Utility of Deceased Expanded-Criteria Donors in Kidney Transplantation: A Single-Center Experience †
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Biostatistical Analysis
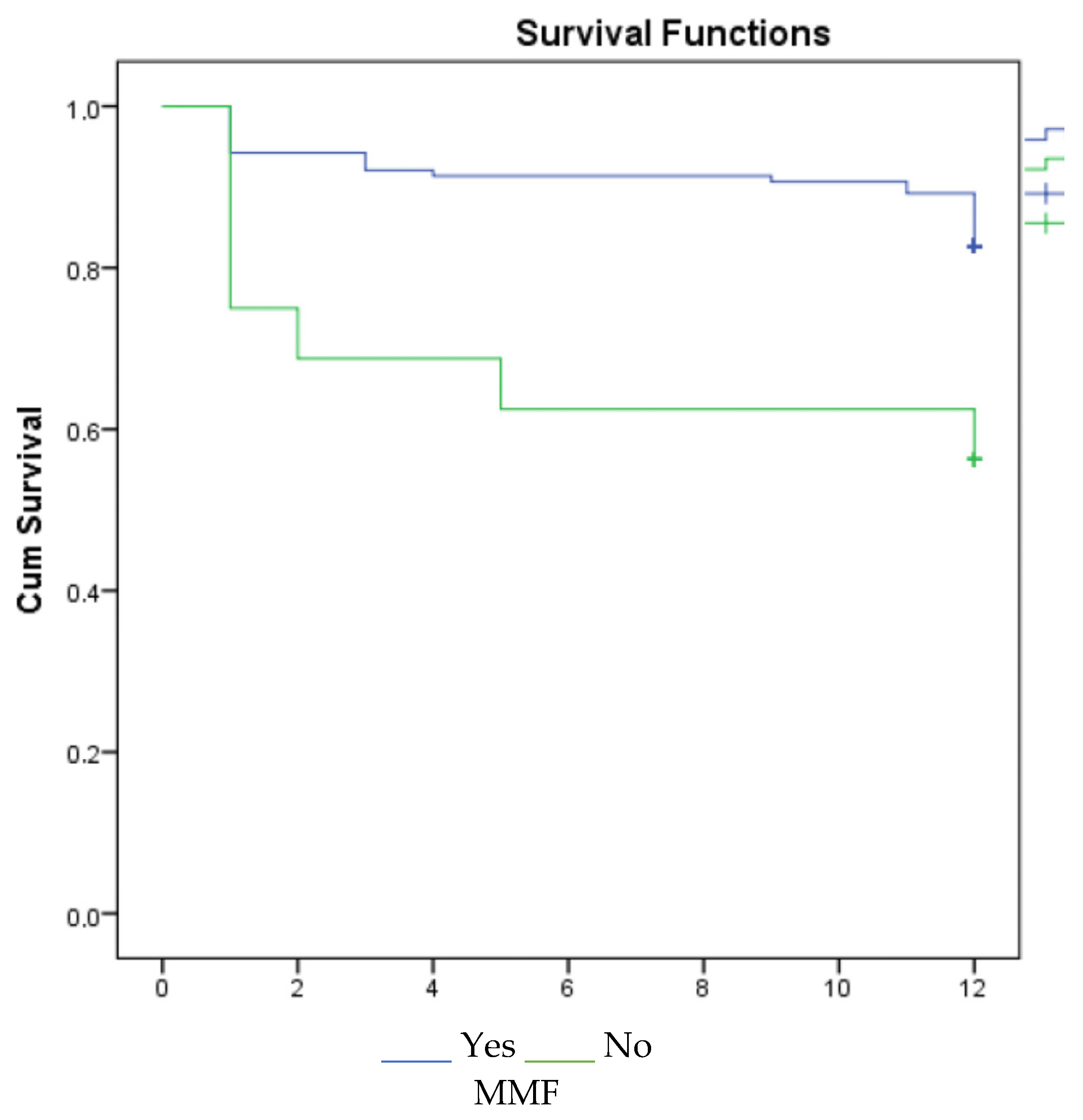
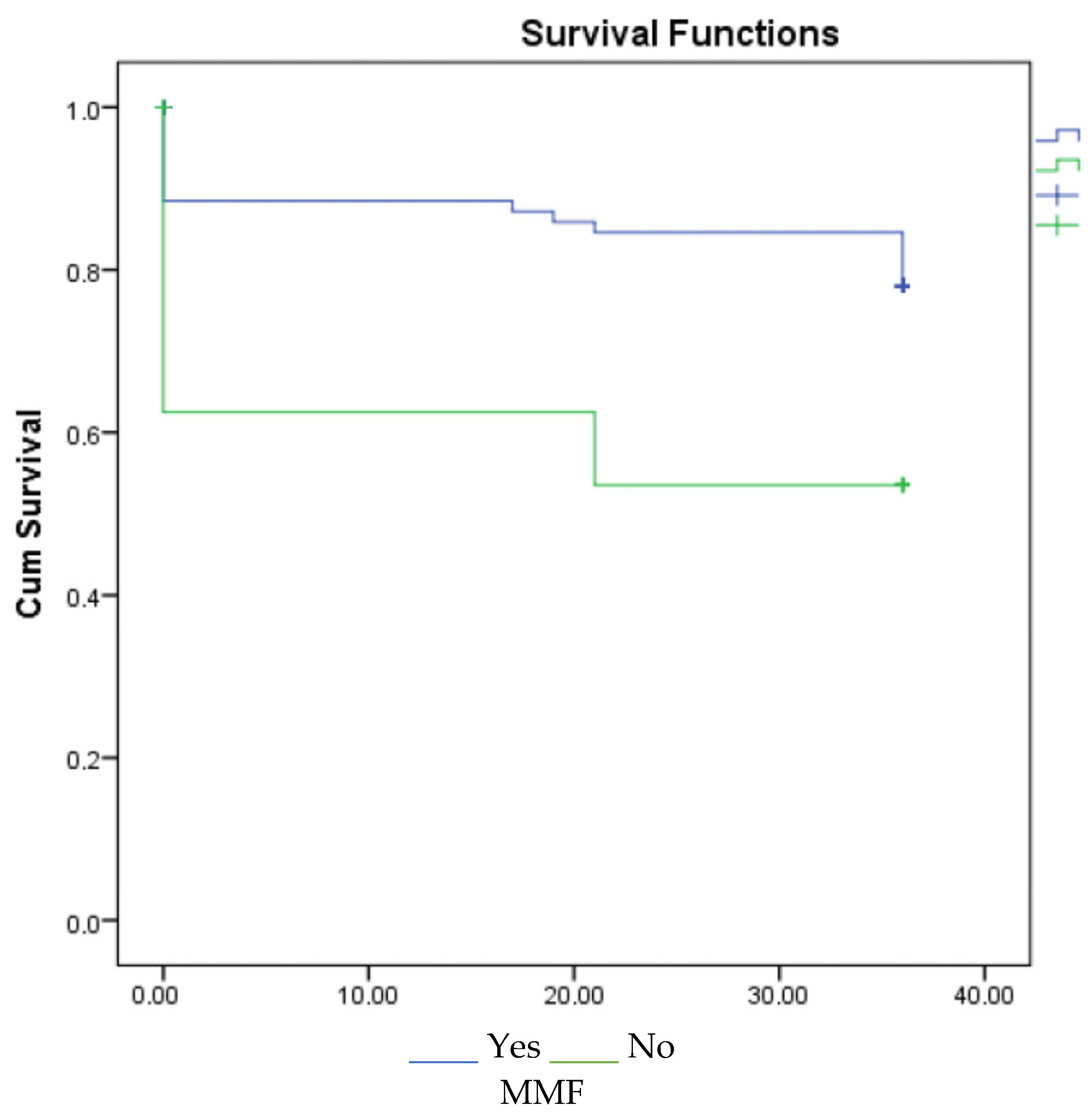
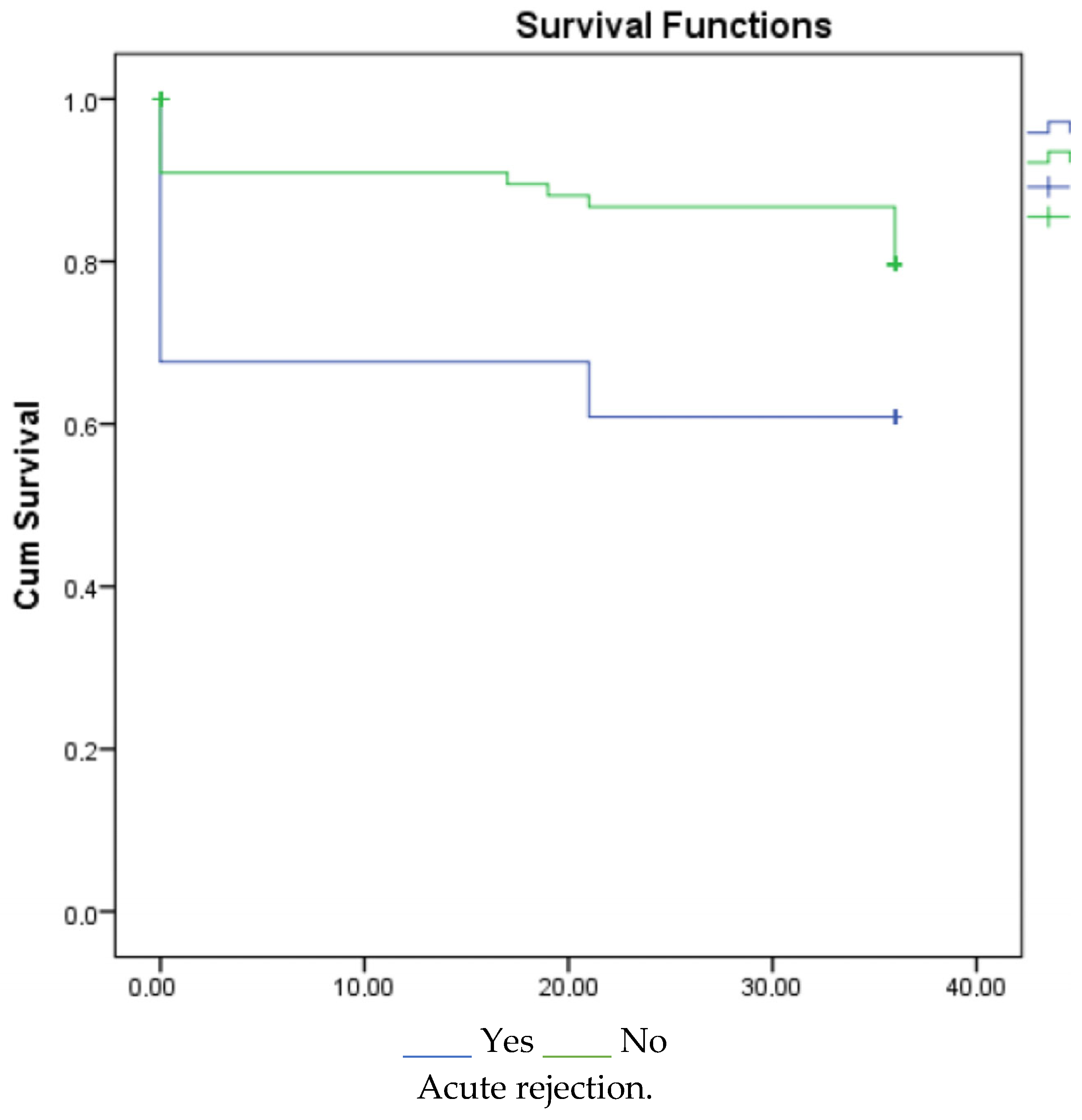
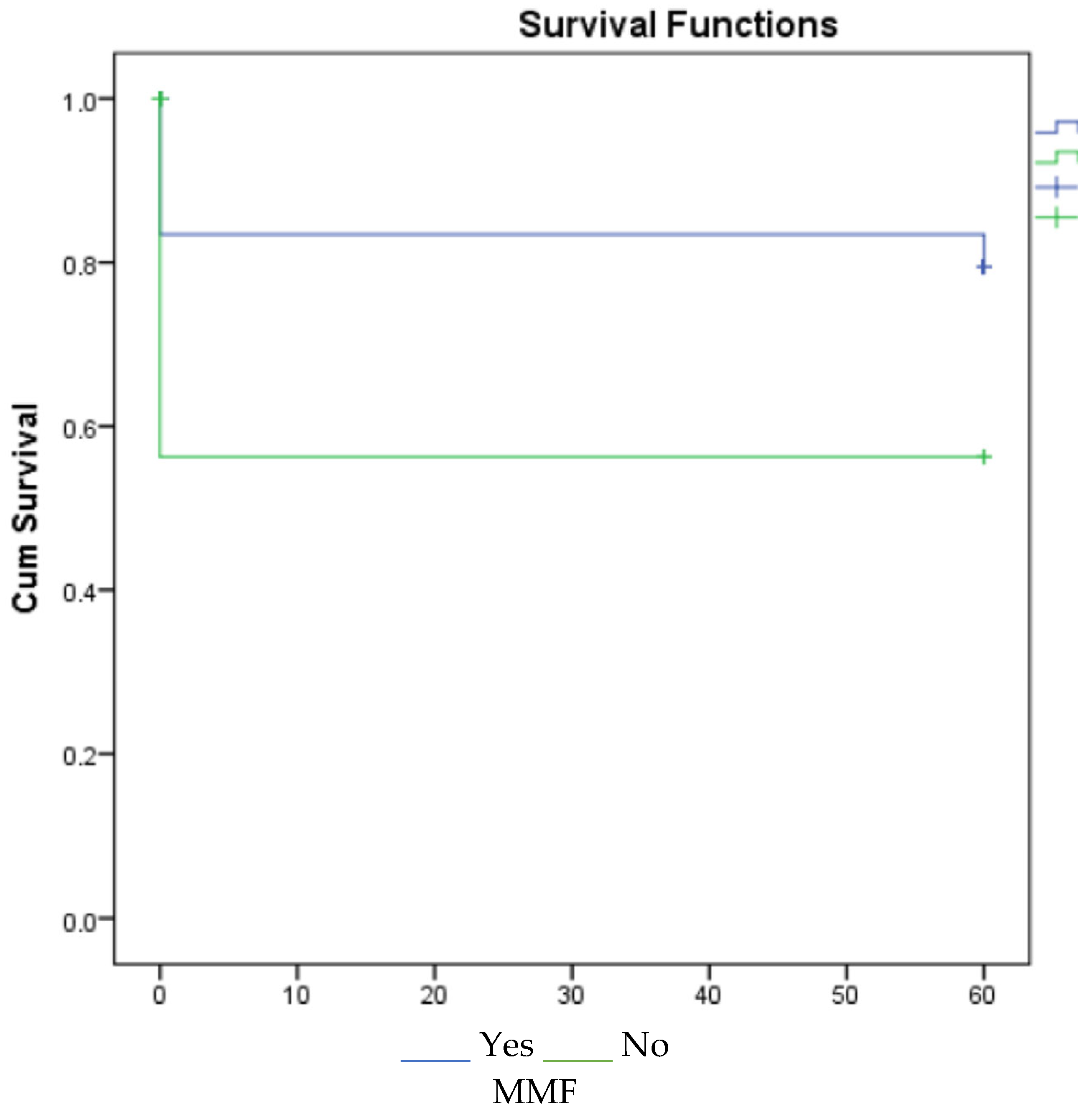
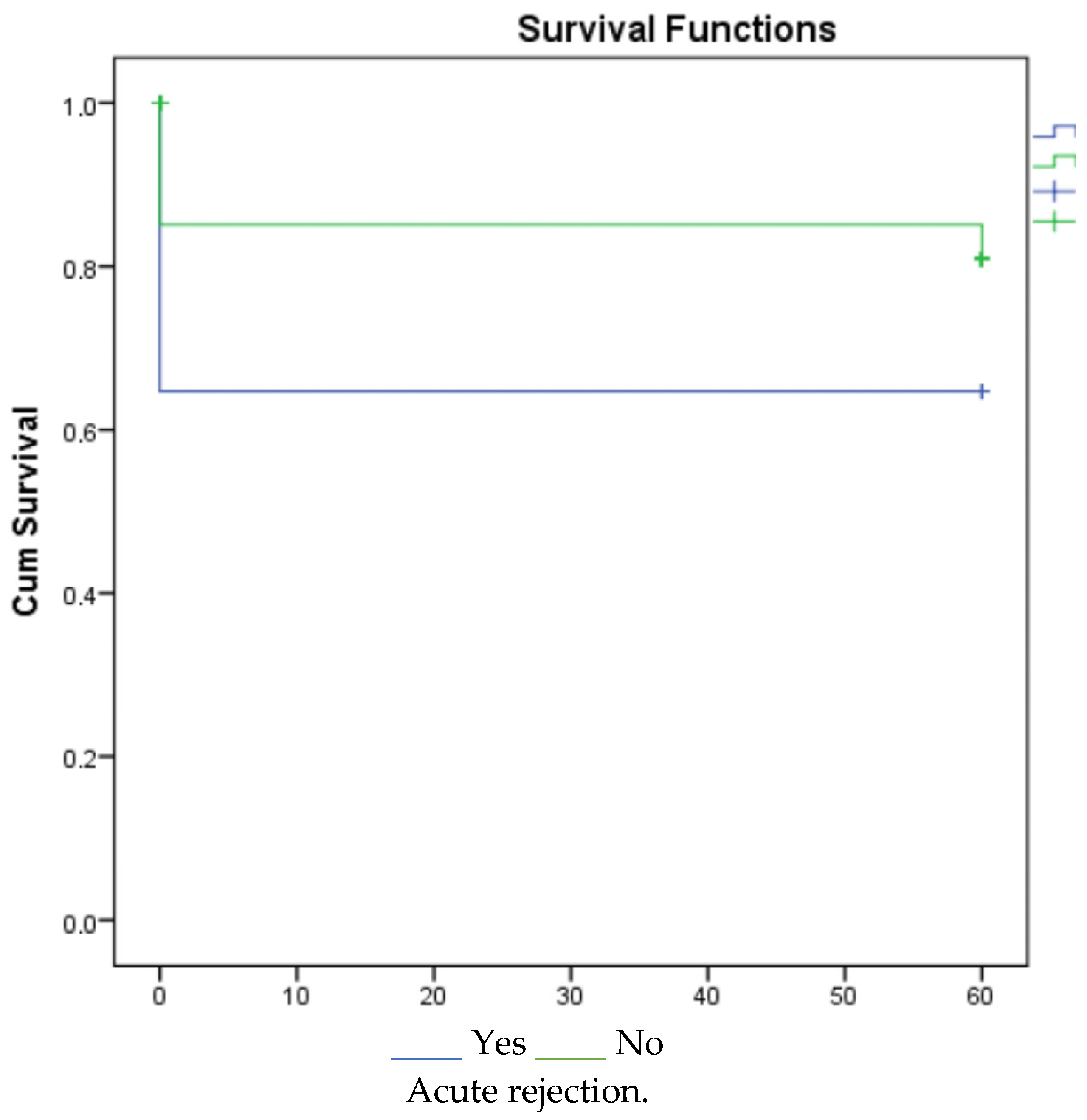
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. 2015, 5, 2–7. [Google Scholar]
- Noble, J.; Jouve, T.; Malvezzi, P.; Süsal, C.; Rostaing, L. Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives in Kidney Transplantation. Front. Immunol. 2020, 10, 3142. [Google Scholar]
- Wang, Z.; Durai, P.; Tiong, H.Y. Expanded criteria donors in deceased donor kidney transplantation—An Asian perspective. Indian J. Urol. 2020, 36, 89–94. [Google Scholar] [PubMed]
- Ko, K.J.; Kim, Y.H.; Kwon, K.H.; Kim, M.H.; Jun, K.W.; Hwang, J.K.; Kim, S.D.; Park, S.C.; Kim, J.I.; Yun, S.S.; et al. Kidney Transplantation Using Expanded-Criteria Deceased Donors: A Comparison With Ideal Deceased Donors and Non-Expanded-Criteria Deceased Donors. Transplant. Proc. 2018, 50, 3222–3227. [Google Scholar]
- Salguero, J.; Chamorro, L.; Gomez-Gomez, E.; Robles, J.E.; Campos, J.P. Midterm Outcomes of Kidney Transplantation from Expanded Criteria Donors After Circulatory Death: A Single-Center Retrospective Cohort Study. Exp. Clin. Transplant. 2023, 21, 481–486. [Google Scholar] [CrossRef]
