The Enhanced Recovery After Surgery Pathway Is Safe, Feasible and Cost-Effective in Delayed Graft Function After Kidney Transplant
Abstract
1. Introduction
2. Materials and Methods
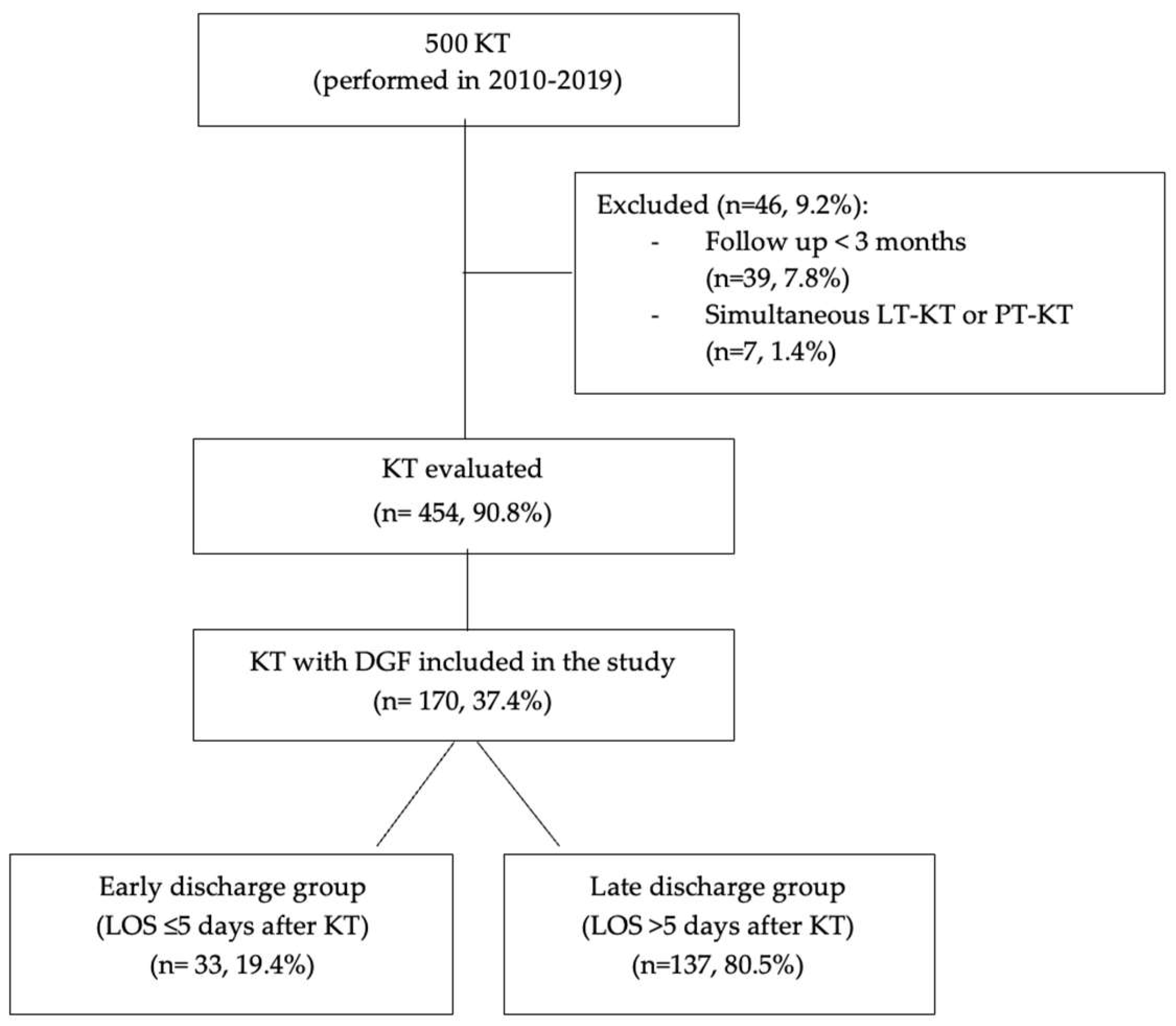
2.1. Study Design
2.2. Data Collection
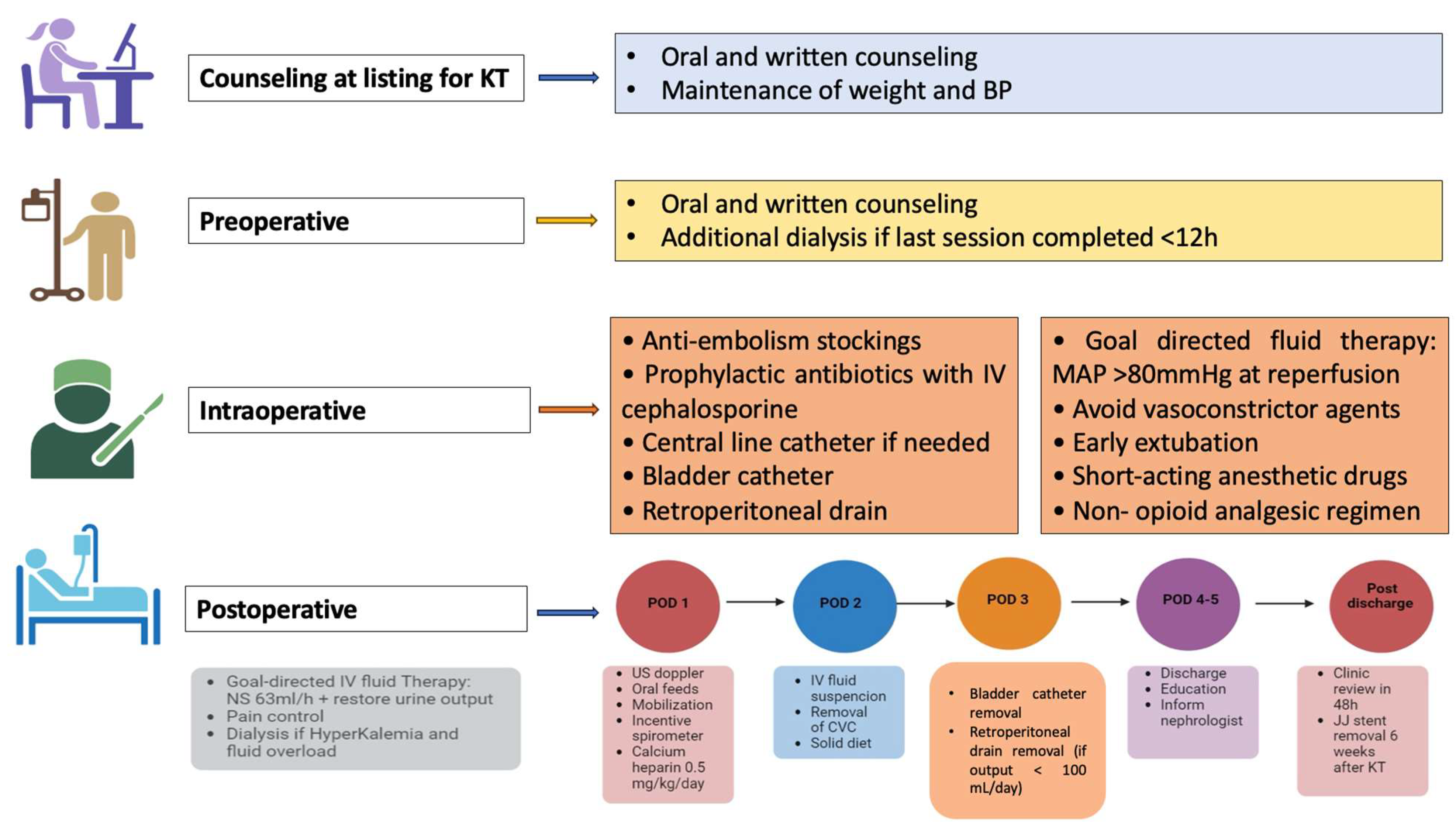
2.3. ERAS Pathway
2.4. Immunosuppressive Therapy
2.5. Outcomes
2.6. Cost Appraisal
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Postoperative Outcomes
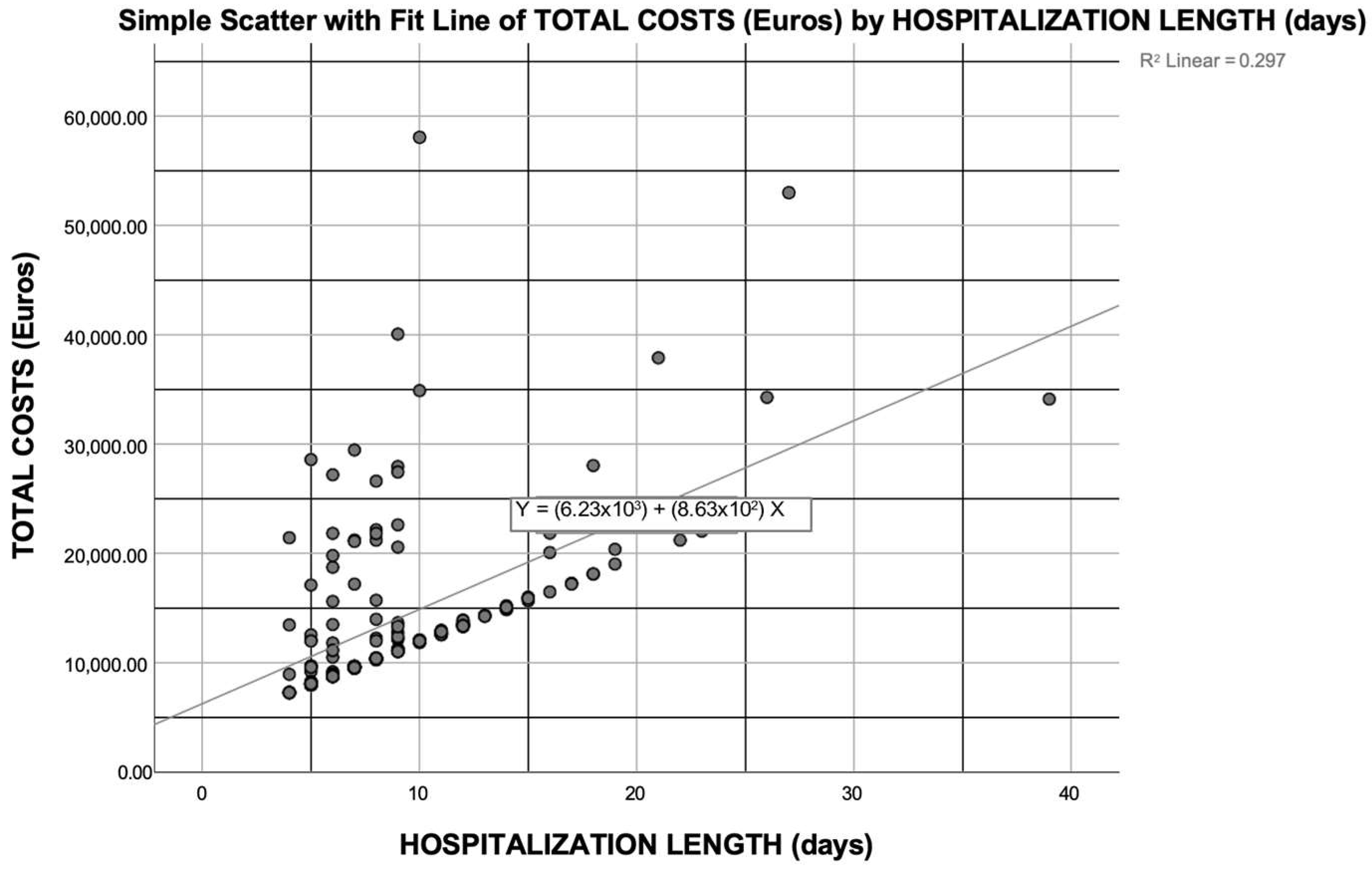
3.3. Cost Analysis of KT Recipients with DGF
3.4. First-Year Post-KT Cost Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERAS | Enhanced recovery after surgery |
| KT | Kidney transplantation |
| DGF | Delayed graft function |
| LOS | Length of hospital stay |
| ECDs | Expanded-criteria donors |
| BMI | Body mass index |
| ESKD | End-stage kidney disease |
| CIT | Cold ischemia time |
| PRA | Panel-reactive antibody |
| CNIs | Calcineurin inhibitors |
| VAT | Value-added tax |
| ADPKD | Autosomal dominant polycystic kidney disease |
| EUR | Euros |
References
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [PubMed]
- Pędziwiatr, M.; Mavrikis, J.; Witowski, J.; Adamos, A.; Major, P.; Nowakowski, M.; Budzyński, A. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med. Oncol. 2018, 35, 95. [Google Scholar] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2019, 43, 659–695. [Google Scholar] [PubMed]
- Greco, M.; Capretti, G.; Beretta, L.; Gemma, M.; Pecorelli, N.; Braga, M. Enhanced Recovery Program in Colorectal Surgery: A Meta-analysis of Randomized Controlled Trials. World J. Surg. 2014, 38, 1531–1541. [Google Scholar]
- ERAS Society (2022). Available online: https://erassociety.org/guidelines/ (accessed on 31 December 2024).
- Halawa, A.; Rowe, S.; Roberts, F.; Nathan, C.; Hassan, A.; Kumar, A.; Suvakov, B.; Edwards, B.; Gray, C. A Better Journey for Patients, a Better Deal for the NHS: The Successful Implementation of an Enhanced Recovery Program After Renal Transplant Surgery. Exp. Clin. Transpl. 2018, 16, 127–132. [Google Scholar]
