Incidence Rates and Risk of Hospital Registered Infections among Schizophrenia Patients before and after Onset of Illness: A Population-Based Nationwide Register Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Descriptive Measures
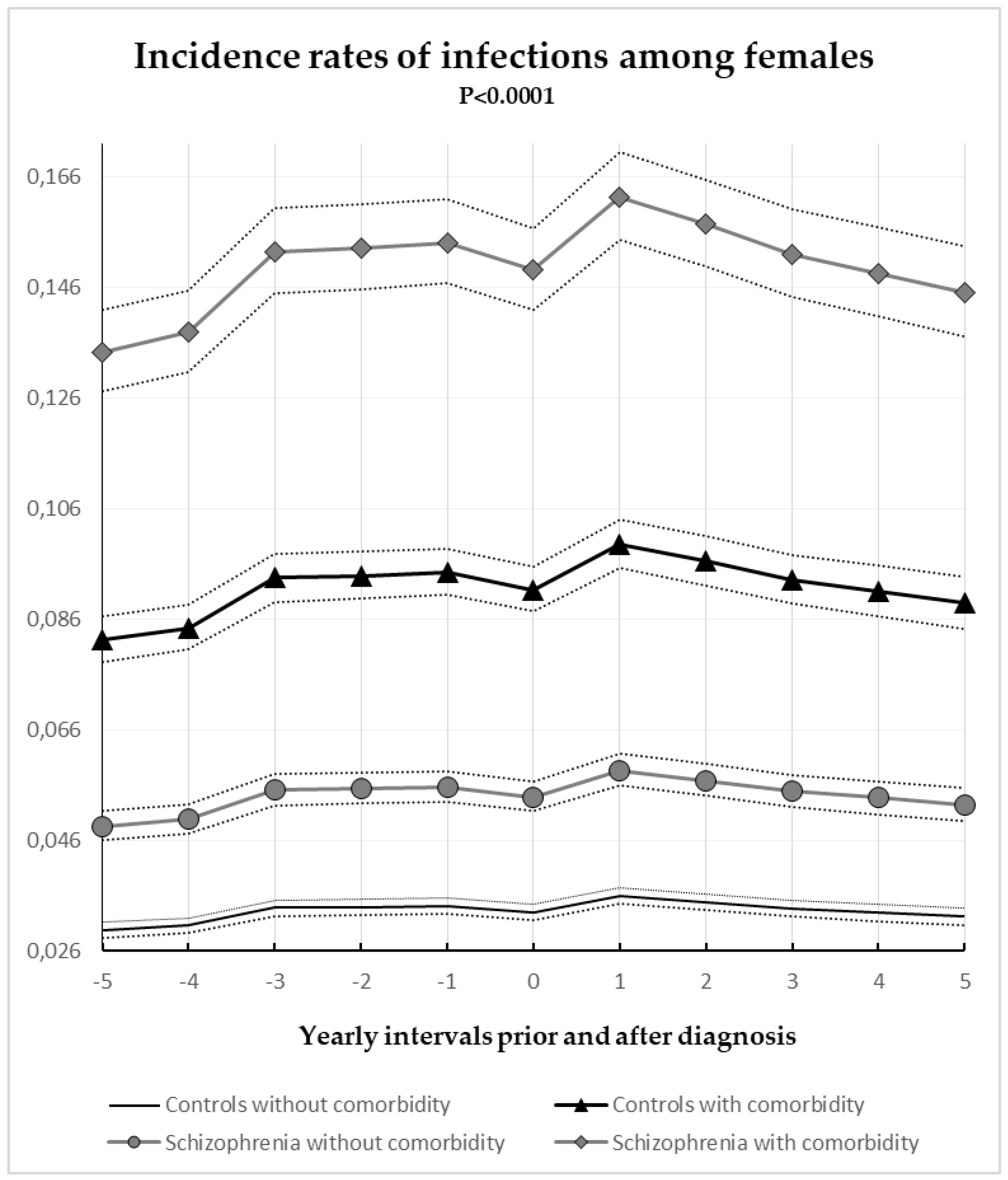
3.2. Gender-Specific Annual Incidence Rates of Hospital Registered Infections among Schizophrenia and Control Subjects with and without Comorbidity
3.3. Time-Dependent Risk of Hospital Registered Infections Overall and Stratified by Type of Infection
4. Discussion
4.1. Principal Findings
4.2. Time and Risk of Infections
4.3. Strengths
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Infections Assessment: | sepsis (ICD-10: A40, A41), hepatitis (ICD-10: B15–B19), gastro-intestinal infections (ICD-10: A00–A09, K35), skin infections (ICD-10: L00–L08, B00–B09, A46), respiratory infections (ICD-10: J00–J18), urinary tract infections (ICD-10: N00, N10, N300, N390), genital infections (ICD-10: N518B, N70, N71, N72, N76, N770D, N771B, N771L), CNS infections (ICD-10: I02, G00–G07, A17, A80–A89, B003, B004, B010, B011, B020, B021, B050, B051, B060, B261, B262, B375, B451, B582, B602, A022C, A548A, A548D, A521A, A521B, A229C, A321, A504, A390, E236A), ottis media (ICD-10: H65–67), HIV (ICD-10: B20–B24), tuberculosis (ICD-10: A15–A19), other type of infection (the remaining infections within the general chapters ICD-10: A, B or M0). |
| Comorbidity Component: | Myocardial Infarction (ICD-10: I21; I22; I23), Congestive Heart Failure (ICD-10: I50; I11.0; I13.0; I13.2), Peripheral Vascular Disease (ICD-10: I70; I71; I72; I73; I74; I77), Cerebrovascular Disease (ICD-10: I60–I69; G45; G46), Dementia (ICD-10: F00–F03; F05.1; G30), Chronic pulmonary diseases (ICD-10: J40–J47; J60–J67; J68.4; J70.1; J70.3; J84.1; J92.0; J96.1; J98.2; J98.3), Connective Tissue Disease (ICD-10: M05; M06; M08; M09; M30; M31; M32; M33; M34; M35; M36; D86), Peptic Ulcer Disease (ICD-10: K22.1; K25–K28), Diabetes Mellitus (ICD-10: E10–E11), Moderate to Severe Chronic Kidney Disease (ICD-10: I12; I13; N00–N05, N07; N11; N14; N17–N19; Q61), Hemiplegia (ICD-10: G81; G82), Cancer (ICD-10: C00–C96), Liver Disease (ICD-10), Obesity (ICD-10: E65; E66), Substance and Alcohol abuse and alcohol related diseases (ICD-10: F10–F19; G31.2; G72.1; I42.6; K29.2; R78.0; T51; Z72.1), Inflammatory bowel syndrome (ICD-10; K50–K52), Pancreatitis (ICD-10; K85; K86.0; K86.1) |
References
- Dalman, C.; Allebeck, P.; Gunnell, D.; Harrison, G.; Kristensson, K.; Lewis, G.; Lofving, S.; Rasmussen, F.; Wicks, S.; Karlsson, H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: A cohort study of more than one million Swedish subjects. Am. J. Psychiatry 2008, 165, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Benros, M.E.; Mortensen, P.B. Hospital contacts with infection and risk of schizophrenia: A population-based cohort study with linkage of Danish national registers. Schizophr. Bull. 2014, 40, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Blomström, Å.; Karlsson, H.; Svensson, A.; Frisell, T.; Lee, B.K.; Dal, H.; Magnusson, C.; Dalman, C. Hospital admission with infection during childhood and risk for psychotic illness—A population-based cohort study. Schizophr. Bull. 2014, 40, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chikritzhs, T. Early childhood infections and risk of schizophrenia. Psychiatry Res. 2012, 200, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Seminog, O.O.; Goldacre, M.J. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax 2013, 68, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Partti, K.; Vasankari, T.; Kanervisto, M.; Perälä, J.; Saarni, S.I.; Jousilahti, P.; Lönnqvist, J.; Suvisaari, J. Lung function and respiratory diseases in people with psychosis: Population-based study. Br. J. Psychiatry 2015, 207, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Davydow, D.S.; Ribe, A.R.; Pedersen, H.S.; Fenger-Grøn, M.; Cerimele, J.M.; Vedsted, P.; Vestergaard, M. Serious Mental Illness and Risk for Hospitalizations and Rehospitalizations for Ambulatory Care-sensitive Conditions in Denmark: A Nationwide Population-based Cohort Study. Med. Care 2016, 54, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Laursen, T.M.; Mortensen, P.B. Association between parental hospital-treated infection and the risk of schizophrenia in adolescence and early adulthood. Schizophr. Bull. 2013, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, H.-C.; Lin, H.-C. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr. Bull. 2011, 37, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-N.; Lu, C.-L.; Yang, H.-H. Increased risks of acute organ dysfunction and mortality in intensive care unit patients with schizophrenia: A nationwide population-based study. Psychosom. Med. 2011, 73, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Ribe, A.R.; Vestergaard, M.; Katon, W.; Charles, M.; Benros, M.E.; Vanderlip, E.; Nordentoft, M.; Laursen, T.M. Thirty-Day Mortality After Infection Among Persons With Severe Mental Illness: A Population-Based Cohort Study in Denmark. Am. J. Psychiatry 2015, 172, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chant, D.; McGrath, J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 2007, 64, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.-J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Laursen, T.M.; Agerbo, E. Comorbidity of schizophrenia and infection: A population-based cohort study. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Myles, N.; Newall, H.D.; Curtis, J.; Nielssen, O.; Shiers, D.; Large, M. Tobacco use before, at, and after first-episode psychosis: A systematic meta-analysis. J. Clin. Psychiatry 2012, 73, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, J.; Löhönen, J.; Koponen, H.; Isohanni, M.; Miettunen, J. Prevalence of alcohol use disorders in schizophrenia—A systematic review and meta-analysis. Acta Psychiatr. Scand. 2009, 120, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.J.; Nielsen, P.R.; Benros, M.E.; Pedersen, C.B.; Mortensen, P.B. Somatic diseases and conditions before the first diagnosis of schizophrenia: A nationwide population-based cohort study in more than 900,000 individuals. Schizophr. Bull. 2015, 41, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.M.; Phillip, N.; Miller, B.J. Urinary tract infections in children and adolescents with acute psychosis. Schizophr. Res. 2017, 183, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Carson, C.M.; Ezeoke, A.; Buckley, P.F.; Miller, B.J. Urinary