Pharmacotherapy for Irritable Bowel Syndrome
Abstract
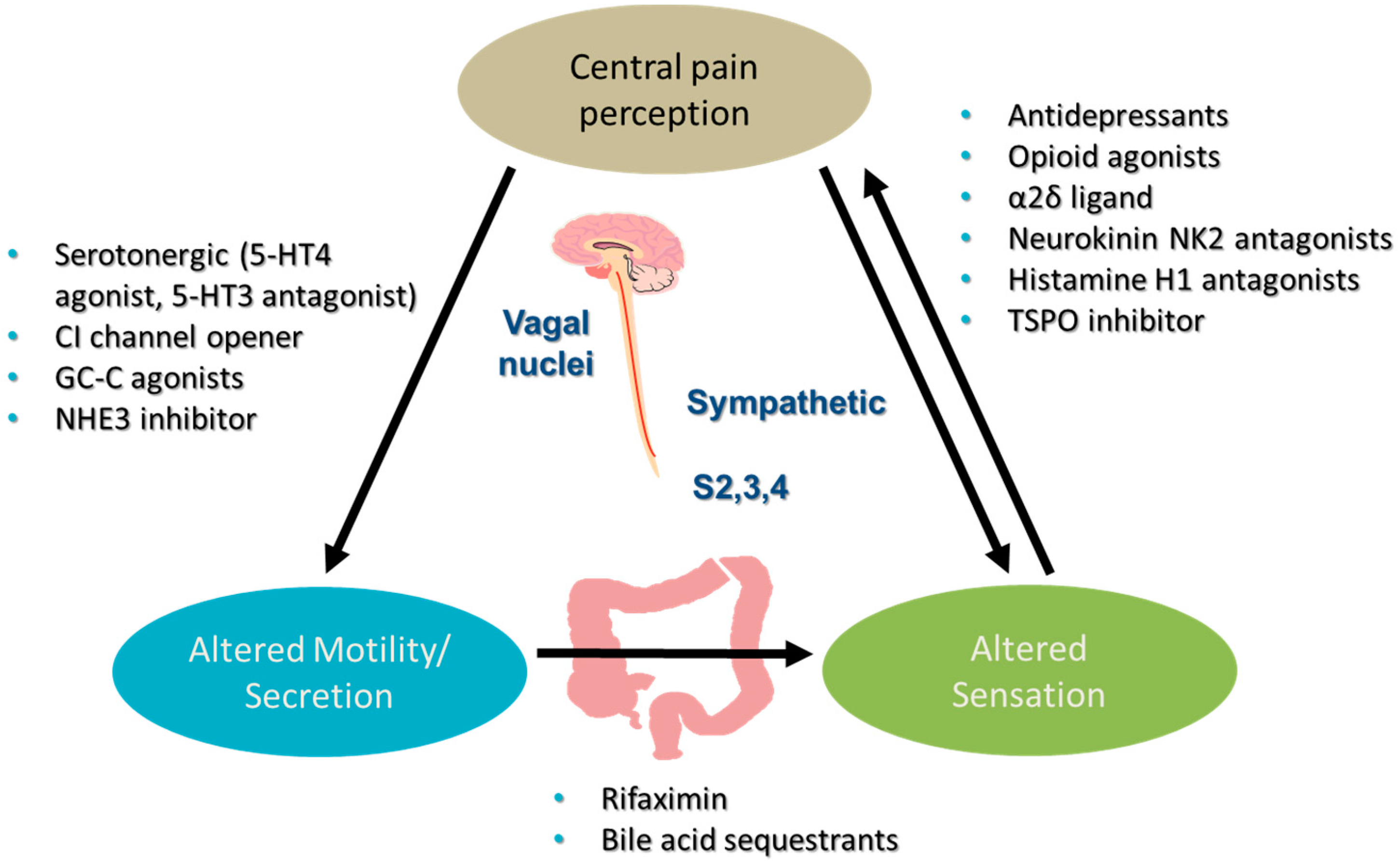
1. Introduction
2. Antispasmodic Drugs
2.1. Mechanism
2.2. Efficacy
2.2.1. Efficacy Focused on Systematic Reviews
2.2.2. Efficacy Focused on Otilonium Bromide Trials
2.2.3. Efficacy Focused on Pinaverium Trials
2.3. Safety
3. Peppermint Oil
3.1. Mechanism
3.2. Efficacy
3.3. Safety
4. Antidepressants
4.1. Mechanism
4.2. Efficacy
4.3. Safety
5. Drugs Acting on Opioid Receptors
5.1. Mechanism
5.2. Efficacy
5.3. Safety
6. 5-HT3 Receptor Antagonists
6.1. Mechanism
6.2. Efficacy
6.3. Safety
7. Experimental Approaches Using Visceral Analgesics
7.1. Histamine H1 Receptor Antagonist, Ebastine
7.2. Neurokinin-2 Receptor Antagonist, Ibodutant
7.3. Selective Inhibitor of Translocator Protein TSPO
8. GABAergic Agents
9. Bile Acid Sequestrants
10. Antibiotics
10.1. Mechanism
10.2. Efficacy
10.3. Safety
11. Intestinal Secretagogues
11.1. Chloride Channel-Related
11.2. Sodium-Hydrogen Exchanger
12. 5-HT4 Receptor Agonists
13. Conclusions
Conflicts of Interest
References
- Ford, A.C.; Forman, D.; Bailey, A.G.; Axon, A.T.R.; Moayyedi, P. Irritable bowel syndrome: A 10-year natural history of symptoms, and factors that influence consultation behavior. Am. J. Gastroenterol. 2008, 103, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Shin, A.; Busciglio, I.; Carlson, P.; Acosta, A.; Bharucha, A.E.; Burton, D.; Lamsam, J.; Lueke, A.; Donato, L.J.; et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol. Motil. 2014, 26, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Review article: Biomarkers and personalized therapy in lower functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2015, 42, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Linden, D.R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.J.; Chey, W.D.; Foxx-Orenstein, A.E.; Quigley, E.M.M.; Schiller, L.R.; Schoenfeld, P.S.; Spiegel, B.M.R.; Talley, N.J.; Moayyedi, P. An evidence-based position statement on the management of irritable bowel syndrome. Am. J. Gastroenterol. 2009, 104 (Suppl. 1), S8–S35. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lembo, A.; Sultan, S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014, 147, 1149–1172. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. Task force on the management of functional bowel disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Aziz, Q.; Creed, F.E.A.; Houghton, L.; Hungin, P.; Jones, R.; Kumar, D.; Rubin, G.; Trudgill, N.; Whorwell, P. Clinical Services Committee of The British Society of Gastroenterology. Guidelines on the irritable bowel syndrome: Mechanisms and practical management. Gut 2007, 56, 1770–1798. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. American College of Gastroenterology monograph on the management of irritable bowel syndrome. Expert Opin. Pharmacother. 2015, 16, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Quartero, A.O.; Meineche-Schmidt, V.; Muris, J.; Rubin, G.; de Wit, N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2005, 2, CD003460. [Google Scholar]
- Tack, J.; Fried, M.; Houghton, L.A.; Spicak, J.; Fisher, G. Systematic review: The efficacy of treatments for irritable bowel syndrome—A European perspective. Aliment. Pharmacol. Ther. 2006, 24, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J.; Spiegel, B.M.R.; Foxx-Orenstein, A.E.; Schiller, L.; Quigley, E.M.M.; Moayyedi, P. Effect of fibre, antispasmodics, and peppermint oil in irritable bowel syndrome: Systematic review and meta-analysis. Br. Med. J. 2008, 337, a2313. [Google Scholar] [CrossRef] [PubMed]