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar]
- McAdams-DeMarco, M.A.; King, E.A.; Luo, X.; Haugen, C.; DiBrito, S.; Shaffer, A.; Kucirka, L.M.; Desai, N.M.; Dagher, N.N.; Lonze, B.E.; et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann. Surg. 2017, 266, 1084–1090. [Google Scholar]
- Bahl, D.; Haddad, Z.; Datoo, A.; Qazi, Y.A. Delayed graft function in kidney transplantation. Curr. Opin. Organ. Transpl. 2019, 24, 82–86. [Google Scholar]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transpl. 2020, 34, e13814. [Google Scholar]
- Formica, R.N., Jr. A critical assessment on kidney allocation systems. Transpl. Rev. 2017, 31, 61–67. [Google Scholar]
- Audard, V.; Matignon, M.; Dahan, K.; Lang, P.; Grimbert, P. Renal transplantation from extended criteria cadaveric donors: Problems and perspectives overview. Transpl. Int. 2008, 21, 11–17. [Google Scholar] [PubMed]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Delayed Graft Function in Kidney Transplant: Risk Factors, Consequences, and Prevention Strategies. J. Pers. Med. 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Mezzolla, V.; Pontrelli, P.; Fiorentino, M.; Stasi, A.; Pesce, F.; Franzin, R.; Rascio, F.; Grandaliano, G.; Stallone, G.; Infante, B.; et al. Emerging biomarkers of delayed graft function in kidney transplantation. Transpl. Rev. 2021, 35, 100629. [Google Scholar]
- Dumbill, R.; Jaques, R.; Robb, M.; Johnson, R.; Ploeg, R.J.; Kaisar, M.E.; Sharples, E.J. Transplant and Recipient Factors in Prediction of Kidney Transplant Outcomes: A UK-Wide Paired Analysis. J. Clin. Med. 2022, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Golder, H.J.; Papalois, V. Enhanced Recovery after Surgery: History, Key Advancements and Developments in Transplant Surgery. J. Clin. Med. 2021, 10, 1634. [Google Scholar] [CrossRef]
