Association between Cardiovascular Risk and Diabetes with Colorectal Neoplasia: A Site-Specific Analysis
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Clinical Assessment
2.3. Questionnaire
2.4. Laboratory Assessment
2.5. Cardiovascular Risk Assessment
2.6. Colonoscopy
2.7. Statistics
3. Results
3.1. Differences by Coronary Risk Profiles
3.2. Cardiovascular Risk Profile and Site-Specific Colonoscopic Results
3.3. Diabetes Mellitus and the Metabolic Syndrome
3.4. Comparison for Sex Differences
4. Discussion
4.1. The Rationale for Site-Specific Risk Factors in CRC
4.2. Site-Specific Risk Factors for CRC
4.3. Diabetes Mellitus Type 2 and Metabolic Syndrome
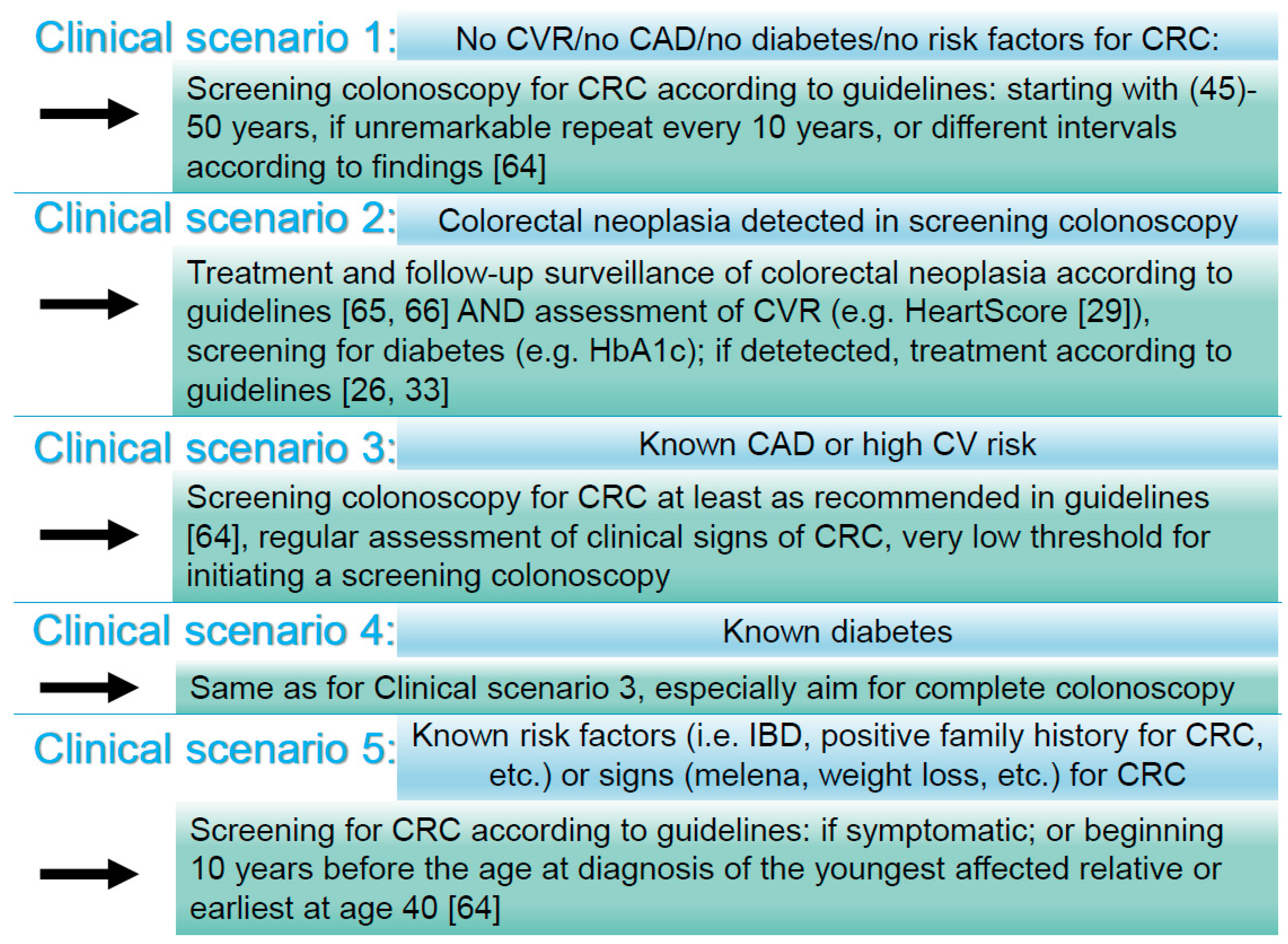
4.4. Number Needed to Screen: Selecting High-Risk Patients for CRC to Increase the Detection-Rate
4.5. Coronary Artery Disease, Cardiovascular Risk, and Colorectal Cancer: Potential Mechanisms, Integration into a Holistic Diagnostic Approach, and Future (Practical) Considerations
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bufill, J.A. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann. Intern. Med. 1990, 113, 779–788. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Potter, J.D. Host factors in carcinogenesis: Certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J. Natl. Cancer Inst. 1985, 75, 185–191. [Google Scholar] [PubMed]
- Minoo, P.; Zlobec, I.; Peterson, M.; Terracciano, L.; Lugli, A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int. J. Oncol. 2010, 37, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Liefers, G.J.; Los, L.C.; Kranenbarg, E.K.; Hermans, J.; Tollenaar, R.A.; Moriya, Y.; van de Velde, C.J.; van Krieken, J.H. Mechanisms of oncogenesis in colon versus rectal cancer. J. Pathol. 2001, 195, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Kuriyama, S.; Nakaya, N.; Shimazu, T.; Ohmori, K.; Nishino, Y.; Tsubono, Y.; Fukao, A.; Tsuji, I. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: The Miyagi Cohort Study. Eur. J. Cancer (Oxford) 2007, 43, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Mizoue, T.; Inoue, M.; Wakai, K.; Nagata, C.; Shimazu, T.; Tsuji, I.; Otani, T.; Tanaka, K.; Matsuo, K.; Tamakoshi, A.; et al. Alcohol drinking and colorectal cancer in Japanese: A pooled analysis of results from five cohort studies. Am. J. Epidemiol. 2008, 167, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Limburg, P.J.; Anderson, K.E.; Johnson, T.W.; Franceschi, S.; Levi, F.; Talamini, R.; La Vecchia, C. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 133–137. [Google Scholar] [PubMed]
