow-up of preeclampsia: The Italian study group on kidney and pregnancy. J. Nephrol. 2017, 30, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Nobumoto, E.; Okimoto, N.; Inoue, S.; Segawa, T.; Hiramatsu, Y. Superimposed preeclampsia in women with chronic kidney disease. Gynecol. Obstet. Investig. 2012, 74, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Nevis, I.F.; Reitsma, A.; Dominic, A.; McDonald, S.; Thabane, L.; Akl, E.A.; Hladunewich, M.; Akbari, A.; Joseph, G.; Sia, W.; et al. Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin. J. Am. Soc. Nephrol. 2011, 6, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Conijn, A.; Attini, R.; Biolcati, M.; Bossotti, C.; Consiglio, V.; Deagostini, M.C.; Todros, T. Pregnancy in chronic kidney disease: Need for a common language. J. Nephrol. 2011, 24, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ma, X.X.; Hao, L.; Liu, L.J.; Lv, J.C.; Zhang, H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin. J. Am. Soc. Nephrol. 2015, 10, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.A.; James, N.T.; Kucirka, L.M.; Boyarsky, B.J.; Garonzik-Wang, J.M.; Montgomery, R.A.; Segev, D.L. Pregnancy outcomes in kidney transplant recipients: A systematic review and meta-analysis. Am. J. Transplant. 2011, 11, 2388–2404. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Attini, R.; Nuzzo, A.M.; Piazzese, A.; Parisi, S.; Ferraresi, M.; Todros, T.; Piccoli, G.B. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013, 83, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Gaglioti, P.; Attini, R.; Parisi, S.; Bossotti, C.; Olearo, E.; Oberto, M.; Ferraresi, M.; Rolfo, A.; Versino, E.; et al. Pre-eclampsia or chronic kidney disease? The flow hypothesis. Nephrol. Dial. Transplant. 2013, 28, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Perni, U.; Sison, C.; Sharma, V.; Helseth, G.; Hawfield, A.; Suthanthiran, M.; August, P. Angiogenic factors in superimposed preeclampsia: A longitudinal study of women with chronic hypertension during pregnancy. Hypertension 2012, 59, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Suwaki, N.; Nakatsukasa, H.; Masumoto, A.; Tateishi, Y.; Hiramatrsu, Y. Circulating angiogenic factors in preeclampsia, gestational week , and preeclampsia superimposed on chronic glomerulonephritis. Am. J. Obstet. Gynecol. 2006, 194, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Seed, P.T.; Lightstone, L.; Nelson-Piercy, C.; Gill, C.; Webster, P.; Poston, L.; Chappell, L.C. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int. 2016, 89, 874–885. [Google Scholar] [CrossRef] [PubMed]
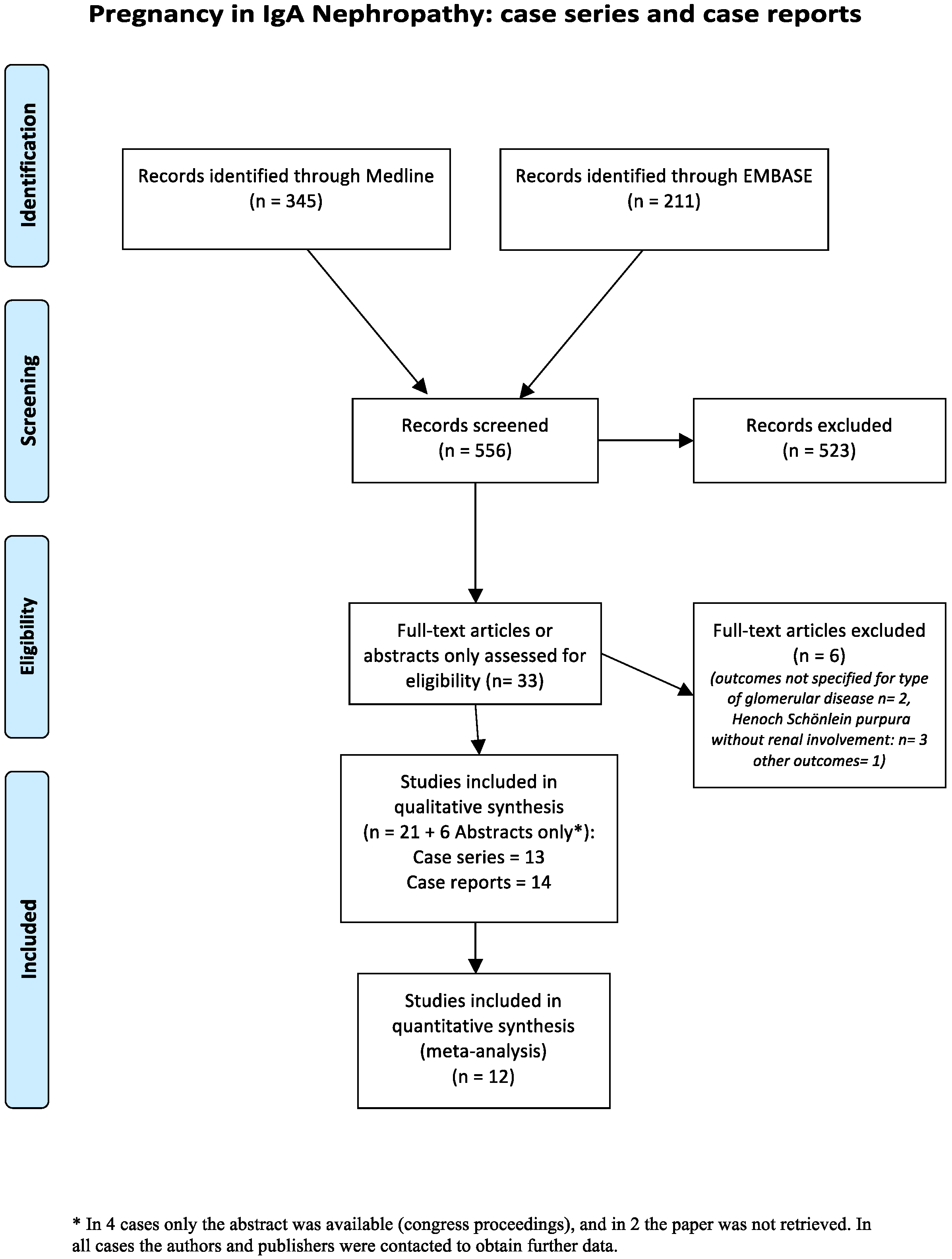
| Author Year [Ref] | Years | Country | Study | Objective, as Stated in the Study | Pregnancies (P) §§ Women (W) |
|---|---|---|---|---|---|
| Park 2017 [27] | 1979–2015 | Korea | Ret | To assess the relationship between pregnancy and renal prognosis in women with IgAN and to investigate further whether obstetric complications are associated with renal prognosis | 59 W 64 P 59 W controls (non-pregnant IgA) |
| O’Shaughnessy 2017 [28] | 1996–2015 | USA | Ret | To investigate the influence of glomerular disease subtype on pregnancy outcomes | 17 W 18 P |