- Kruszyna, T.; Niekowal, B.; Kraśnicka, M.; Sadowski, J. Enhanced Recovery After Kidney Transplantation Surgery. Transpl. Proc. 2016, 48, 1461–1465. [Google Scholar]
- Espino, K.A.; Narvaez, J.R.F.; Ott, M.C.; Kayler, L.K. Benefits of multimodal enhanced recovery pathway in patients undergoing kidney transplantation. Clin. Transplant. 2018, 32, e13173. [Google Scholar]
- Dias, B.H.; Rana, A.A.M.; Olakkengil, S.A.; Russell, C.H.; Coates, P.T.H.; Clayton, P.A.; Bhattacharjya, S. Development and implementation of an enhanced recovery after surgery protocol for renal transplantation. ANZ J. Surg. 2019, 89, 1319–1323. [Google Scholar]
- Elsabbagh, A.M.; Ghoneim, I.; Moiz, A.; Welch, K.; Brown, J.S. Enhanced Recovery After Surgery Pathway in Kidney Transplantation: The Road Less Traveled. Transpl. Direct. 2022, 8, e1333. [Google Scholar]
- Angelico, R.; Romano, F.; Riccetti, C.; Pellicciaro, M.; Toti, L.; Favi, E.; Cacciola, R.; Manzia, T.M.; Tisone, G. The Enhanced Recovery after Surgery (ERAS) Pathway Is a Safe Journey for Kidney Transplant Recipients during the “Extended Criteria Donor” Era. Pathogens 2022, 11, 1193. [Google Scholar] [CrossRef]
- Quinino, R.M.E.; Agena, F.; Paula, F.J.; Nahas, W.C.; David-Neto, E. Comparative analysis of kidney transplant costs related to recovery of renal function after the procedure. J. Bras. Nefrol. 2021, 43, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Tsapepas, D.; King, K.L.; Husain, S.A.; Corvino, F.A.; Dillon, A.; Wang, W.; Mayne, T.J.; Mohan, S. Financial impact of delayed graft function in kidney transplantation. Clin. Transpl. 2020, 34, e14022. [Google Scholar] [CrossRef]
- Ojo, A.O.; Wolfe, R.A.; Held, P.J.; Port, F.K.; Schmouder, R.L. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 1997, 63, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transpl. 2011, 11, 2279–2296. [Google Scholar] [CrossRef]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, J.; Lajoie, G.; Cattran, D.; Fenton, S.; Zaltzman, J.; Cardella, C.; Cole, E. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation 1999, 67, 1162–1167. [Google Scholar] [CrossRef]