- Yuhara, H.; Steinmaus, C.; Cohen, S.E.; Corley, D.A.; Tei, Y.; Buffler, P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011, 106, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, J.; Park, J.W.; Park, S.; Kim, J.; Oh, J.H.; Shin, A. Diabetes Mellitus and Site-specific Colorectal Cancer Risk in Korea: A Case-control Study. J. Prev. Med. Public Health 2016, 49, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.A.; Freedman, D.M.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control 2008, 19, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Joo, J.; Bak, J.; Yang, H.R.; Kim, J.; Park, S.; Nam, B.H. Site-specific risk factors for colorectal cancer in a Korean population. PLoS ONE 2011, 6, e23196. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Jim, M.H.; Lam, K.F.; Morris, J.S.; Siu, D.C.W.; Tong, T.; Ng, F.H.; Wong, S.Y.; Hui, W.M.; Chan, C.K.; et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007, 298, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kang, H.Y.; Choi, S.Y.; Kim, M.N.; Yang, J.I.; Chung, S.J.; Yang, S.Y.; Kim, Y.S.; Kim, J.S. Colorectal adenoma is associated with coronary artery calcification in a Korean population. Atherosclerosis 2015, 242, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, R.; Svaerke, C.; Sorensen, H.T.; Sandler, R.S.; Baron, J.A. Risk of colorectal cancer in patients with acute myocardial infarction and stroke: A nationwide cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, Y.J.; Shim, J.Y.; Lee, H.R. The association between coronary calcification and adenomatous polyps of colon in Korean adults. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Xu, Q.L.; Xu, C.Y.; Wu, C.; Yu, L.F. Prevalence of colorectal neoplasm in Chinese patients with high-risk coronary artery disease classified by the Asia-Pacific Colorectal Screening score. J Dig. Dis. 2015, 16, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kim, Y.S.; Chung, S.J.; Song, J.H.; Choi, S.Y.; Park, M.J.; Yim, J.Y.; Lim, S.H.; Kim, D.; Kim, C.H.; et al. Association between colorectal adenoma and coronary atherosclerosis detected by CT coronary angiography in Korean men; a cross-sectional study. J. Gastroenterol. Hepatol. 2010, 25, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Niederseer, D.; Stadlmayr, A.; Huber-Schonauer, U.; Plöderl, M.; Schmied, C.; Lederer, D.; Patsch, W.; Aigner, E.; Datz, C. Cardiovascular Risk and Known Coronary Artery Disease Are Associated With Colorectal Adenoma and Advanced Neoplasia. J. Am. Coll. Cardiol. 2017, 69, 2348–2350. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Salzl, P.; Weiss, W.; Müller, C.; Bannert, C.; Knoflach, P.; Häfner, M.; Peck-Radosavljevic, M.; Trauner, M.; Gschwantler, M. Empfehlungen der ÖGGH zur Darmkrebsvorsorge und Nachsorge nach koloskopischer Polypektomie. J. Gastroenterol. Hepatol. Erk. 2012, 10, 29–30. [Google Scholar]