| Su 2017 [29] | 2003–2014 | China | Pro | To assess the effects of pregnancy on kidney disease progression and risk factors for adverse pregnancy outcomes in patients with IgAN | 104 W 116 P 309 W controls (non-pregnant IgA) |
| Tocos 2017 [30] | 2000–2016 | Italy | Pro | To evaluate the maternofetal outcomes in different glomerulonephritis | 27 W § 33 P 1418 P controls (low risk) |
| Liu 2014 [31] | 2003–2012 | China | Matched-cohort | To evaluate the safety of pregnancy in women with IgAN, as well as their risk factors for adverse pregnancy outcomes, as compared to non-pregnant women with IgAN | 62 W 69 P 62 W controls (non-pregnant IgA) |
| Oh 2011 [32] | 2004–2009 | Korea | Ret | To investigate whether higher week at conception predicts a faster decline in maternal renal outcomes and to identify whether a week reduction prior to pregnancy attenuates the deterioration of postnatal maternal outcomes | 52 W |
| Suetsugu 2011 (**) [33] | NR | Japan | Ret | To explore the clinical characteristics of predictive factors for hypertension in biopsy-proven IgA nephropathy patients with superimposed preeclampsia | 34 W |
| Shimizu 2010 [34] | 1995–2006 | Japan | Pro | To evaluate the impact of the CKD staging in patients with IgAN on pregnancy and delivery | 29 W 29 P 45 W controls (non-pregnant IgA) |
| Waness 2010 [35] | 2000–2006 | Saudi Arabia | Pro | To examine the natural history of pregnancies and their impact on renal function in Saudi females affected by IgAN | 12 W 12 P |
| Limardo 2010 [36] | 1974–2003 | Italy | Ret multicenter cohort | To compare the long-term outcome of kidney disease in women with IgAN and preserved kidney function (sCr <1.2 mg/dL) who did and did not become pregnant. Data on 10 pregnant and 12 non pregnant women with sCr >1.2 mg/dL also gathered | 136 W 229 P 87 W controls (non-pregnant IgA) |
| Donggyu 2010 (*) [37] | 1987–2008 | Korea | Ret | To clarify the influence of pregnancy on the natural course of IgAN | 25 W 28 P |
| Ronkainen 2006 [38] | NR | Finland | Ret | To evaluate renal survival, morbidity, pregnancy complications and factors predicting outcome after childhood IgAN | 10 W 22 P |
| Ronkainen 2002 [39] | NR | Finland | Ret | To assess long-term outcome of children with renal involvement at onset of Henoch-Schönlein purpura by comparison with those who have mainly extra-renal symptoms at referral | 14 W 23 P |
| Overall number of women, pregnancies and controls | 581 W 729 P § 562 non pregnant IgA controls 1418 low risk controls | ||||
| Author Year [Ref] | Control Policies |
|---|---|
| Su 2017 [29] | Follow up at least once a month before delivery, and every 1–6 months after delivery, with minimum follow up 12 months postpartum or until dialysis treatment |
| Tocos 2017 [30] | Follow up at least once monthly if week , hypertension or kidney function reduction |
| Liu 2014 [31] | Follow up every ≤1 month; eGFR decline; determination time-averaged MAP and week every 3 months |
| Shimizu 2010 [34] | BP, week , blood analysis and eGFR at the baseline at the time of detection of pregnancy; at 16, 22 and 30 weeks of pregnancy; at the time of delivery; and at 3 months and 1, 2 and 3 years after delivery |