- Clave, P.; Acalovschi, M.; Triantafillidis, J.K.; Uspensky, Y.P.; Kalayci, C.; Shee, V.; Tack, J. Randomised clinical trial: Otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2011, 34, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Longanesi, A.; Blasi, A.; Monello, S.; Cestari, R.; Missale, G.; Corazziari, E.; Badiali, G.; Pescatori, M.; Anastasio, G.; et al. Clinical and functional evaluation of the efficacy of otilonium bromide: A multicenter study in Italy. Ital. J. Gastroenterol. 1991, 23 (Suppl. 1), 60–63. [Google Scholar] [PubMed]
- Battaglia, G.; Morselli-Labate, A.M.; Camarri, E.; Francavilla, A.; De Marco, F.; Mastropaolo, G.; Naccarato, R. Otilonium bromide in irritable bowel syndrome: A double-blind, placebo-controlled, 15-week study. Aliment. Pharmacol. Ther. 1998, 12, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Glende, M.; Morselli-Labate, A.M.; Battaglia, G.; Evangelista, S. Extended analysis of a double-blind, placebo-controlled, 15-week study with otilonium bromide in irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2002, 14, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lai, Y.; Lu, W.; Li, B.; Fan, H.; Yan, Z.; Gong, C.; Wan, X.; Wu, J.; Huang, D.; et al. Pinaverium reduces symptoms of irritable bowel syndrome in a multi-center, randomized controlled trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Liotta, R.; Mule, F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014, 740, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; MacDonald, J.K.; Levesque, B.G. Peppermint oil for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2014, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Cash, B.D.; Epstein, M.S.; Shah, S.M. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig. Dis. Sci. 2016, 61, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, P.; Zimmermann, T.; Sattel, H. Medically unexplained physical symptoms, anxiety and depression: A meta-analytic review. Psychosom. Med. 2003, 65, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Langhorst, J.; Gass, F.; Theysohn, N.; Benson, S.; Engler, H.; Gizewski, E.R.; Forsting, M.; Elsenbruch, S. Placebo analgesia in patients with functional and organic abdominal pain: A fMRI study in IBS, UC and healthy volunteers. Gut 2015, 64, 418–427. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H.J.; Tramer, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef]
- Saarto, T.; Wiffen, P.J. Antidepressants for neuropathic pain. Cochrane Database Syst. Rev. 2007, 4, CD005454. [Google Scholar]
- Gorard, D.A.; Libby, G.W.; Farthing, M.J. Influence of antidepressants on whole gut orocaecal transit times in health and irritable bowel syndrome. Aliment. Pharmacol. Ther. 1994, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Morgan, V.; Pickens, D.; Gautam, S.; Kessler, R.; Mertz, H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005, 54, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, H.; Merat, S.; Rashidioon, A.; Ghoddoosi, A.; Malekzadeh, R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: A double-blind randomized-controlled study. Aliment. Pharmacol. Ther. 2005, 22, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, H.; Merat, S.; Momtahen, S.; Kazzazi, A.S.; Ghaffari, N.; Olfati, G.; Malekzadeh, R. Clinical trial: The effect of amitriptyline in patients with diarrhea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 27, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Tabas, G.; Beaves, M.; Wang, J.; Friday, P.; Mardini, H.; Arnold, G. Paroxetine to treat irritable bowel syndrome not responding to high fiber diet: A double-blind placebo-controlled trial. Am. J. Gastroenterol. 