- Oruç, A.; Ersoy, A.; Ayar, Y.; Akgür, S.; Yildiz, A. Exclusion Reasons of Cadaveric Kidney Transplantation Candidates. Turk. J. Nephrol. 2018, 27, 82–86. [Google Scholar]
- Çoban, Ş.; Yıldız, S.; Bozkaya, E.; Derici, Z.S.; Ünlü, M.; Çelik, A.; Sifil, A.; Cavdar, C.; Camsari, T. Evaluation of Morbidity and Patient and Graft Survival in Kidney Transplant Recipients: Experience of Dokuz Eylul University Hospital. Turk. J. Nephrol. 2017, 26, 41–47. [Google Scholar]
- Sexton, D.J.; O’Kelly, P.; Kennedy, C.; Denton, M.; de Freitas, D.G.; Magee, C.; O’Seaghdha, C.M.; Conlon, P.J. Assessing the discrimination of the Kidney Donor Risk Index/Kidney Donor Profile Index scores for allograft failure and estimated glomerular filtration rate in Ireland’s National Kidney Transplant Programme. Clin. Kidney J. 2019, 12, 569–573. [Google Scholar]
- Goussous, N.; De Leon, F.; Alghannam, K.; Howard, B.C.; Than, P.A.; Wang, A.X.; Sageshima, J.; Perez, R.V. Outcomes Using High KDPI Kidneys in Recipients Over 65 y of Age. Transpl. Direct. 2024, 10, e1738. [Google Scholar] [CrossRef]
- Lentine, K.L.; Smith, J.M.; Lyden, G.R.; Miller, J.M.; Dolan, T.G.; Bradbrook, K.; Larkin, L.; Temple, K.; Handarova, D.K.; Weiss, S.; et al. OPTN/SRTR 2022 Annual Data Report: Kidney. Am. J. Transpl. 2024, 24 (Suppl. S1), S19–S118. [Google Scholar] [CrossRef]