- Remuzzi, G.; Cravedi, P.; Perna, A.; Dimitrov, B.D.; Turturro, M.; Locatelli, G.; Rigotti, P.; Baldan, N.; Beatini, M.; Valente, U.; et al. Long-term outcome of renal transplantation from older donors. N. Engl. J. Med. 2006, 354, 343–352. [Google Scholar] [CrossRef]
- Available online: https://www.agenziaentrate.gov.it/portale/web/guest/iva-regole-generali-aliquote-esenzioni-pagamento/norme-generali-e-aliquote (accessed on 31 December 2024).
- Melih, K.V.; Boynuegri, B.; Mustafa, C.; Nilgun, A. Incidence, Risk Factors, and Outcomes of Delayed Graft Function in Deceased Donor Kidney Transplantation. Transpl. Proc. 2019, 51, 1096–1100. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Hippen, B.; Gill, J.; Heilman, R.; Stewart, D.; Reddy, K.S.; Mohan, S.; Wiseman, A.; Cooper, M. Current opinions on DGF management practices: A survey of the United States and Canada. Clin. Transpl. 2023, 37, e14949. [Google Scholar] [CrossRef]
- Phillips, B.L.; Ibrahim, M.; Greenhall, G.H.B.; Mumford, L.; Dorling, A.; Callaghan, C.J. Effect of delayed graft function on longer-term outcomes after kidney transplantation from donation after circulatory death donors in the United Kingdom: A national cohort study. Am. J. Transpl. 2021, 21, 3346–3355. [Google Scholar]
- Budhiraja, P.; Reddy, K.S.; Butterfield, R.J.; Jadlowiec, C.C.; Moss, A.A.; Khamash, H.A.; Kodali, L.; Misra, S.S.; Heilman, R.L. Duration of delayed graft function and its impact on graft outcomes in deceased donor kidney transplantation. BMC Nephrol. 2022, 23, 154. [Google Scholar]
- Tan, J.H.S.; Bhatia, K.; Sharma, V.; Swamy, M.; van Dellen, D.; Dhanda, R.; Khambalia, H. Enhanced recovery after surgery recommendations for renal transplantation: Guidelines. Br. J. Surg. 2022, 110, 57–59. [Google Scholar]
- Massie, A.B.; Luo, X.; Chow, E.K.; Alejo, J.L.; Desai, N.M.; Segev, D.L. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am. J. Transpl. 2014, 14, 2310–2316. [Google Scholar]