2004, 99, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Broekaert, D.; Fischler, B.; van Oudenhove, L.; Gevers, A.M.; Janssens, J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006, 55, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Vij, J.C.; Jiloha, R.C.; Kumar, N.; Madhu, S.V.; Malika, V.; Anand, B.S. Effect of antidepressant drug (doxepin) on irritable bowel syndrome patients. Indian J. Psychiatry 1991, 33, 243–246. [Google Scholar]
- Drossman, D.A.; Toner, B.B.; Whitehead, W.E.; Diamant, N.E.; Dalton, C.B.; Duncan, S.; Emmott, S.; Proffitt, V.; Akman, D.; Frusciante, K.; et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003, 125, 19–31. [Google Scholar] [CrossRef]
- Ladabaum, U.; Sharabidze, A.; Levin, T.R.; Zhao, W.K.; Chung, E.; Bacchetti, P.; Jin, C.; Grimes, B.; Pepin, C.J. Citalopram is not effective therapy for nondepressed patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2010, 8, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Franzen, M.D.; Nickell, P.V.; Ransom, D.; Lebovitz, P.J. An open-label trial of duloxetine in patients with irritable bowel syndrome and comorbid generalized anxiety disorder. Int. J. Psychiatry Clin. Pract. 2014, 18, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Weinland, S.R.; Morris, C.B.; Dalton, C.; Hu, Y.; Whitehead, W.E.; Toner, B.B.; Diamant, N.; Leserman, J.; Bangdiwala, S.I.; Drossman, D.A. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am. J. Gastroenterol. 2010, 105, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lin, C.L.; Sung, F.C.; Liang, J.A.; Kao, C.H. Antidepressant treatment and risk of dementia: A population-based, retrospective case-control study. J. Clin. Psychiatry 2016, 77, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Breining, A.; Bonnet-Zamponi, D.; Zerah, L.; Micheneau, C.; Riolacci-Dhoyen, N.; Chan-Chee, C.; Deligne, J.; Harlin, J.M.; Boddaert, J.; Verny, M.; et al. Exposure to psychotropics in the French older population living with dementia: A nationwide population-based study. Int. J. Geriatr. Psychiatry 2017, 32, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lembo, A.; Katzka, D.A. Opioids in gastroenterology: Treating adverse effects and creating therapeutic benefits. Clin. Gastroenterol. Hepatol. 2017, 15, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Kasich, A.M. Treatment of diarrhea in irritable colon, including preliminary observations with a new antidiarrheal agent, diphenoxylate hydrochloride (Lomotil). Am. J. Gastroenterol. 1961, 35, 46–49. [Google Scholar] [PubMed]
- Lavo, B.; Stenstam, M.; Nielsen, A.L. Loperamide in treatment of irritable bowel syndrome—A double-blind placebo controlled study. Scand. J. Gastroenterol. 1987, 130, 77–80. [Google Scholar] [CrossRef]
- Hovdenak, N. Loperamide treatment of the irritable bowel syndrome. Scand. J. Gastroenterol. 1987, 130, 81–84. [Google Scholar] [CrossRef]
- Efskind, P.S.; Bernklev, T.; Vatn, M.H. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand. J. Gastroenterol. 1996, 31, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Dove, L.S.; Lembo, A.; Randall, C.W.; Fogel, R.; Andrae, D.; Davenport, J.M.; McIntyre, G.; Almenoff, J.S.; Covington, P.S. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 2013, 145, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.J.; Lacy, B.E.; Zuckerman, M.J.; Schey, R.; Dove, L.S.; Andrae, D.A.; Davenport, J.M.; McIntyre, G.; Lopez, R.; Turner, L.; et al. Eluxadoline for irritable bowel syndrome with diarrhea. N. Engl. J. Med. 2016, 374, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Wade, P.R.; Kirchgessner, A.L.; Tamir, H. 5-HT receptor subtypes outside the central nervous system. Roles in the physiology of the gut. Neuropsychopharmacology 1990, 3, 385–395. [Google Scholar] [PubMed]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Viramontes, B.E.; Camilleri, M.; McKinzie, S.; Pardi, D.S.; Burton, D.; Thomforde, G.M. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am. J. Gastroenterol. 2001, 96, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Hicks, G.A.; Coldwell, J.R.; Schindler, M.; Ward, P.A.; Jenkins, D.; Lynn, P.A.; Humphrey, P.P.; Blackshaw, L.A. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J. Physiol. 2002, 544, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Montori, V.M.; Keller, J.; West, C.P.; Layer, P.; Camilleri, M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: A systematic review and meta-analysis of randomised controlled trials. Clin. Gastroenterol. Hepatol. 2008, 6, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Brandt, L.J.; Young, C.; Chey, W.D.; Foxx-Orenstein, A.E.; Moayyedi, P. Efficacy of 5-HT-3 antagonists and 5-HT-4 agonists in irritable bowel syndrome: Systematic review and meta-analysis. Am. J. Gastroenterol. 2009, 104, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Kinoshita, Y.; Okumura, T.; Ida, M.; Akiho, H.; Nakashima, Y.; Nishida, A.; Haruma, K. Ramosetron reduces symptoms of irritable bowel syndrome with diarrhea and improves quality of life in women. Gastroenterology 2016, 150, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, K.; Harasawa, S.; Hongo, M.; Hiwatashi, N.; Sasaki, D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand. J. Gastroenterol. 2008, 43, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Garsed, K.; Chernova, J.; Hastings, M.; Lam, C.; Marciani, L.; Singh, G.; Henry, A.; Hall, I.; Whorwell, P.; Spiller, R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014, 63, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chey, W.D.; Harris, L.; Olden, K.; Surawicz, C.; Schoenfeld, P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: Systematic review of clinical trials and post-surveillance marketing data. Am. J. Gastroenterol. 2006, 101, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016, 150, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Luria, X. Comparative clinical studies with ebastine: Efficacy and tolerability. Drug Saf. 1999, 21 (Suppl. 1), 63–67. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, M.; Akyuz, F.; Tack, J. Targeting tachykinin receptors for the treatment of functional gastrointestinal disorders with a focus on irritable bowel syndrome. Neurogastroenterol Motil. 2015, 27, 1354–1370. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Schumacher, K.; Tonini, G.; Scartoni, S.; Capriati, A.; Maggi, C.A. The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 2017, 66, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Duffy, K.; Sharpe, J.; Nabata, T.; Bruce, M. Randomised clinical trial: Exploratory phase 2 study of ONO-2952 in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017, 45, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, J.H.; Cho, S.W. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2005, 22, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Iturrino, J.; Camilleri, M.; Busciglio, I.; Burton, D.; Zinsmeister, A.R. Effect of the α2δ ligand, pregabalin, on colonic sensory and motor functions in healthy adults. Am. J. Physiol. 2011, 301, G377–G378. [Google Scholar]