- Heemann, U.; Abramowicz, D.; Spasovski, G.; Vanholder, R.; European Renal Best Practice Work Group on Kidney Transplantation. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: A European Renal Best Practice (ERBP) position statement. Nephrol. Dial. Transpl. 2011, 26, 2099–2106. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern Med. 2007, 147, 573–577. [Google Scholar] [PubMed]
- Regıstry of the Nephrology, Dıalysıs and Transplantatıon in Turkey, Regıstry 2019 and 2020. In Mınıstry of Health and Turkısh Socıety of Nephrology Joınt Report; Suleymanlar, G., Ates, K., Seyahi, N., Kocyigit, I., Eds.; Turkish Society of Nephrology Registry Committee: Ankara, Turkey, 2020; pp. 52–53. [Google Scholar]
- Monteoliva, P.B.; Redondo-Pachón, D.; García, E.M.; Calabia, E.R. Kidney transplant outcome of expanded criteria donors after circulatory death. Nefrol. Engl. Ed. 2021, 42, 135–144. [Google Scholar]
- Ma, M.K.; Lim, W.H.; Craig, J.C.; Russ, G.R.; Chapman, J.R.; Wong, G. Mortality among Younger and Older Recipients of Kidney Transplants from Expanded Criteria Donors Compared with Standard Criteria Donors. Clin. J. Am. Soc. Nephrol. 2016, 11, 128–136. [Google Scholar] [PubMed]
- Kuhn, C.; Lang, B.M.; Lörcher, S.; Karolin, A.; Binet, I.; Beldi, G.; Golshayan, D.; Hadaya, K.; Mueller, T.F.; Schaub, S.; et al. Outcome of kidney transplantation from very senior donors in Switzerland—A national cohort study. Transpl. Int. 2021, 34, 689–699. [Google Scholar]
- Molinari, M.; Kaltenmeier, C.; Liu, H.; Ashwat, E.; Jorgensen, D.; Puttarajappa, C.; Wu, C.M.; Mehta, R.; Sood, P.; Shah, N.; et al. Function and longevity of renal grafts from high-KDPI donors. Clin. Transpl. 2022, 36, e14759. [Google Scholar]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am. J. Transpl. 2019, 19, 425–433. [Google Scholar]