- Stadlmayr, A.; Aigner, E.; Steger, B.; Scharinger, L.; Lederer, D.; Mayr, A.; Strasser, M.; Brunner, E.; Heuberger, A.; Hohla, F.; et al. Nonalcoholic fatty liver disease: An independent risk factor for colorectal neoplasia. J. Intern. Med. 2011, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; de Backer, G.; de Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Bond, J.H. Polyp guideline: Diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am. J. Gastroenterol. 2000, 95, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Zauber, A.G. The advanced adenoma as the primary target of screening. Gastrointest. Endosc. Clin. North Am. 2002, 12, 1–9. [Google Scholar] [CrossRef]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; de Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed]
- Versteylen, M.O.; Joosen, I.A.; Shaw, L.J.; Narula, J.; Hofstra, L. Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester to predict coronary atherosclerosis and cardiovascular events. J. Nucl. Cardiol. 2011, 18, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Gervaz, P.; Bucher, P.; Morel, P. Two colons-two cancers: Paradigm shift and clinical implications. J. Surg. Oncol. 2004, 88, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Morikawa, T.; Kuchiba, A.; Imamura, Y.; Qian, Z.R.; Nishihara, R.; Liao, X.; Waldron, L.; Hoshida, Y.; Huttenhower, C.; et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012, 61, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Benedix, F.; Meyer, F.; Kube, R.; Gastinger, I.; Lippert, H. Right- and left-sided colonic cancer different tumour entities. Zentralblatt Chir. 2010, 135, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Laird-Fick, H.S.; Chahal, G.; Olomu, A.; Gardiner, J.; Richard, J.; Dimitrov, N. Colonic polyp histopathology and location in a community-based sample of older adults. BMC Gastroenterol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, J.Y.; Zhao, L.N.; Yu, T.; Zhong, W.; Xia, Z.S.; Shan, T.D.; Ouyang, H.; Yang, H.S.; Chen, Q.K. Diabetes mellitus increases the risk of colorectal neoplasia: An updated meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Insulin and colon cancer. Cancer Causes Control 1995, 6, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Kiunga, G.A.; Raju, J.; Sabljic, N.; Bajaj, G.; Good, C.K.; Bird, R.P. Elevated insulin receptor protein expression in experimentally induced colonic tumors. Cancer Lett. 2004, 211, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, K.C.; Walsh, S.J.; Roche, M.; Jiang, Y.; Syrowatka, A.; Collins, K.D. Diabetes and sex-specific colorectal cancer risks in Newfoundland and Labrador: A population-based retrospective cohort study. Can. J. Public Health 2013, 104, e101–e107. [Google Scholar] [PubMed]