tract infections in acute psychosis. J. Clin. Psychiatry 2014, 75, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Graham, K.L.; Bodenheimer, C.M.; Culpepper, N.H.; Waller, J.L.; Buckley, P.F. A prevalence study of urinary tract infections in acute relapse of schizophrenia. J. Clin. Psychiatry 2013, 74, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nosè, M.; Recla, E.; Trifirò, G.; Barbui, C. Antipsychotic drug exposure and risk of pneumonia: A systematic review and meta-analysis of observational studies. Pharmacoepidemiol. Drug Saf. 2015, 24, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-J.; Yang, S.-Y.; Liao, Y.-T.; Chen, W.J.; Lee, W.-C.; Shau, W.-Y.; Chang, Y.-T.; Tsai, S.-Y.; Chen, C.-C. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr. Bull. 2013, 39, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Kim, M.; Mitchell, C.; Inskip, H. Twenty-five year mortality of a community cohort with schizophrenia. Br. J. Psychiatry 2010, 196, 116–121. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, T.H. Early detection and intervention of schizophrenia: Rationale and research. Br. J. Psychiatry Suppl. 1998, 172, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Birchwood, M.; Todd, P.; Jackson, C. Early intervention in psychosis. The critical period hypothesis. Br. J. Psychiatry Suppl. 1998, 172, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Frank, L. Epidemiology. When an entire country is a cohort. Science 2000, 287, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B.; Gøtzsche, H.; Møller, J.O.; Mortensen, P.B. The Danish Civil Registration System. A cohort of eight million persons. Dan. Med. Bull. 2006, 53, 441–449. [Google Scholar] [PubMed]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Mors, O.; Perto, G.P.; Mortensen, P.B. The Danish Psychiatric Central Research Register. Scand. J. Public Health 2011, 39, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Helweg-Larsen, K. The Danish Register of Causes of Death. Scand. J. Public Health 2011, 39, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Uggerby, P.; Østergaard, S.D.; Røge, R.; Correll, C.U.; Nielsen, J. The validity of the schizophrenia diagnosis in the Danish Psychiatric Central Research Register is good. Dan. Med. J. 2013, 60, A4578. [Google Scholar] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kaka, A.S.; Filice, G.A.; Kuskowski, M.; Musher, D.M. Does active hepatitis C virus infection increase the risk for infection due to Staphylococcus aureus? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Catts, V.S.; Catts, S.V.; O’Toole, B.I.; Frost, A.D.J. Cancer incidence in patients with schizophrenia and their first-degree relatives—A meta-analysis. Acta Psychiatr. Scand. 2008, 117, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Agerbo, E.; Byrne, M.; Eaton, W.W.; Mortensen, P.B. Marital and labor market status in the long run in schizophrenia. Arch. Gen. Psychiatry 2004, 61, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, H.; Søndergaard Pedersen, H.; Fenger-Grøn, M.; Mors, O.; Nordentoft, M.; Vestergaard, M.; Munk Laursen, T. Increased use of primary care during 6 years of prodromal schizophrenia. Acta Psychiatr. Scand. 