- Arias-Cabrales, C.E.; Pérez-Sáez, M.J.; Redondo-Pachón, D.; Buxeda, A.; Burballa, C.; Duran, X.; Mir, M.; Crespo, M.; Pascual, J. Relevance of KDPI value and acute rejection on kidney transplant outcomes in recipients with delayed graft function—A retrospective study. Transpl. Int. 2020, 33, 1071–1077. [Google Scholar]
- Jalalzadeh, M.; Mousavinasab, N.; Peyrovi, S.; Ghadiani, M.H. The impact of acute rejection in kidney transplantation on long-term allograft and patient outcome. Nephro-Urol. Mon. 2015, 7, e24439. [Google Scholar]
- Peeters, L.E.J.; Andrews, L.M.; Hesselink, D.A.; de Winter, B.C.M.; van Gelder, T. Personalized immunosuppression in elderly renal transplant recipients. Pharmacol. Res. 2018, 130, 303–307. [Google Scholar]
- Diekmann, F.; Gutiérrez-Dalmau, A.; López, S.; Cofán, F.; Esforzado, N.; Ricart, M.J.; Rossich, E.; Saval, N.; Torregrosa, J.V.; Oppenheimer, F.; et al. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: Comparison of two CNI-free protocols. Nephrol. Dial. Transpl. 2007, 22, 2316–2321. [Google Scholar]
- Knight, S.R.; Russell, N.K.; Barcena, L.; Morris, P.J. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: A systematic review. Transplantation 2009, 87, 785–794. [Google Scholar] [PubMed]