- Imperiale, T.F.; Monahan, P.O.; Stump, T.E.; Glowinski, E.A.; Ransohoff, D.F. Derivation and Validation of a Scoring System to Stratify Risk for Advanced Colorectal Neoplasia in Asymptomatic Adults: A Cross-sectional Study. Ann. Intern. Med. 2015, 163, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Betes, M.; Munoz-Navas, M.A.; Duque, J.M.; Angós, R.; Macías, E.; Súbtil, J.C.; Herraiz, M.; de la Riva, S.; Delgado-Rodríguez, M.; Martínez-González, M.A. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am. J. Gastroenterol. 2003, 98, 2648–2654. [Google Scholar] [PubMed]
- Cai, Q.C.; Yu, E.D.; Xiao, Y.; Bai, W.Y.; Chen, X.; He, L.P.; Yang, Y.X.; Zhou, P.H.; Jiang, X.L.; Xu, H.M.; et al. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am. J. Epidemiol. 2012, 175, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Polkowski, M.; Kraszewska, E.; Rupinski, M.; Butruk, E.; Regula, J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut 2014, 63, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Lin, O.S.; Kozarek, R.A.; Schembre, D.B.; Ayub, K.; Gluck, M.; Cantone, N.; Soon, M.S.; Dominitz, J.A. Risk stratification for colon neoplasia: Screening strategies using colonoscopy and computerized tomographic colonography. Gastroenterology 2006, 131, 1011–1109. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Hoffmeister, M.; Brenner, H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin. Gastroenterol. Hepatol. 2014, 12, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.G.; Ho, K.Y.; Chiu, H.M.; Zhu, F.; Ching, J.Y.; Wu, D.C.; Matsuda, T.; Byeon, J.S.; Lee, S.K.; Goh, K.L.; et al. The Asia-Pacific Colorectal Screening score: A validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011, 60, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.; Rupinski, M.; Kraszewska, E.; Polkowski, M.; Pachlewski, J.; Orlowska, J.; Nowacki, M.P.; Butruk, E. Colonoscopy in Colorectal-Cancer Screening for Detection of Advanced Neoplasia. New Eng. J. Med. 2006, 355, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Egred, M.; Viswanathan, G.; Davis, G. Myocardial infarction in young adults. Postgrad. Med. J. 2005, 81, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.; Chan, A.T. Statins and colorectal cancer. Clin. Gastroenterol. Hepatol. 2013, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Smiechowski, B.; Azoulay, L.; Yin, H.; Pollak, M.N.; Suissa, S. The Use of Metformin and Colorectal Cancer Incidence in Patients with Type II Diabetes Mellitus. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sørensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer RiskA Population-Based, Case–Control StudyNSAID Use and Colon Cancer Risk. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Reinhart, K.; Pramhas, S.; Wiener, C.; Gal, O.; Bannert, C.; Hassler, M.; Kozbial, K.; Dunkler, D.; Trauner, M.; et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011, 306, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Méndez, O.; Peg, V.; Salvans, C.; Pujals, M.; Fernández, Y.; Abasolo, I.; Pérez, J.; Matres, A.; Valeri, M.; Gregori, J.; et al. Extracellular HMGA1 Promotes Tumor Invasion and Metastasis in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Chiefari, E.; Salerno, N.; Ventura, V.; D’Ascoli, G.L.; Arcidiacono, B.; Ambrosio, G.; Bilotta, F.L.; Torella, D.; Foti, D.; et al. HMGA1 is a novel candidate gene for myocardial infarction susceptibility. Int. J. Cardiol. 2017, 227, 331–334. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 diabetes mellitus and cardiovascular disease: Genetic and epigenetic links. Front. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; de Rosa, S.; Tripolino, C.; Ambrosio, G.; Covello, C.; Abramo, E.; Carallo, C.; Mongiardo, A.; Spaccarotella, C.; Torella, D.; et al. Delayed flow-mediated vasodilation and critical coronary stenosis. J. Investig. Med. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal Cancer Screening for Average-Risk Adults: 2018 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Moss, A.; Hassan, C.; Bhandari, P.; Dumonceau, J.M.; Paspatis, G.; Jover, R.; Langner, C.; Bronzwaer, M.; Nalankilli, K.; et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017, 49, 270–297. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Quintero, E.; Dumonceau, J.M.; Regula, J.; Brandão, C.; Chaussade, S.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; Gimeno-García, A.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013, 45, 842–851. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2014; American Cancer Society: Atlanta, GA, USA, 2014. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the Future of Cardiovascular Disease in the United States A Policy Statement From the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.K.; Cardarelli, K.M. Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethn. Dis. 2007, 17, 143–152. [Google Scholar] [PubMed]
- Ollberding, N.J.; Nomura, A.M.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Racial/ethnic differences in colorectal cancer risk: The multiethnic cohort study. Int. J. Cancer 2011, 129, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Lanti, M. Comparison of the Framingham risk function-based coronary chart with risk function from an Italian population study. Eur. Heart J. 2000, 21, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [PubMed]
| Variable | No CAD History | CAD History | p |
|---|---|---|---|
| N | 1990 | 108 | |
| Age (years, [mean ± SD]) | 58.7 ± 9.7 | 66.6 ± 7.5 | <0.001 |
| Males (n, [%]) | 982 (49.4) | 69 (63.9) | 0.003 |