| Waness 2010 [35] | Monthly measures of BP, 24 h week , sCr, CCr; close monitoring and follow up |
| Limardo 2010 [36] | Information gathered at time of biopsy and every 5 year thereafter: CCr, 24 h week , body weight, BP, therapy with ACEIs/ARBs or immunosuppressants |
| Donggyu 2010 (*) [37] | sCr followed up max 3 years after delivery |
| Author Year [Ref] | All Cases Considered | Live Births Only | All Cases | All Cases, or As Stated | |||||
|---|---|---|---|---|---|---|---|---|---|
| P | Abort. Spont. Induced | Still Birth | Live Birth | Neo. Death | Preterm <37 weeks Early <34 weeks Extreme <28 weeks | NICU | PE-HT | Other | |
| Park 2017 [27] | 64 | NR | NR | NR | NR | Preterm: 21 (33%) Early: 8 (13%) | NR | PE: 13 (20%) | LBW <2500 g: 16 (25%) LBW <1500 g: 6 (9%) SGA <10th: 6 (9%) |
| O’Shaughnessy 2017 [28] | 18 | NA | 0 | 18 (100%) | 2 (11%) | Preterm <37 w: 6 (33%), 5/6 induced/CS on maternal indication Preterm <32 w: 4 (22%) | NR | PE: 6 (33%) | Median GA: 37.5 week (36–39) Median BW: 2627 g (2136–3315) IUGR <10th: 2 (11%) IUGR<3rd: 0 Apgar 1 min: 8 (7–9) Apgar 5 min: 9 (9–9) |
| Su 2017 [29] | 116 | 5 (4%) spont 2 (2%) induc | 18 (16%) | 90 (78%) | 1 (1%) | Preterm: 13 (11%) | NR | GHT: 26/89 (29%) Severe PE: 12 (10%) PtU >3.5 g/day: 19/110 (17%) | CS: 62 (53%) Mean GA: 37.8 week ± 2.4 Mean BW: 3035 g ± 668 † LBW <2500 g: 16 (17%) † LBW <1500 g: 3 (3%) † |
| Tocos 2017 [30] | 33 cases | NA | NA | 33 (100%) | NR | Preterm: 12 (36%) Early: 4 (12%) †† Extreme: 1 (3%) †† | NR | GHT: 7/24 (29%) ††† PE: 4/17 (24%) ††† | CS: 9 (27%) LBW: 10 (30%) SGA <10th: 4 (12%) †† SGA <5th: 1 (3%) †† |
| 1418 controls | NA | NA | 1418 (100%) | NR | Preterm: 89 (6%) Early: 13 (1%) Extreme: 2 (0.1%) | NR | HT: 66 (5%) PE: 25 (2%) PtU: 25 (2%) | CS: 379 (27%) GA: 39 weeks (25–42) BW: 3232 ± 476 g SGA <10th: 120 (8%) SGA <5th: 45 (3%) | |
| Liu 2014 [31] | 69 | 8 (12%) § | 2 (3%) | 59 (86%) | NR | Preterm: 7 (10%) | NR | Severe PE: 6 W (10%) | CS: 42 (61%) LBW: 8/59 (14%) Mean BW: 2972 ± 654 g |
| Oh 2011 [32] | 52 | §§ | NR | NR | NR | Preterm: 8 (15.4%) | 4 (7.7%) | HT: <8 weeks 31 (60%) | CS: 24 (46.2%) LBW: 13 (25%) |
| Suetsugu 2011 (**) [33] | 34 | NR | NR | NR | 1 (3%) | NR | NR | Superimp. PE: 13 (38.2%) | BW negatively correlated with glomerular sclerosis, sCr and BUN. |
| Shimizu 2010 [34] | 29 | 0 | 0 | 29 (100%) | 0 | 0 Gestation 38.0 ± 2 weeks | NR | No PE | CS: 5 (17.2%) BW: 2911.2 ± 138.7 g LBW: 0 |
| Waness 2010 [35] | 12 | 0 | 0 | 12 (100%) | NR | 0 | NR | HT: 12 (100%) PE: 3 (25%) HELLP: 1 (8.3%) | CS: 2 (HELLP and PE) BW: 3.1 kg LBW: 0 Apgar: normal (1’ and 5’) |
| Limardo 2010 [36] | 229 | 15 spon 13 indu | 5 (2.2%) | 195 (85%) | 1 (0.4%) | Preterm: 20 (10%) | NR | HT: 43/201 (21%) PE: 17 W (13%) | Mean BW: 3039 ± 610 g LBW: 22/195 (11%) |
| Donggyu 2010 (*) [37] | 28 | NR | NR | NR | NR | NR | NR | PE: 4 W (of 5 with sCr >2.0 mg/dL) | NR |
| Ronkainen 2006 [38] | 22 | At least 2 spont | NR | 20 | NR | Preterm: at least 6 (30%) Extreme: at least 2 (10%) | NR | HT: 10 (46%) Severe PE: 1 W (10%) PtU: 12 (55%) | 6 (30%) of 20 live born infants from mothers with HT and/or week premature |