- Su, V.C.H.; Greanya, E.D.; Ensom, M.H. Impact of Mycophenolate Mofetil Dose Reduction on Allograft Outcomes in Kidney Transplant Recipients on Tacrolimus-Based Regimens: A Systematic Review. Ann. Pharmacother. 2011, 45, 248–257. [Google Scholar] [PubMed]

| Donor Type | SCD (n = 92) | ECD (n = 63) | p |
|---|---|---|---|
| Gender (n, %) | |||
| Male | 63 (68.50%) | 38 (60.30%) | 0.381 |
| Female | 29 (31.50%) | 25 (39.70%) | |
| Age (n) | 41 (20–59) | 62 (42–86) | p < 0.001 |
| Donor height (cm) | 170 (150–196) | 170 (150–185) | 0.133 |
| Donor kg | 70 (50–115) | 75 (52–115) | 0.065 |
| Last urine (cc) | 180 (10–1000) | 100 (20–1400) | 0.023 |
| 24 h urine (cc) | 4255 (750–14,950) | 3200 (800–7500) | 0.030 |
| Donor BMI | 24.71 ± 3.24 | 26.96 ± 3.64 | p < 0.001 |
| DM in donor (n, %) | |||
| Yes | 0 (0%) | 27 (42.90%) | p < 0.001 |
| No | 92 (100%) | 36 (57.10%) | |
| HT in donor (n, %) | |||
| Yes | 8 (8.70%) | 59 (93.70%) | p < 0.001 |
| No | 84 (91.30%) | 4 (6.30%) | |
| Cause of death (n, %) | |||
| CVA | 62 (67.40%) | 60 (95.20%) | p < 0.001 |
| Trauma | 24 (26.10%) | 1 (1.60%) | |
| Other | 6 (6.50%) | 2 (3.20%) | |
| İntensive care Hospitalization (day) | 3 (1–21) | 3 (1–10) | 0.134 |
| Cold ischemia (hour) | 10 (1–21) | 12 (4–36) | 0.020 |
| HbsAg (n, %) | |||
| Positive | 2 (2.20%) | 3 (4.80%) | 0.397 |
| Negative | 90 (97.80%) | 60 (95.20%) | |
| antiHbs (n, %) | |||
| Positive | 39 (42.40%) | 53 (57.60%) | 0.241 |
| Negative | 20 (31.70%) | 43 (68.30%) | |
| Yes | 8 (8.70%) | 59 (93.70%) | p < 0.001 |
| No | 84 (91.30%) | 4 (6.30%) | |