| BMI (kg/m2) | 27.1 ± 4.8 | 28.2 ± 4.5 | 0.026 |
| Systolic blood pressure (mmHg) | 133.7 ± 19.2 | 131.7 ± 18.6 | 0.280 |
| Diastolic blood pressure (mmHg) | 79.6 ± 9.0 | 80.9 ± 11.1 | 0.229 |
| Smoking status (never/ever/current, n, [%]) | 968 (48.6)/703 (35.4)/319 (16.0) | 49 (45.4)/46 (42.6)/13 (12) | 0.250 |
| Hypertension (n, [%]) | 1242 (62.4) | 70 (64.8) | 0.615 |
| IFG (n, [%]) | 143 (7.3) | 24 22.2 | <0.001 |
| IGT (n, [%]) | 330 (16.5) | 21 (19.4) | 0.437 |
| HOMA-IR | 2.8 ± 6.1 | 4.6 ± 6.5 | 0.003 |
| HbA1c [%] | 5.8 ± 0.7 | 6.1 ± 0.7 | <0.001 |
| Diabetes (n, [%]) | 262 (13.2) | 35 (32.4) | <0.001 |
| Metabolic Syndrome (n, [%]) | 385 (19.4) | 39 (36.1) | <0.001 |
| FRS | 7.1 ± 6.0 | 11.5 ± 6.8 | <0.001 |
| HS | 3.0 ± 3.6 | 4.8 ± 3.6 | <0.001 |
| Aspirin (n, [%]) | 264 (13.2) | 83 (76.9) | <0.001 |
| CAD History | Total N | Subjects with Any Adenoma N (%) | N to be Screened | OR (95% CI) | p-Value | Subjects with Advanced Neoplasia | N to be Screened | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| No | 1990 | 526 (26.4) | 3.8 | Reference | 75 (3.8) | 26.5 | Reference | ||
| Yes | 108 | 38 (35.2) | 2.8 | 1.51 (1.10–2.27) | 0.047 | 10 (9.6) | 10.8 | 2.62 (1.31–5.20) | 0.007 |
| Risk Score | Any Adenoma | p-Value | Advanced Neoplasia | p-Value |
|---|---|---|---|---|
| FRS (% points) | 1.07 (1.06–1.09) | <0.001 | 1.07 (1.04–1.12) | <0.001 |
| HS (% points) | 1.09 (1.07–1.14) | <0.001 | 1.09 (1.05–1.14 | <0.001 |
| Any Adenoma | Advanced Neoplasia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N (%) | N to be Screened | OR (95% CI) | Sensitivity | Specificity | p | N (%) | N to be Screened | OR (95% CI) | p | Sensitivity | Specificity | |
| FRS | 1990 | 526 (26.4) | 3.8 | 75 (3.8) | 26.5 | ||||||||
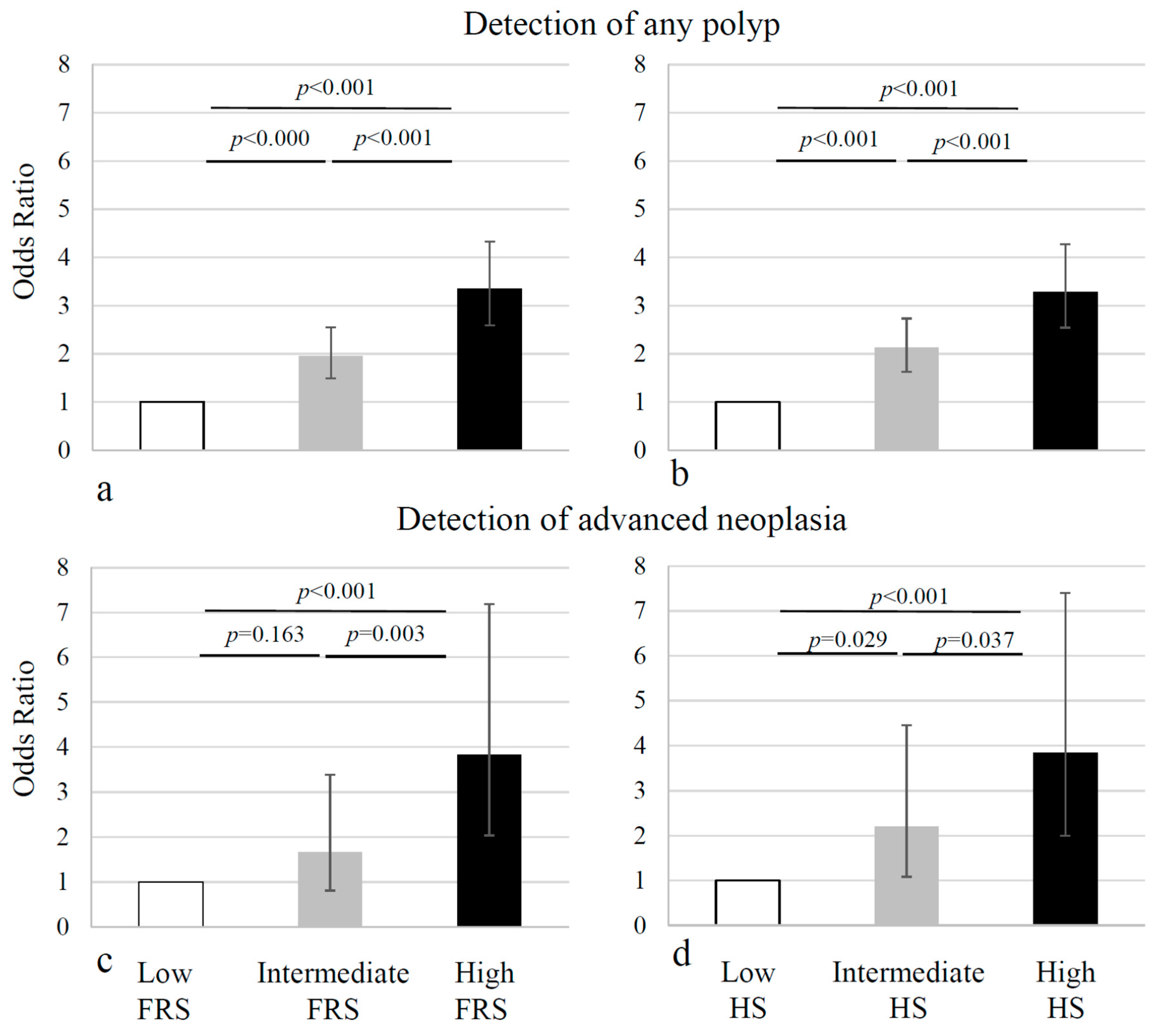
| Low (0–3) | 711 | 111 (15.6) | 6.4 | Reference | 13 (1.8) | 54.7 | Reference | ||||||
| Intermediate (4–8) | 633 | 168 (26.5) | 3.8 | 1.95 (1.49–2.55) | <0.001 | 19 (3.0) | 33.3 | 1.66 (0.81–3.39) | 0.163 | ||||
| High (>8) | 646 | 247 (38.2) | 2.6 | 3.35 (2.59–4.33) | 47% | 73% | <0.001 | 43 (6.7) | 15.0 | 3.83 (2.04–7.19) | <0.001 | 57% | 69% |
| HS | 1990 | 526 (26.4) | 3.8 | 75 (3.8) | 26.5 | ||||||||
| Low (<1.077) | 711 | 109 (15.3) | 6.5 | Reference | 12 (1.7) | 59.2 | Reference | ||||||
| Intermediate (1.077–3.192) | 633 | 176 (27.8) | 3.6 | 2.13 (1.63–2.78) | <0.001 | 23 (3.6) | 27.5 | 2.20 (1.08–4.45) | 0.029 | ||||
| High (3.192) | 646 | 241 (37.3) | 2.7 | 3.29 (2.54–4.26) | 46% | 72% | <0.001 | 40 (6.2) | 16.2 | 3.84 (2.00–7.40) | <0.001 | 53% | 68% |
| FRS-Tertiles | N | N (%) with Any Adenoma | N to be Screened | OR (95% CI) | p-Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Proximal Colon | 1990 | 325 (16.3) | 6.1 | ||||
| Low | 711 | 69 (9.7) | 10.3 | ||||