- Bizard, F.; Boudemaghe, T.; Delaunay, L.; Léger, L.; Slim, K. Medico-economic impact of enhanced rehabilitation after surgery: An exhaustive, nation-wide claims study. BMC Health Serv. Res. 2021, 21, 1341. [Google Scholar]
- Stone, A.B.; Grant, M.C.; Pio Roda, C.; Hobson, D.; Pawlik, T.; Wu, C.L.; Wick, E.C. Implementation Costs of an Enhanced Recovery After Surgery Program in the United States: A Financial Model and Sensitivity Analysis Based on Experiences at a Quaternary Academic Medical Center. J. Am. Coll. Surg. 2016, 222, 219–225. [Google Scholar]
- Stowers, M.D.; Lemanu, D.P.; Hill, A.G. Health economics in Enhanced Recovery After Surgery programs. Can. J. Anaesth. 2015, 62, 219–230. [Google Scholar] [CrossRef]
- Helanterä, I.; Isola, T.; Lehtonen, T.K.; Åberg, F.; Lempinen, M.; Isoniemi, H. Association of Clinical Factors with the Costs of Kidney Transplantation in the Current Era. Ann. Transpl. 2019, 24, 393–400. [Google Scholar]
- Axelrod, D.A.; Schnitzler, M.A.; Xiao, H.; Irish, W.; Tuttle-Newhall, E.; Chang, S.-H.; Kasiske, B.L.; Alhamad, T.; Lentine, K.L. An economic assessment of contemporary kidney transplant practice. Am. J. Transpl. 2018, 18, 1168–1176. [Google Scholar]
- Cheng, X.S.; Han, J.; Braggs-Gresham, J.L.; Held, P.J.; Busque, S.; Roberts, J.P.; Tan, J.C.; Scandling, J.D.; Chertow, G.M.; Dor, A. Trends in Cost Attributable to Kidney Transplantation Evaluation and Waiting List Management in the United States, 2012–2017. JAMA Netw. Open 2022, 5, e221847. [Google Scholar] [CrossRef]
- Loubeau, P.R.; Loubeau, J.M.; Jantzen, R. The economics of kidney transplantation versus hemodialysis. Prog. Transpl. 2001, 11, 291–297. [Google Scholar]
- Ellimoottil, C.; Ye, Z.; Chakrabarti, A.K.; Englesbe, M.J.; Miller, D.C.; Wei, J.T.; Mathur, A.K. Understanding Inpatient Cost Variation in Kidney Transplantation: Implications for Payment Reforms. Urology 2016, 87, 88–94. [Google Scholar] [PubMed]