| KDPI (%) | 56 (19–88) | 97 (65–100) | <0.001 |
| KDRI (%) | 1.06 (0.73–1.57) | 1.93 (1.16–3.50) | <0.001 |
| HLA mismatch (n) | 2 (1–6) | 2 (1–6) | 0.865 |
| Recipient Age (n) | SCD (n = 92) 42.50 ± 13.27 | ECD (n = 63) 42.84 ± 14.34 | 0.879 |
|---|---|---|---|
| HT in recipient (n, %) | |||
| Yes | 0 (0%) | 27 (42.90%) | p < 0.001 |
| No | 92 (100%) | 36 (57.10%) | |
| DM in recipient (n, %) | |||
| Yes | 19 (20.70%) | 16 (25.40%) | 0.618 |
| No | 73 (79.30%) | 47 (74.60%) | |
| Hospitalization after Tx (n, %) | |||
| UTI | 20 (21.70%) | 16 (25.40%) | 0.926 |
| Pneumonia | 25 (27.20%) | 15 (23.80%) | |
| Surgical complication | 11 (12%) | 9 (14.30%) | |
| Rejection | 8 (8.70%) | 6 (9.50%) | |
| Other | 8 (8.70%) | 3 (4.80%) | |
| None | 20 (21.70%) | 14 (22.20%) | |
| Graft loss (n, %) | |||
| Yes | 12 (13%) | 10 (16.10%) | 0.763 |
| No | 80 (87%) | 52 (83.90%) | |
| Survey | |||
| Alive | 76 (82.60%) | 48 (76.20%) | 0.162 |
| Rejection | 5 (5.40%) | 9 (14.30%) | |
| Death | 11 (12%) | 6 (9.50%) |
| SCD (n = 92) | ECD (n = 63) | p | |
|---|---|---|---|
| CNI (n, %) | |||
| Cyclosporine | 49 (53.30%) | 26 (41.30%) | 0.142 |
| Tacrolimus | 43 (46.70%) | 37 (58.70%) | |
| MMF (n, %) | |||
| Yes | 84 (91.30%) | 55 (87.30%) | 0.592 |
| No | 8 (8.70%) | 8 (12.70%) | |
| mTOR (n, %) | |||
| Yes | 6 (6.50%) | 6 (9.50%) | 0.549 |
| No | 86 (93.50) | 57 (90.50%) | |
| Primary non-functioning kidney (n, %) | |||
| Yes | 0 (0%) | 5 (7.90%) | 0.010 |