| Ronkainen 2002 [39] | 23 | NR | NR | NR | NR | NR | NR | HT and/or PtU: 16 (70%) | NR |
| Summary data | 729 | 45/485 (9.3%) | 25/473 (5.3%) | 456/528 (86.3%) | 5/426 (1.2%) | Preterm 95/608 (15.6%) Early: 18/135 (13.3%) Extreme: 3/53 (5.6%) | 4/52 (7.7%) | PIH: 98/348 (28.2%) PE: 79/523 (15.1%) | CS: 144/311 (46.3%) LBW: 85/530 (16.0%) IUGR/SGA <10th: 12/115 (10.4%) |
| Author Year [Ref] | Age (years) | Kidney Function at Baseline | Other Maternal Outcomes and Main Results |
|---|---|---|---|
| Park 2017 [27] | 28 (24–31) (cases) | eGFR: 80.0 (61.0–105.6) sCr: 0.90 mg/dL (0.70–1.00) PtU: 1.09 g/day (0.46–2.02) HT: 36 (61%) | Renal survival rate with gestational complications: 55.3% at 10 y; 46.1% at 20 years Renal survival rate without gestational complications: 97.3% at 10 y; 97.3% at 20 years Obstetric complications (PE, LBW and/or preterm birth), not pregnancy itself, associated with CKD progression, especially if eGFR <60, preexisting HT and PtU >1 g/day (all significant) |
| 26 (23–32) (controls) | eGFR: 85.0 (64.7–102.0) sCr: 0.80 mg/dL (0.70–1.00) PtU: 0.87 g/day (0.43–1.60) HT: 33 (56%) | Renal survival rate: 80.3% at 10 years; 70.4% at 20 years | |
| O’Shaughnessy 2017 [28] | 31.3 (23.0–33.8) | eGFR: 72 (61–90) (9/18 P) sCr: 1.0 mg/dL (0.8–1.2) (9/18 P) PtU spot: 1.3 g (0.9–4.1) (8/18 P) | ≥200% increase PtU (2–12 m postpartum): 2/6 (33.3%) ≥150% increase sCr (2–12 m postpartum): 1/8 (12.5%) ESRD 1 year postpartum: 2 (11.1%) § Active IgAN during pregnancy: 12 (66.7%). Dialysis during pregnancy: 0 |
| Su 2017 [29] | 27.2 ± 3.5 (cases) | eGFR: 102.6 ± 23.9 PtU: 1.04 g/day (0.03–7.25) HT: 15 (14%) Follow up: 67 ± 34 months | Persistent HT postpartum: 12/89 (13%). Irreversible PtU worsening: 7 (6%) PtU at pregnancy start or first trimester: risk factor for severe PE and infant loss ESRD: 4 (4%) § ESRD/>50% decrease eGFR: 7 (7%) Significant decrease kidney function after pregnancy in CKD stage 3–4 only |
| 28.7 ± 6.3 (controls) | eGFR: 94.5 ± 26.7 PtU: 1.29 g/day (0.02–11.78) HT: 52 (17%) Follow up: 65 ± 34 months | ESRD: 18 (6%) § ESRD/>50% decrease eGFR: 31 (10%) | |
| Tocos 2017 [30] | 31.9 ± 5.2 (cases) | eGFR: 89.9 ± 32.7 sCr: 0.87 mg/dL (0.50–2.88) PtU ≥0.5 g/day: 13 (41%) HT: 9 (27%) | Worsening CKD stage during pregnancy: 1/33 (3%) §§ Increased risk of PE but not of preterm delivery suggests late maternal PE |
| 31.2 ± 5.5 (controls) | HT: none | ||
| Liu 2014 [31] | 27.3 ± 3.6 (cases) | eGFR: 102.3 ± 21.9 PtU: 1.27 (0.06–7.25) g/day HT: 7 (11%) | HT after pregnancy 8 (13%); MAP during follow up 86.4 ± 8.6 Kidney disease progression: 4 (6%); decrease eGFR >50%: 3 (5%); ESRD: 1 (2%) § Mean change eGFR: −2.5 mL/min (−6.7 to 0.06) PtU during follow up: 0.67 g/day (0.10–6.72) Proteinuria in pregnancy borderline significant for adverse pregnancy outcomes |
| 27.8 ± 4.4 (controls) | eGFR: 103.4 ± 20.8 PtU: 1.09 (0.06–8.37) g/day HT: 4 (6%) | MAP during follow up 85.4 ± 7.3; Kidney disease progression: 6 (10%) decrease eGFR >50%: 4 (7%); ESRD: 2 (3%) § Mean change eGFR −2.4 –−7.1 to 2.4) mL/min PtU during follow up: 0.68 (0.07–4.30) g/day | |