| Variables | Overall Population (n = 170) | Early Discharge Group (n = 33, 19.4%) | Late Discharge Group (n = 137, 80.5%) | p Value |
|---|---|---|---|---|
| n (%) or Median (Sample Min–Max) | n (%) or Median (Sample Min–Max) | n (%) or Median (Sample Min–Max) | ||
| Recipient | ||||
| Age (years) | 58 (25–75) | 54 (34–75) | 63 (29–74) | 0.162 |
| Age > 60 years | 81 (47.6%) | 12 (36.3%) | 69 (50.4%) | 0.176 |
| Gender (male) | 111 (65.2%) | 23 (69.7%) | 88 (64.2%) | 0.685 |
| BMI | 24 (16–35) | 24 (17–35) | 25 (18–32) | 0.673 |
| Obesity (BMI ≥30) | 24 (14.1%) | 7 (21.2%) | 17 (12.4%) | 0.262 |
| Cause of ESKD: | 0.598 | |||
| -Glomerulonephritis | 58 (34.1%) | 15 (45.5%) | 43 (31.2%) | |
| -ADPKD | 40 (23.5%) | 7 (21.2%) | 33 (24.1%) | |
| -Arterial hypertension | 19 (11.1%) | 2 (6.1%) | 17 (12.4%) | |
| -Pyelonephritis | 10 (5.8%) | 2 (6.1%) | 8 (5.8%) | |
| -Unknown ESRD | 14 (8.2%) | 4 (12.1%) | 10 (7.3%) | |
| -Diabetes | 12 (7.06%) | 2 (6.1%) | 10 (7.3%) | |
| -Congenital malformation | 4 (2.3%) | 0 (0%) | 4 (2.9%) | |
| -Other causes * | 13 (7.6%) | 1 (3%) | 12 (8.8%) | |
| Median time on waiting list (days) | 807 (13–3821) | 988 (83–2816) | 611 (13–3185) | 0.152 |
| Comorbidities: | 83 (48.8%) | 12 (36.3%) | 71 (51.8%) | 0.124 |
| -Arterial hypertension | 49 (28.8%) | 6 (18.1%) | 43 (35.5%) | 0.198 |
| -Cardiovascular diseases | 34 (20%) | 4 (12.1%) | 30 (21.9%) | 0.237 |
| -DMII | 17 (10%) | 5 (15.1%) | 12 (8.8%) | 0.329 |
| -Comorbidities ≥ 2 | 12 (7.05%) | 2 (6.06%) | 10 (7.3%) | 1.000 |
| Donor | ||||
| Type of donor: | 1.000 | |||
| -Brain dead donor | 168 (98.8%) | 33 (100%) | 135 (98.5%) | |
| -Living donor | 2 (1.2%) | 0 (0%) | 2 (1.5%) | |
| Age (years) | 59 (15–83) | 54 (34–76) | 62 (27–83) | 0.289 |
| Age > 60 years | 82 (48.2%) | 12(36.4%) | 70 (51.1%) | 0.174 |
| Cause of death: | 0.298 | |||
| -Cerebral hemorrhage | 108 (63.5%) | 21 (63.6%) | 87 (63.5%) | |