| No | 92 (100%) | 58 (92.10%) | |
| Acute rejection (n, %) | |||
| Yes | 19 (20.70%) | 15 (23.80%) | 0.788 |
| No | 73 (79.30%) | 48 (76.20%) | |
| DGF (n, %) | |||
| Yes | 13 (14.10%) | 14 (22.20%) | 0.276 |
| No | 79 (85.90%) | 49 (77.80%) | |
| ATN (n, %) | |||
| Yes | 31 (33.70%) | 20 (31.70%) | 0.936 |
| No | 61 (66.30%) | 43 (68.30%) |
| SCD (n = 92) | ECD (n = 63) | p | |
|---|---|---|---|
| Serum creatinine first month (mg/dL) | 1.30 (0.53–8.90) | 1.61 (0.46–14.80) | 0.014 |
| Serum creatinine sixth month * | 0.08 (−1:1.28) | −0.03 (−0.84:1.15) | 0.061 |
| Serum creatinine twelfth month * | −0.02 (−1:3.24) | 0.01 (−0.85:5.96) | 0.208 |
| Serum creatinine final* | −0.04 (−1:9.34) | 0 (−0.82:6.01) | 0.510 |
| GFR first month (ml/min/m2) | 57.67 (7–202) | 44 (3.57–202) | 0.005 |
| GFR sixth month * | −0.07 (−1:65.26) | 0.04 (−0.59:4.13) | 0.010 |
| GFR twelfth month * | −0.05 (−1:7.18) | −0.07 (−0.90:5.66) | 0.572 |
| GFR final month * | −0.07 (−1:6.73) | −0 (−0.90:4.77) | 0.445 |
| SCD (n = 92) | ECD (n = 63) | |
|---|---|---|
| Serum urea (mg/dL) | 42 (6–114) | 57 (15–137) |
| Serum creatinine (mg/dL) | 1.10 (0.47–6.20) | 1.30 (0.50–4.02) |
| Hgb (g/dL) | 11.10 (6.50–17.40) | 12.60 (7.42–15.50) |
| Wbc (103/µL) | 14.19 (1.10–29.20) | 14 (1.51–46) |
| Na (mmol/L) | 151.50 (12–199) | 153 (68–211) |
| K (mmol/L) | 3.69 (1.50–6.40) | 3.60 (2.40–4.90) |
| Post-transplant surgical complications | ||
| Lymphocele | 31 (33.70%) | 21 (33.30%) |
| Hernia | 1 (1.10%) | 3 (4.80%) |