| Oh 2011 [32] | 30.5 (25.0–39.0) | eGFR: 91.2 (24.1–157.0) mL/min MAP: 89.6–99.3 mmHg | eGFR after delivery 77.8 (19.8–150.0) Median ΔGFR with ≤30% reduction week prior to conception: 13% Median ΔGFR with >30% reduction week prior to conception: 8.7% MAP during pregnancy 96.7–102 Significant increase sCr (0.8–1.0 mg/dL) and PtU (0.7–1.5 g/g) after delivery |
| Suetsugu 2011 (*) [33] | NR | NR | Superimposed PE: preconception SBP, sCr, BUN higher; CCr and eGFR lower Delivery: sCr, BUN, uric acid higher; CCr and eGFR lower (significant) At delivery correlation between BP and histological severity, week and sCr |
| Shimizu 2010 [34] | 27.3 ± 4.0 (cases) | eGFR mL/min CKD1: 97.3 ± 9.4 CKD2: 74.1 ± 4.5 CKD3: 54.4 ± 11.6 | eGFR 3 year after delivery (mL/min): CKD1: 93.0 ± 1.6; CKD2: 78.2 ± 11.8; CKD3: 58.5 ± 14.4; Overall: baseline 68.9 ± 14.4—three years after 68.5 ± 14.9 sCr baseline—3 year after delivery (mg/dL): 1: 0.68–0.64; 2: 0.75–0.72; 3:0.94–0.90. Overall: 0.83 ± 0.20–0.75 ± 0.14 PtU baseline—3 year after delivery (g/day): CKD1: 0.19 ± 0.1–0.20 ± 0.28; CKD2: 0.39 ± 0.22–0.48 ± 0.44; CKD3: 0.77 ± 0.31–0.38 ± 0.33 (**) BP constant in all CKD groups |
| 28.1 ± 5.1 (controls) | eGFR: 70.9 ± 20.7 | eGFR after 3 years (mL/min): 68.6 ± 14.4 sCr baseline—after 3 years (mg/dL): 0.8 ± 0.15–0.88 ± 0.16. PtU baseline—after 3 years (g/day): 0.85 ± 0.65–0.40 ± 0.26 No new onset hypertension | |
| Waness 2010 [35] | 28.6 | CCr 88.6 mL/min sCr: 0.99 mg/dL BP: 128.2/82.1 mmHg PtU 535.2 mg/day | In 3rd trimester: BP 163.7/90.3 mmHg PtU 2179.2 mg/day CCr 77.4 mL/mins Cr 84.3 mmol/L |
| Limardo 2010 [36] | 26.72 ± 4.27 (cases) | sCr 0.87 ± 0.15 CCr 92 ± 17 PtU 1.0 (0–6) g/day HT: 27 (20%) | After 10 years: 36% on steroids and/or immuno-depressors; 61% on ACEI or ARBs Significant CCr decrease (−1.2 mL/min/year) in women with PtU >1 g/day at diagnosis, not modified by number of pregnancies, hypertension, PE Doubling of sCr in 13 (9.6%); start of dialysis in 3 (3.4%) §; new-onset HT in 34 (31%) of 109 previously normotensive women |
| 26.19 ± 5.15 (controls) | sCr 0.86 ± 0.16 CCr 89 ± 18 PtU 0.5 (0–7.6) g/day HT: 10 (11%) | After 10 years: 29% on steroids and/or immune-depressors; 47% on ACEI or ARBs Doubling of sCr in 7 (8%); start of dialysis in 2 (1.5%) §; new-onset HT in 16 of 77 (21%) previously normotensive women | |
| Donggyu 2010 * [37] | NR | NR | PE in 4 of 5 women with sCr >2.0 mg/dL at delivery ESRD within 2 years in 2/2 W with sCr >2.5 mg/dL in postpartum All women with sCr <2.5 mg/dL in postpartum had stable sCr 3 year after delivery |
| Ronkainen 2006 [38] | NR | NR | ESRD 2.6 year after delivery in 1 hypertensive woman with slightly impaired renal function before pregnancy § |
| Ronkainen 2002 [39] | NR | NR | HT or PtU in pregnancy: 9 (64.3%), of whom 5 reported poor outcome (not specified); no poor outcome reported in women without HT or PtU in pregnancy |
| Summary data baseline | GFR or CCr >100 mL/min in 2/9 study reporting on this item GFR <100 mL/min in 7/9 studies PtU ≥0.5 g/day in 6/7; <0.5 g /day in 1/7 studies (**) Hypertension in 11–61% in 4 studies, in other not clearly defined at baseline | ||