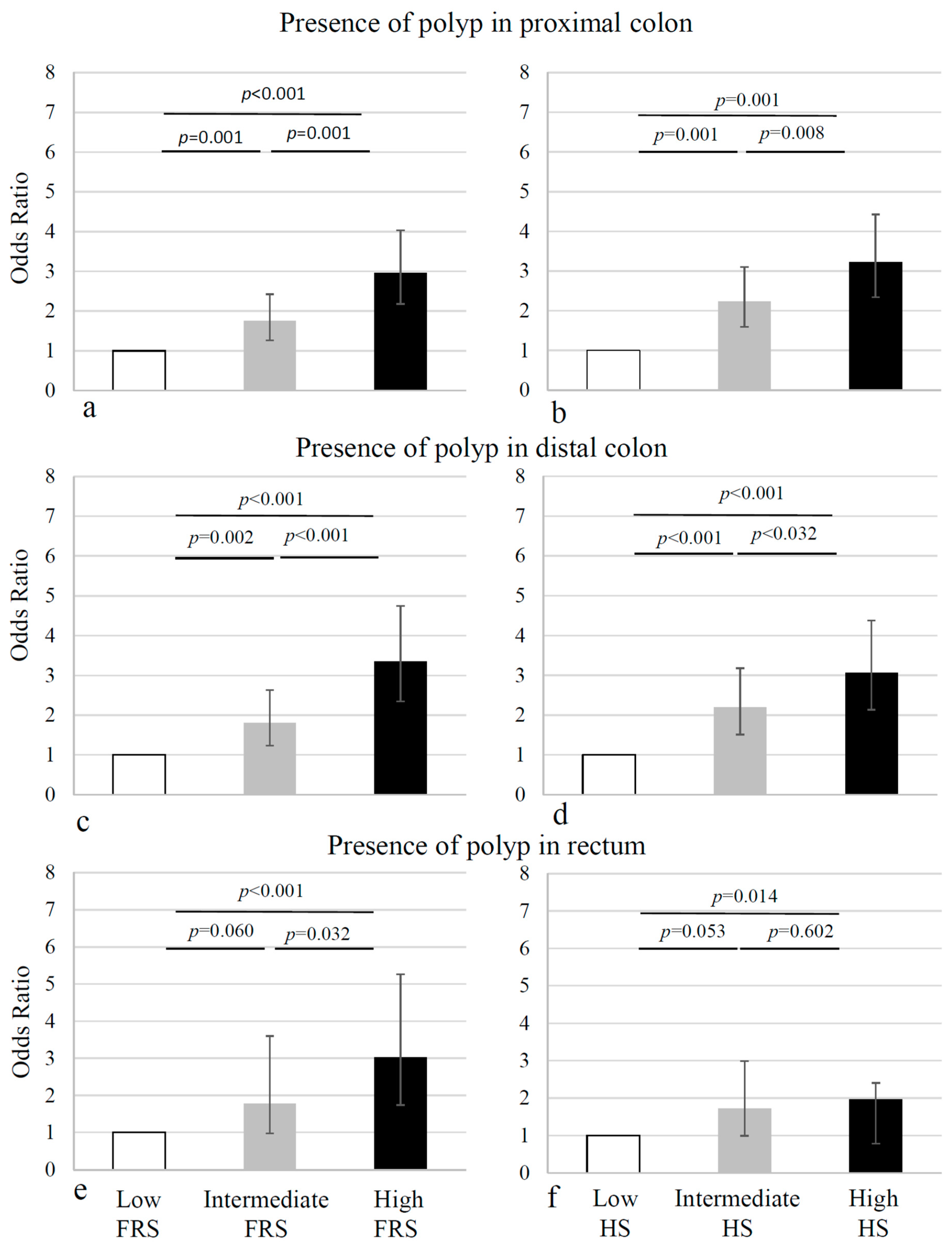
| Intermediate | 633 | 100 (15.8) | 6.3 | Reference | 0.001 | ||
| High | 646 | 156 (24.2) | 4.1 | 1.75 (1.26–2.42) | <0.001 | 48% | 71% |
| Distal Colon | 1990 | 247 (12.4) | 8.1 | 2.96 (2.18–4.03) | |||
| Low | 711 | 48 (6.8) | 14.8 | Reference | |||
| Intermediate | 633 | 73 (11.5) | 8.7 | 1.80 (1.23–2.63) | 0.002 | ||
| High | 646 | 126 (19.5) | 5.1 | 3.35 (2.35–4.76) | <0.001 | 51% | 70% |
| Rectum | 1990 | 93 (4.7) | 21.4 | ||||
| Low | 711 | 18 (2.5) | 39.5 | Reference | |||
| Intermediate | 633 | 28 (4.4) | 22.6 | 1.78 (0.98–3.25) | 0.060 | ||
| High | 646 | 47 (7.3) | 13.7 | 3.02 (1.74–5.26) | <0.001 | 51% | 68% |
| HS-Tertiles | N | N (%) with Any Adenoma | N to be Screened | OR (95% CI) | p-Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Proximal Colon | 1990 | 325 (16.3) | 6.1 | ||||
| Low | 711 | 62 (8.7) | 11.5 | ||||
| Intermediate | 633 | 111 (17.5) | 5.7 | Reference | <0.001 | ||
| High | 646 | 152 (23.5) | 4.3 | 2.23 (1.60–3.10 | <0.001 | 47% | 70% |
| Distal Colon | 1990 | 247 (12.4) | 8.1 | 3.22 (2.34–4.43) | |||
| Low | 711 | 47 (6.6) | 15.1 | ||||
| Intermediate | 633 | 85 (13.4) | 7.4 | Reference | <0.001 | ||
| High | 646 | 115 (17.8) | 5.6 | 2.19 (1.51–3.18) | <0.001 | 47% | 70% |
| Rectum | 1990 | 93 (4.7) | 21.4 | 3.06 (2.14–4.38) | |||
| Low | 711 | 22 (3.1) | 32.3 | ||||
| Intermediate | 633 | 33 (5.2) | 19.2 | Reference | 0.053 | ||
| High | 646 | 38 (5.9) | 17 | 1.72 (0.99–2.99) | 0.014 | 41% | 68% |
| IFG [yes/no] | IGT [yes/no] | HOMA [index] | FRS [%] | HS [%] | Polyps [n] | Tubular Adenoma [yes/no]] | Advanced Neoplasia [yes/no] | Adenoma in Proximal Colon [n] | Adenoma in Distal Colon [n] | Adenoma in Rectum [n] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFG [yes/no] | — | ||||||||||
| — | |||||||||||
| IGT [yes/no] | 0.123 | — | |||||||||
| <0.001 | — | ||||||||||
| HOMA [index] | 0.283 | 0.105 | — | ||||||||
| <0.001 | <0.001 | — | |||||||||
| FRS [%] | 0.176 | 0.15 | 0.25 | — | |||||||
| <0.001 | <0.001 | <0.001 | — | ||||||||
| HS [%] | 0.184 | 0.14 | 0.208 | 0.802 | — | ||||||
| <0.001 | <0.001 | <0.001 | <0.001 | — | |||||||
| polyps [n] | 0.031 | 0.054 | 0.111 | 0.238 | 0.237 | — | |||||
| 0.16 | 0.013 | <0.001 | <0.001 | <0.001 | — | ||||||
| tubular adenoma [yes/no]] | 0.039 | 0.045 | 0.095 | 0.212 | 0.222 | 0.92 | — | ||||
| 0.077 | 0.04 | <0.001 | <0.001 | <0.001 | <0.001 | — | |||||
| advanced neoplasia [yes/no] | 0.005 | 0.053 | 0.053 | 0.1 | 0.096 | 0.317 | 0.087 | — | |||
| 0.833 | 0.015 | 0.047 | <0.001 | <0.001 | <0.001 | <0.001 | — | ||||
| adenoma in proximal colon [n] | 0.02 | 0.043 | 0.07 | 0.179 | 0.191 | 0.728 | 0.707 | 0.223 | — | ||
| 0.36 | 0.047 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | — | |||