2016, 134, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Goodwin, R.D.; Okkels, N.; Gustafsson, L.N.; Taha, F.; Cole, S.W.; Munk-Jørgensen, P. Depression and the risk of severe infections: Prospective analyses on a nationwide representative sample. Int. J. Epidemiol. 2016, 45, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Ophoff, R.A.; Steinberg, S.; Andreassen, O.A.; Cichon, S.; Rujescu, D.; Werge, T.; Pietiläinen, O.P.H.; Mors, O.; Mortensen, P.B.; et al. Common variants conferring risk of schizophrenia. Nature 2009, 460, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Bernstein, H.-G.; Schiltz, K.; Müller, U.J.; Westphal, S.; Drexhage, H.A.; Bogerts, B. Immune system and glucose metabolism interaction in schizophrenia: A chicken-egg dilemma. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Dalman, C.; Wicks, S.; Lee, B.K.; Karlsson, H. Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl. Psychiatry 2013, 3, e228. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Agerbo, E.; Skogstrand, K.; Hougaard, D.M.; Meyer, U.; Mortensen, P.B. Neonatal levels of inflammatory markers and later risk of schizophrenia. Biol. Psychiatry 2015, 77, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Ozcan, S.; Gardner, R.M.; Rustogi, N.; Wicks, S.; van Rees, G.F.; Leweke, F.M.; Dalman, C.; Karlsson, H.; Bahn, S. Schizophrenia-risk and urban birth are associated with proteomic changes in neonatal dried blood spots. Transl. Psychiatry 2017, 7, 1290. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Trabjerg, B.B.; Meier, S.; Mattheisen, M.; Mortensen, P.B.; Mors, O.; Børglum, A.D.; Hougaard, D.M.; Nørgaard-Pedersen, B.; Nordentoft, M.; Agerbo, E. Influence of Polygenic Risk Scores on the Association Between Infections and Schizophrenia. Biol. Psychiatry 2016, 80, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Dalman, C.; Kappelmann, N.; Stochl, J.; Dal, H.; Kosidou, K.; Jones, P.B.; Karlsson, H. Association of Childhood Infection With IQ and Adult Nonaffective Psychosis in Swedish Men: A Population-Based Longitudinal Cohort and Co-relative Study. JAMA Psychiatry 2018, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, S.; Bernardo, M.; Bonnin, C.M.; Bioque, M.; Cabrera, B.; Mezquida, G.; Solé, B.; Vieta, E.; Torrent, C. The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study. Eur. Neuropsychopharmacol. 2016, 26, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Muck-Jørgensen, P.; Mors, O.; Mortensen, P.B.; Ewald, H. The schizophrenic patient in the somatic hospital. Acta Psychiatr. Scand. Suppl. 2000, 96–99. [Google Scholar] [CrossRef]
- Secher, R.G.; Hjorthoj, C.R.; Austin, S.F.; Thorup, A.; Jeppesen, P.; Mors, O.; Nordentoft, M. Ten-Year Follow-up of the OPUS Specialized Early Intervention Trial for Patients With a First Episode of Psychosis. Schizophr. Bull. 2015, 41, 617–626. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of the Study Population | Schizophrenia N (%) | Control N (%) | Total N (%) | p Value |
|---|---|---|---|---|
| Year of Birth | ||||
| 1975–1979 | 2516 | 25,160 | 27,676 | |
| 1980–1985 | 2784 | 27,840 | 30,624 | |
| 1986–1990 | 2552 | 25,520 | 28,072 | |
| Gender | ||||
| Male | 4772 | 47,720 | 52,492 | |
| Female | 3080 | 30,800 | 33,880 | |
| Comorbidity Component | ||||
| Myocardial Infarction | 13 (0.17%) | 25 (0.03%) | 38 (0.04%) | <0.0001 |