| Summary data progression | ESRD: 11/330 (3.3%) cases vs 22/458 (4.8%) controls, reported on by 5 studies of whom 3 provided a control group § Park: no significant difference between cases and non-pregnant controls. Significant better renal survival in cases without vs with obstetric complications, and in cases without obstetric complications vs non pregnant controls Su: no significant difference in incidence ESRD or eGFR decrease between cases and non-pregnant controls Liu: no difference between cases and non-pregnant controls over follow-up Shimizu: no difference in eGFR decrease between pregnancy and non-pregnancy Limardo: no significant difference in all outcomes (start of dialysis, doubling of serum creatinine, new onset hypertension) | ||
| Author Year [Ref] | Country | Age (years) | sCr-GFR-PtU | Other Data at Referral | Main Drugs in Pregnancy |
|---|---|---|---|---|---|
| Kaul 2016 * [40] | India | NR | NR | IgAN new-onset | Steroids, fish oil |
| NR | NR | IgAN new-onset | Steroids, fish oil | ||
| NR | NR | IgAN new-onset | Steroids, fish oil | ||
| Lim 2016 * [41] | USA | NR | NR | IgAN (diagnosed several years postpartum) | NR |
| Sun 2015 [42] | China | 26 | PtU 1–2+ | IgAN new-onset | NR |
| Nagai 2015 [43] | Japan | 37 | PtU postpartum | HSPN postpartum | NR |
| Liang 2015 * [44] | USA | 32 | PtU 2 g/day | IgAN new-onset | NR |
| Zand 2014 [45] | USA | 18 | sCr 1.8 mg/dL | IgAN | NR |
| Cornelis 2013 [46] | The Netherlands | 21 | CCr 20–25 mL/min | IgAN | Methyldopa, labetalol, EPO, thyroid hormones, oral iron |
| Hou 2013 [47] | USA | 28 | CCr 79 mL/min PtU 1.13 g/day | IgAN new-onset | Methyldopa, labetalol, hydralazine, magnesium (31 week), steroid prophylaxis (31 week) |
| Goifrè 2007 [48] | Italy | 25 | sCr 2.2 mg/dL PtU 1 g/day | IgAN | ASA, oral iron, vitamins, vaginal dinoprostone gel (36 week) |
| 30 | sCr 8 mg/dL | EPO, vit D, calcium carbonate, ritodrin (29 week) | |||
| 32 | HD | EPO, vit D, calcium carbonate, ritodrin (30 week) | |||
| Tanno 2007 [49] | Japan | 31 | sCr 0.8 mg/dL | HSPN recurrence in renal allograft | Methyldopa, amlodipine Immunosuppressors (not clear) |
| Barquero-Romero 2006 [50] | Spain | 36 | sCr 0.50 mg/dL PtU 1+ | HSPN new-onset | Methylprednisolone |
| Koizumi 2004 [51] | Japan | 30 | PtU + | HSPN new-onset | Low dose oral steroids for 3 week |
| Cusi 2003 [52] | Italy | 29 | sCr 1.5 mg/dL PtU 1.2 g/day | IgAN | Methyldopa, nifedipine, clonidine, EPO, steroid prophylaxis (26 week) |
| Amir 2002 (*) [53] | Saudi Arabia | NR | sCr 2.7 mg/dL PtU 5.4 g/day | IgAN with P-ANCA | Cyclophosphamide, prednisone |
| Summary data | 28.8 (18–37) | sCr <1.0 mg/dL: 2/9 (22.2%) sCr ≥1.0 mg/dL: 5/9 (55.6%) CCr <90 mL/min: 2/9 (22.2%) CCr ≥90 mL/min: 0 PtU >= 0.5 g/day: 5/5 (100%) reporting quantitatively | IgAN: 12 (new-onset: 6) HSPN: 4 (new-onset: 3) | Antihypertensive agent: 4/10 Immunosuppressors: 4/10 | |
| Author Year [Ref] | Pts | GW | Parity | Delivery | Indication for Delivery | NICU | APGAR 1–5 min Infant Outcomes | Sex | Weight (g) | Centile * |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaul 2016 * [40] | 3 | NR | NR | NR | NR | NR | All live births | NR | NR | NR |