- Houghton, L.A.; Fell, C.; Whorwell, P.J.; Jones, I.; Sudworth, D.P.; Gale, J.D. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut 2007, 56, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A.; Almazar, A.E.; Tilkes, K.; Choung, R.S.; Locke, G.R., III; Zinsmeister, A.; Talley, N.J. A placebo-controlled trial of pregabalin for irritable bowel syndrome. Am. J. Gastroenterol. 2016, 111, S236. [Google Scholar]
- Straube, S.; Derry, S.; Moore, R.A.; McQuay, H.J. Pregabalin in fibromyalgia: Meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology 2010, 49, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Mumtaz, S.; Bholah, H.; Chowdhury, F.U.; Sanders, D.S.; Ford, A.C. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on Rome III criteria. Clin. Gastroenterol. Hepatol. 2015, 13, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Valentin, N.; Camilleri, M.; Altayar, O.; Vijayvargiya, P.; Acosta, A.; Nelson, A.D.; Murad, M.H. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: A systematic review and meta-analysis. Gut 2016, 65, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Acosta, A.; Busciglio, I.; Boldingh, A.; Dyer, R.B.; Zinsmeister, A.R.; Lueke, A.; Gray, A.; Donato, L.J. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Bajor, A.; Tornblom, H.; Rudling, M.; Ung, K.A.; Simren, M. Increased colonic bile acid exposure: A relevant factor for symptoms and treatment in IBS. Gut 2015, 64, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Chang, C.; Chua, K.S.; Mirocha, J.; DiBaise, J.; Rao, S.; Amichai, M. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig. Dis. Sci. 2014, 59, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cheeseman, F.; Vanner, S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011, 60, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 3503–3506. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Maneerattanaporn, M.; Kim, H.M.; Chey, W.D. The efficacy and safety of rifaximin for the irritable bowel syndrome: A systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Chang, L.; Lembo, A.; Aggarwal, K.; Bortey, E.; Paterson, C.; Forbes, W.P. Effects of rifaximin on urgency, bloating, and abdominal pain in patients with IBS-D: A randomized, controlled, repeat treatment study. Gastroenterology 2015, 148 (Suppl. 1), S69. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M.; Shin, A.; Linker Nord, S.; O’Neill, J.; Gray, A.V.; Lueke, A.J.; Donato, L.J.; Burton, D.B.; Szarka, L.A.; et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Gastroenterology 2016, 150, S948–S949. [Google Scholar] [CrossRef]
- Pimentel, M.; Cash, B.D.; Lembo, A.; Wolf, R.A.; Israel, R.J.; Schoenfeld, P. Repeat rifaximin for irritable bowel syndrome: No clinically significant changes in stool microbial antibiotic sensitivity. Dig. Dis. Sci. 2017. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Shi, K.Q.; Huang, S.; Wang, L.R.; Lin, Y.Q.; Huang, G.Q.; Chen, Y.P.; Braddock, M.; Zheng, M.H. Systematic review with network meta-analysis: The comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment. Pharmacol. Ther. 2015, 41, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Chey, W.D.; Johanson, J.F.; Fass, R.; Scott, C.; Panas, R.; Ueno, R. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome—Results of two randomized, placebo-controlled studies. Aliment. Pharmacol. Ther. 2009, 29, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.J.; Schneier, H.A.; Shiff, S.J.; Kurtz, C.B.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Lavins, B.J.; Currie, M.G.; Fitch, D.A.; et al. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med. 2011, 365, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J.; Shiff, S.J.; Kurtz, C.B.; Currie, M.G.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Fitch, D.A.; et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lembo, A.J.; Shiff, S.J.; Lavins, B.J.; Currie, M.G.; Jia, X.D.; Shi, K.; MacDougall, J.E.; Shao, J.Z.; Eng, P.; et al. 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol. 2012, 107, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Shailubhai, K.; Barrow, L.; Talluto, C.; Comiskey, S.; Foss, J.; Feng, R.; Joslyn, A.; Jacob, G. Plecanatide, a guanylate cyclase C agonist improves bowel habits and symptoms associated with chronic constipation in a phase IIa clinical study. Am. J. Gastroenterol. 2011, 106 (Suppl. 2), S502. [Google Scholar]
- Miner, P.B., Jr.; Koltun, W.D.; Wiener, G.J.; De La Portilla, M.; Prieto, B.; Shailubhai, K.; Layton, M.B.; Barrow, L.; Magnus, L.; Griffin, P.H. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am. J. Gastroenterol. 2017, 112, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.; Dorn, S.D.; Krause, R.; Eng, P.; Kirshoff, R.; Nguyen, A.; Griffin, P. Efficacy and safety of plecanatide in patients with irritable bowel syndrome with constipation: Results from 2 randomized, double-blind, placebo-controlled clinical trials. Gastroenterology 2017, 152 (Suppl. 1), S1309–S1310. [Google Scholar] [CrossRef]
- Chey, W.D.; Lembo, A.J.; Rosenbaum, D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: A phase 2, randomized, placebo-controlled efficacy and safety trial. Am. J. Gastroenterol. 2017, 112, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Keller, J.; Loeffler, H.; Kreiss, A. Tegaserod in the treatment of irritable bowel syndrome (IBS) with constipation as the prime symptom. Ther. Clin. Risk Manag. 2007, 3, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ohmiya, N.; Miyahara, R.; Ando, T.; Watanabe, O.; Kawashima, H.; Itoh, A.; Hirooka, Y.; Niwa, Y.; Goto, H. Are symptomatic changes in irritable bowel syndrome correlated with the capsule endoscopy transit time? A pilot study using the 5-HT4 receptor agonist mosapride. Hepatogastroenterology 2011, 58, 453–458. [Google Scholar] [PubMed]
- Mansour, N.M.; Ghaith, O.; El-Halabi, M.; Sharara, A.I. A prospective randomized trial of mosapride vs. placebo in constipation-predominant irritable bowel syndrome. Am. J. Gastroenterol. 2012, 107, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Kolar, G.; Erwin, P.; West, C.P.; Murad, M.H. Systematic review with meta-analysis: Highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment. Pharmacol. Ther. 2014, 39, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Acosta, A.; Camilleri, M.; Boldingh, A.; Burton, D.; Ryks, M.; Rhoten, D.; Zinsmeister, A.R. A randomized trial of 5-hydroxytryptamine4-receptor agonist, YKP10811, on colonic transit and bowel function in functional constipation. Clin. Gastroenterol. Hepatol. 2015, 13, 701–708. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Mechanism of Action | Efficacy | Quality of Data | Adverse Events | Limitations of Data |
|---|---|---|---|---|---|
| Antispasmodic drugs | Smooth muscle relaxation | May be effective | Low | More likely with antispasmodics in a meta-analysis of 22 RCTs, particularly dry mouth, dizziness, and blurred vision | No high-quality trials, heterogeneity between studies, possible publication bias, and only a small number of RCTs assessing each individual antispasmodic |