| Congestive Heart Failure | 10 (0.13%) | 37 (0.05%) | 47 (0.05%) | 0.0003 |
| Peripheral Vascular Disease | 18 (0.23%) | 95 (0.12%) | 113 (0.13%) | 0.02 |
| Cerebrovascular Disease | 46 (0.59%) | 178 (0.23%) | 224 (0.26%) | <0.0001 |
| Dementia | 4 (0.05%) | 7 (0.01%) | 11 (0.01%) | 0.01 |
| Chronic Pulmonary Diseases | 434 (5.53%) | 2125 (2.71%) | 2539 (2.96%) | <0.0001 |
| Connective Tissue Disease | 43 (0.55%) | 325 (0.41%) | 368 (0.43%) | 0.09 |
| Peptic Ulcer Disease | 67 (0.85%) | 179 (0.23%) | 246 (0.28%) | <0.0001 |
| Diabetes Mellitus | 134 (1.71%) | 523 (0.67%) | 657 (0.76%) | <0.0001 |
| Moderate to Severe Chronic Kidney Disease | 43 (0.55%) | 230 (0.29%) | 273 (0.32%) | 0.0004 |
| Hemiplegia | 19 (0.24%) | 141 (0.18%) | 160 (0.19%) | ns |
| Cancer | 44 (0.56%) | 476 (0.61%) | 520 (0.60%) | ns |
| Liver Disease | 44 (0.56%) | 79 (0.10%) | 123 (0.14%) | <0.0001 |
| Obesity | 308 (3.92%) | 1556 (1.98%) | 1864 (2.16%) | <0.0001 |
| Substance and Alcohol Abuse and Alcohol Related Diseases | 1613 (20.54%) | 2744 (3.49%) | 4357 (5.04%) | <0.0001 |
| Inflammatory Bowel Syndrome | 152 (1.94%) | 929 (1.18%) | 1081 (1.25%) | <0.0001 |
| Pancreatitis | 27 (0.34%) | 85 (0.11%) | 112 (0.13%) | <0.0001 |
| Type of Infection | Incidence Rate Ratios (95% CI) | |
|---|---|---|
| Basic Model * | Adjusted Model ** | |
| Any infection *** | 1.99 (1.93–2.06) | 1.63 (1.58–1.68) |
| Sepsis a | 2.32 (1.86–2.88) | 1.34 (1.07–1.66) |
| Gastrointestinal | 1.49 (1.39–1.59) | 1.24 (1.17–1.33) |
| Skin | 2.42 (2.32–2.52) | 1.88 (1.81–1.96) |
| Respiratory | 1.92 (1.81–2.03) | 1.43 (1.35–1.52) |
| Urological | 1.97 (1.82–2.14) | 1.80 (1.65–1.95) |
| Genital | 1.89 (1.71–2.08) | 1.91 (1.73–2.11) |
| Otitis media | 1.41 (1.27–1.56) | 1.29 (1.17–1.43) |
| Tuberculosis | 3.15 (2.27–4.36) | 2.35 (1.69–3.27) |
| CNS Infections b | 1.20 (0.96–1.51) | 0.98 (0.78–1.23) |
| Other Types | 1.31 (1.23–1.38) | 1.17 (1.10–1.23) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankiewicz-Dulacz, M.; Stenager, E.; Chen, M.; Stenager, E. Incidence Rates and Risk of Hospital Registered Infections among Schizophrenia Patients before and after Onset of Illness: A Population-Based Nationwide Register Study. J. Clin. Med. 2018, 7, 485. https://doi.org/10.3390/jcm7120485
Pankiewicz-Dulacz M, Stenager E, Chen M, Stenager E. Incidence Rates and Risk of Hospital Registered Infections among Schizophrenia Patients before and after Onset of Illness: A Population-Based Nationwide Register Study. Journal of Clinical Medicine. 2018; 7(12):485. https://doi.org/10.3390/jcm7120485
Chicago/Turabian StylePankiewicz-Dulacz, Monika, Egon Stenager, Ming Chen, and Elsebeth Stenager. 2018. "Incidence Rates and Risk of Hospital Registered Infections among Schizophrenia Patients before and after Onset of Illness: A Population-Based Nationwide Register Study" Journal of Clinical Medicine 7, no. 12: 485. https://doi.org/10.3390/jcm7120485
APA StylePankiewicz-Dulacz, M., Stenager, E., Chen, M., & Stenager, E. (2018). Incidence Rates and Risk of Hospital Registered Infections among Schizophrenia Patients before and after Onset of Illness: A Population-Based Nationwide Register Study. Journal of Clinical Medicine, 7(12), 485. https://doi.org/10.3390/jcm7120485