| Lim 2016 * [41] | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sun 2015 [42] | 1 | 40 | Primi | CS | NR | NR | NR | NR | NR | NR |
| Nagai 2015 [43] | 1 | At term | NR | Vaginal | None | NR | NR | NR | NR | NR |
| Liang 2015 * [44] | 1 | 36 + 5 | Gravi 7 Para 1 P1051 | Vaginal induced | Presumed superimposed PE | NR | NR | F | NR | NR |
| Zand 2014 [45] | 1 | 32 | NR | Vaginal | None | NR | Healthy | M | NR | NR |
| Cornelis 2013 [46] | 1 | 36 | Primi | Vaginal assisted | Sudden HT | YES | 9 and 10 Wet lung syndrome, NICU non-invasive ventilatory support 1 day. Discharged 8 days later. | M | 2480 | 25 |
| Hou 2013 [47] | 1 | 31 | Gravi 2 Para 0 | CS | PE, failed induction | NR | 3 and 8 Normal development 11 years later. | F | 1596 | 64 |
| Goifrè 2007 [48] | 1 | 38 | Gravi 1 Para 0 | Vaginal | None | YES | 8 and 9 NICU, discharged 20 days later. | M | 3150 | 45 |
| 33 | Gravi 2 Para 1 | CS | NR | YES | 7 and 8 NICU for RD (ventilatory support for 6 h); discharged 20 days later. | M | 2190 | 65 | ||
| 33 | Gravi 3 Para 2 | CS | NR | YES | 5 and 8 NICU for RD (ventilatory support for 2 days); discharged 30 days later. | M | 2500 | 90 | ||
| Tanno 2007 [49] | 1 | 28 | NR | CS | Impaired umbilical flow and fetal growth | NR | No obvious anomalies | NR | 999 | 39 ** |
| Barquero-Romero 2006 [50] | 1 | 39 | Multi | Vaginal | None | NR | Healthy at 3 m follow up | M | 3380 | 41 |
| Koizumi 2004 [51] | 1 | 40 | Primi | Vaginal | None | NR | Healthy | M | 2986 | 11 |
| Cusi 2003 [52] | 1 | 31 | NR | Vaginal | None | NR | 8 and 9 Healthy | F | 1626 | 68 |
| Amir 2002 (*) [53] | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Summary data | 16 (18 cases) | <37 w: 8/13 (61.5%) ≥37 w: 5/13 (38.5%) | Primi: 4/9 (44.4%) Multi: 5/9 (55.6%) | Vaginal: 8/13 (61.5%) CS: 5/13 (38.5%) | Maternal complications: 3/10 (30%) Fetal complications: 1/10 (10%) None: 6/10 (60%) | All reported cases had favorable outcomes; NICU reported in 4 cases | F: 3/10 (30%) M: 7/10 (70%) | <2500 g: 5/9 (55.6%); <1500 g: 1/9 (11.1%) | AGA: 9/9 (100%) (calculated upon INeS charts) | |
| Author Year [Ref] | PE/Other | Maternal Outcomes, as Reported in the Paper |
|---|---|---|
| Kaul 2016 * [40] | NR | All 3 patients treated with steroids and fish oil, complete remission in all 3 patients |
| Lim 2016 * [41] | HT and PtU at 22 weeks, presumed PE. No follow up postpartum. | Several years postpartum (age 25) presentation with severe HT and cardiac failure, pulmonary edema, hematuria, week , small hypo-echoic kidneys on ultrasound. Kidney biopsy: IgAN with severe atrophy and fibrosis |
| Sun 2015 [42] | None | 5 days postpartum atypical hemolytic uremic syndrome (AKI, nephrotic syndrome, thrombocytopenia and hemolytic anemia), HD for 5 weeks |
| Nagai 2015 [43] | 6th month HSP purpura, during pregnancy normal urinalysis | 1 m postpartum HSPN without renal dysfunction, and anti-PL-7 anti-synthetase syndrome with interstitial lung disease and subclinical myopathy |
| Liang 2015 * [44] | HT, week and hematuria | Normalization BP; persistent PtU and hematuria; biopsy proven IgAN 1.5 years later |