| adenoma in distal colon [n] | 0.031 | 0.038 | 0.117 | 0.165 | 0.156 | 0.64 | 0.592 | 0.221 | 0.169 | — | |
| 0.152 | 0.081 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | — | ||
| adenoma in rectum [n] | 0.024 | 0.028 | 0.033 | 0.096 | 0.081 | 0.367 | 0.305 | 0.15 | 0.036 | 0.115 | — |
| 0.264 | 0.194 | 0.216 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.096 | <0.001 | — |
| Males | Females | p-Value | |
|---|---|---|---|
| n | 1051 | 1047 | n.a. |
| Age (years; mean ± SD) | 58.8 ± 9.4 | 59.5 ± 10.1 | 0.152 |
| CAD (n [%]) | 69 (6.6) | 39 (3.7) | 0.003 |
| FRH (%; mean ± SD) | 10.6 ± 6.1 | 4.0 ± 4.1 | <0.001 |
| HS (%; mean ± SD) | 3.7 ± 3.9 | 2.5 ± 3.3 | <0.001 |
| Arterial hypertension (n [%]) | 701 (66.7) | 611 (58.4) | <0.001 |
| BMI (kg/m2; mean ± SD) | 27.6 ± 4.1 | 26.8 ± 5.5 | <0.001 |
| Diabetes mellitus (n [%]) | 161 (15.3) | 136 (13.0) | 0.126 |
| Any adenoma | 371 (35.3%) | 218 (20.8%) | <0.001 |
| Any adenoma (total number of adenomas detected) (n [%]) | 630 | 338 | <0.001 |
| Advanced neopasia (n [%]) | 67 (6.4) | 41 (3.9) | 0.011 |
| Adenoma in proximal colon (n [%]) | 220 (20.9) | 130 (12.4) | <0.001 |
| Adenoma in distal colon (n [%]) | 166 (15.8) | 100 (9.6) | <0.001 |
| Adenoma in rectum (n [%]) | 71 (6.8) | 30 (2.9) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederseer, D.; Bracher, I.; Stadlmayr, A.; Huber-Schönauer, U.; Plöderl, M.; Obeid, S.; Schmied, C.; Hammerl, S.; Stickel, F.; Lederer, D.; et al. Association between Cardiovascular Risk and Diabetes with Colorectal Neoplasia: A Site-Specific Analysis. J. Clin. Med. 2018, 7, 484. https://doi.org/10.3390/jcm7120484
Niederseer D, Bracher I, Stadlmayr A, Huber-Schönauer U, Plöderl M, Obeid S, Schmied C, Hammerl S, Stickel F, Lederer D, et al. Association between Cardiovascular Risk and Diabetes with Colorectal Neoplasia: A Site-Specific Analysis. Journal of Clinical Medicine. 2018; 7(12):484. https://doi.org/10.3390/jcm7120484
Chicago/Turabian StyleNiederseer, David, Isabelle Bracher, Andreas Stadlmayr, Ursula Huber-Schönauer, Martin Plöderl, Slayman Obeid, Christian Schmied, Sabrina Hammerl, Felix Stickel, Dieter Lederer, and et al. 2018. "Association between Cardiovascular Risk and Diabetes with Colorectal Neoplasia: A Site-Specific Analysis" Journal of Clinical Medicine 7, no. 12: 484. https://doi.org/10.3390/jcm7120484
APA StyleNiederseer, D., Bracher, I., Stadlmayr, A., Huber-Schönauer, U., Plöderl, M., Obeid, S., Schmied, C., Hammerl, S., Stickel, F., Lederer, D., Patsch, W., Aigner, E., & Datz, C. (2018). Association between Cardiovascular Risk and Diabetes with Colorectal Neoplasia: A Site-Specific Analysis. Journal of Clinical Medicine, 7(12), 484. https://doi.org/10.3390/jcm7120484