| İleus | 2 (2.20%) | 0 (0%) |
| Other | 11 (12%) | 8 (12.70%) |
| None | 47 (51.10%) | 31 (49.20%) |
| Post-transplant medical complications | ||
| Drug | 3 (3.30%) | 3 (4.80%) |
| Hyperparathyroidism (secondary or tertiary) | 2 (2.20%) | 1 (1.60%) |
| TXGMP | 1 (1.10%) | 0 (0%) |
| AVN | 9 (9.80%) | 2 (3.20%) |
| None | 77 (83.70%) | 57 (90.50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayar, Y.; Ersoy, A.; Isiktas Sayilar, E.; Yildiz, A.; Can, F.E.; Oruc, A. Utility of Deceased Expanded-Criteria Donors in Kidney Transplantation: A Single-Center Experience. J. Clin. Med. 2025, 14, 3232. https://doi.org/10.3390/jcm14093232
Ayar Y, Ersoy A, Isiktas Sayilar E, Yildiz A, Can FE, Oruc A. Utility of Deceased Expanded-Criteria Donors in Kidney Transplantation: A Single-Center Experience. Journal of Clinical Medicine. 2025; 14(9):3232. https://doi.org/10.3390/jcm14093232
Chicago/Turabian StyleAyar, Yavuz, Alparslan Ersoy, Emel Isiktas Sayilar, Abdülmecit Yildiz, Fatma Ezgi Can, and Aysegul Oruc. 2025. "Utility of Deceased Expanded-Criteria Donors in Kidney Transplantation: A Single-Center Experience" Journal of Clinical Medicine 14, no. 9: 3232. https://doi.org/10.3390/jcm14093232
APA StyleAyar, Y., Ersoy, A., Isiktas Sayilar, E., Yildiz, A., Can, F. E., & Oruc, A. (2025). Utility of Deceased Expanded-Criteria Donors in Kidney Transplantation: A Single-Center Experience. Journal of Clinical Medicine, 14(9), 3232. https://doi.org/10.3390/jcm14093232