| Zand 2014 [45] | HT, anemia, atypical hemolytic uremic syndrome, start HD | On HD; living kidney donor transplant 5 months later |
| Cornelis 2013 [46] | 26 week start intensive HD for rapidly progressive deterioration kidney function; sudden HT 35 + 5 weeks | 2 weeks postpartum restart HD; 1 year later living-donor kidney transplant |
| Hou 2013 [47] | HT, PE 31 weeks | 1 year later deterioration kidney function; 11 years later evaluation for kidney transplant |
| Goifrè 2007 [48] | Anemia | Pre HD (CKD in 1st pregnancy) |
| Anemia; start HD end 1st trimester; polyhydramnios 28 weeks | On HD | |
| Anemia, polyhydramnios 30 weeks | On HD; 1 year later: kidney transplant | |
| Tanno 2007 [49] | 17 week HT and PtU, microhematuria; worsening of kidney function at 24 weeks and 28 weeks | Postpartum decrease BP and sCr, PtU from 6.0 to 1.0 g/day 6 months postpartum kidney biopsy: HSPN recurrence in renal allograft with additional focal segmental membranous nephropathy with C1q deposition |
| Barquero-Romero 2006 [50] | HSP at 36 weeks, good response to steroid | Healthy at 3 months follow up |
| Koizumi 2004 [51] | Elevated levels CRP and ALT/AST | Normalization of blood analysis and urinanalysis |
| Cusi 2003 [52] | HT, anemia | Persistence of HT and anemia |
| Amir 2002 (*) [53] | 11 week rapidly progressive GN: sCr 2.7 mg/dL, PtU 5.4 g/24 h | Good response to cyclophosphamide and prednisone: sCr 1.4 mg/dL, PtU 0.516 g/day, 18 months after diagnosis no significant clinical problems and stable kidney function |
| Summary data | PE: 1 Pregnancy induced HT: 6 Start HD in pregnancy: 3 | Different outcomes of the kidney function also depending upon the disease |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, G.B.; Kooij, I.A.; Attini, R.; Montersino, B.; Fassio, F.; Gerbino, M.; Biolcati, M.; Cabiddu, G.; Versino, E.; Todros, T. A Systematic Review on Materno-Foetal Outcomes in Pregnant Women with IgA Nephropathy: A Case of “Late-Maternal” Preeclampsia? J. Clin. Med. 2018, 7, 212. https://doi.org/10.3390/jcm7080212
Piccoli GB, Kooij IA, Attini R, Montersino B, Fassio F, Gerbino M, Biolcati M, Cabiddu G, Versino E, Todros T. A Systematic Review on Materno-Foetal Outcomes in Pregnant Women with IgA Nephropathy: A Case of “Late-Maternal” Preeclampsia? Journal of Clinical Medicine. 2018; 7(8):212. https://doi.org/10.3390/jcm7080212
Chicago/Turabian StylePiccoli, Giorgina Barbara, Isabelle Annemijn Kooij, Rossella Attini, Benedetta Montersino, Federica Fassio, Martina Gerbino, Marilisa Biolcati, Gianfranca Cabiddu, Elisabetta Versino, and Tullia Todros. 2018. "A Systematic Review on Materno-Foetal Outcomes in Pregnant Women with IgA Nephropathy: A Case of “Late-Maternal” Preeclampsia?" Journal of Clinical Medicine 7, no. 8: 212. https://doi.org/10.3390/jcm7080212
APA StylePiccoli, G. B., Kooij, I. A., Attini, R., Montersino, B., Fassio, F., Gerbino, M., Biolcati, M., Cabiddu, G., Versino, E., & Todros, T. (2018). A Systematic Review on Materno-Foetal Outcomes in Pregnant Women with IgA Nephropathy: A Case of “Late-Maternal” Preeclampsia? Journal of Clinical Medicine, 7(8), 212. https://doi.org/10.3390/jcm7080212