| -Head trauma | 33 (19.4%) | 3 (9.1%) | 30 (21.9%) | |
| -Ischemic stroke | 15 (8.8%) | 5 (15.2%) | 10 (7.3%) | |
| -Anoxic encephalopathy | 10 (5.8%) | 3 (9.1%) | 7 (5.1%) | |
| -Other causes | 4 (2.3%) | 1 (3%) | 3 (2.2%) | |
| Comorbidities: | ||||
| -Cardiovascular disease | 41 (24.1%) | 9 (27.3%) | 32 (23.4%) | 0.654 |
| -Arterial hypertension | 76 (44.7%) | 17 (51.5%) | 59 (43.1%) | 0.437 |
| -≥2 comorbidities | 33 (19.4%) | 5 (15.2%) | 28 (20.4%) | 0.627 |
| ECD | 106 (62.3%) | 19 (57.6%) | 87 (63.5%) | 0.553 |
| Transplant | ||||
| Type of KT: | 0.186 | |||
| -Single KT | 163 (95.8%) | 33(100%) | 130 (94.9%) | |
| -Dual KT: unilateral/bilateral | 7 (4.1%): 4 (2.3%)/3 (1.7%) | 0 (0%) | 7 (5.1%) 4 (2.9%)/3 (2.2%) | |
| Re-transplant | 20 (11.7%) | 6 (18.2%) | 14 (10.2%) | 0.204 |
| Sequential KT after LT | 1 (0.5%) | 0 (0%) | 1 (0.7%) | 0.624 |
| Pre-implant renal biopsy: | 99 (58.2%) | 19 (57.6%) | 80 (58.4%) | 0.932 |
| -Renal biopsy score ≤ 3 | 56 (32.9%) | 9 (27.2%) | 47 (34.3%) | 0.443 |
| -Renal biopsy score > 3 | 43 (25.2%) | 10 (3.04%) | 33(24.08%) | 0.371 |
| Median CIT (hours) | 9 (1–29 | 8.3 (2.06–15.5) | 9.6 (1–23) | 0.060 |
| CIT ≥ 10 h | 69 (40.5%) | 11 (33.3%) | 58 (42.3%) | 0.431 |
| Variables | Overall Population (n = 170) | Early Discharge Group (n = 33, 19.4%) | Late Discharge Group (n = 137, 80.5%) | p Value |
|---|---|---|---|---|
| n (%) or Median (Sample Min–Max) | n (%) or Median (Sample Min–Max) | n (%) or Median (Sample Min–Max) | ||
| Outcomes | ||||
| LOS (days) | 8 (4–39) | 5 (4–5) | 9 (6–39) | <0.001 |
| Postoperative in-hospital dialytic sessions during first hospitalization | 3 (1–9) | 2 (0–3) | 3 (1–9) | <0.001 |
| KT recipients who had a single postoperative (in-hospital) dialysis session within 3 months after KT | 20 (11.7%) | 7 (21%) | 13 (9.7%) | 0.07 |
| Postoperative dialytic sessions at peripheral center within 3 months after discharge | 1 (0–12) | 1 (0–9) | 1 (0–12) | 0.451 |