| Peppermint oil | Smooth muscle relaxation | Effective | Moderate | No increase in adverse events in a meta-analysis of 4 RCTs | Heterogeneity between studies |
| Antidepressants | Central sensory modulation | Effective | Moderate | More likely with antidepressants in a meta-analysis of 17 RCTs, particularly dry mouth and drowsiness | Few high-quality trials, heterogeneity between studies, possible publication bias, and some atypical trials included |
| Ibodutant | Neurokinin NK2 antagonist | May be effective | Moderate | Promising visceral analgesic in a phase 2B trial | Awaiting phase 3 trials |
| Ebastine | Histamine H1 antagonist | May be effective | Low | Promising visceral analgesic in a single center trial | Awaiting phase 2B trials |
| TSPO inhibitor | May be effective | Low | Modest efficacy in a single proof of concept trial | Awaiting phase 2B trials | |
| Loperamide | μ-opioid agonist | Unknown | Low | Limited data | Few RCTs, with a small number of participants, not all of whom had IBS |
| Eluxadoline | Mixed opioid receptor modulator | Effective | High. | Serious events included acute pancreatitis and sphincter of Oddi spasm. Nausea and headache commoner with active therapy | Only a modest benefit over placebo in published RCTs; no benefit over placebo in terms of abdominal pain |
| Alosetron, ramosetron, ondansetron | 5-HT3 receptor antagonists | Effective | High | Serious events with alosetron included ischemic colitis and severe constipation. Ramosetron and ondansetron may be safer, although constipation commoner with active therapy. | Fewer RCTs of ramosetron and ondansetron; ondansetron may have no benefit over placebo in terms of abdominal pain |
| Cholestyramine, colestipol, colesevelam | Bile acid sequestrants | Unknown | Low | Limited data | No published RCTs |
| Rifaximin | Non-absorbable antibiotic | Effective | Moderate | No increase in adverse events in a meta-analysis of 5 RCTs | Only a modest benefit over placebo in published RCTs |
| Lubiprostone | Cl-C2 channel agonist | Effective | Moderate | Nausea commoner with active therapy, occurring in 8% of patients | Only a modest benefit over placebo in published RCTs |
| Linaclotide | GC-C receptor agonist | Effective | High | Diarrhea commoner with active therapy, occurring in 20% of pts | None |
| Plecanatide | GC-C receptor agonist | Effective | High | Diarrhea commoner with active therapy, occurring in ~6% of pts | None |
| Tenapanor | NHE3 inhibitor | Effective | Moderate | Diarrhea commoner with active therapy, occurring in 12% of pts | Awaiting phase 2B/3 trials |
| Prucalopride | 5-HT4 receptor agonist | Effective | high | Diarrhea, cramping, and cardiovascular AEs with “old generation” drugs in this class | Data available for tegaserod and mosapride, not for “new generation” drugs in this class: prucalopride, naronapride, velusetrag, YKP10811 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilleri, M.; Ford, A.C. Pharmacotherapy for Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 101. https://doi.org/10.3390/jcm6110101
Camilleri M, Ford AC. Pharmacotherapy for Irritable Bowel Syndrome. Journal of Clinical Medicine. 2017; 6(11):101. https://doi.org/10.3390/jcm6110101
Chicago/Turabian StyleCamilleri, Michael, and Alexander C. Ford. 2017. "Pharmacotherapy for Irritable Bowel Syndrome" Journal of Clinical Medicine 6, no. 11: 101. https://doi.org/10.3390/jcm6110101
APA StyleCamilleri, M., & Ford, A. C. (2017). Pharmacotherapy for Irritable Bowel Syndrome. Journal of Clinical Medicine, 6(11), 101. https://doi.org/10.3390/jcm6110101