| KT recipients who had ≤4 dialysis sessions at peripheral center within 3 months after KT | 161 (94.7%) | 31 (93%) | 130 (97.7%) | 0.258 |
| Early complications (≤3 months): | 52 (30.5%) | 11 (33.3%) | 41 (29.9%) | 0.680 |
| -Infectious | 46 (27%) | 8 (24.2%) | 38 (27.7%) | 0.828 |
| -Urological | 12 (7%) | 6 (18.2%) | 6 (4.4%) | 0.013 |
| -Vascular | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Late complications (4–12 months): | 66 (38.8%) | 14 (42.4%) | 52 (37.9%) | 0.692 |
| -Infectious | 59(34.7%) | 10 (30.3%) | 49 (35.7%) | 0.685 |
| -Urological | 16 (9.4%) | 7 (21.2%) | 9 (6.56%) | 0.017 |
| -Vascular | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Reoperations (after KT): | ||||
| -Early (≤3 months) | 2 (1.1%) | 1 (3%) | 1 (0.7%) | 0.351 |
| -Late (4–12 months) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Transplant nephrectomy: | ||||
| -Early (≤3 months) | 1 (0.5%) | 0 (0%) | 1 (0.7%) | 1 |
| -Late (4–12 months) | 3 (1.7%) | 1 (3%) | 2 (1.4%) | 0.479 |
| Interventional radiology procedures: | ||||
| 8 (4.7%) | 1 (3%) | 7 (5.1%) | 1 |
| 5 (2.9%) | 0 (0%) | 5 (3.6%) | 0.584 |
| 4 (2.3%) | 1 (3%) | 3 (2.1%) | 0.582 |
| 8 (4.7%) | 4 (12.1%) | 4 (2.9%) | 0.046 |
| 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| 8 (4.7%) | 4 (12.1%) | 4 (2.9%) | 0.046 |
| 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Readmission rates after KT: | 79 (46.4%) | 10 (30.3%) | 69 (50.3%) | 0.051 |
| -Early (≤3 months) | 61 (35.8%) | 9 (27.2%) | 52 (38%) | 0.313 |
| -Late (4–12 months) | 18 (10.5%) | 1 (3%) | 17 (12.4%) | 0.203 |
| Time of readmission since KT (days) | 8 (1–352) | 1 (0–3) | 48 (2–352) | <0.001 |
| Outpatient clinic reviews within 3 months after KT | 7 (2–16) | 8 (3–15) | 7 (2–16) | 0.359 |
| Cost Variables | Early Discharge Group (n = 33, 19.4%) | Late Discharge Group (n = 137, 80.5%) | ||
|---|---|---|---|---|
| Median (Sample Min–Max) | Median (Sample Min–Max) | Δ | p Value | |
| Costs of KT hospital admission | EUR 3870.12 (3104.12–3961.1) | EUR 6974.24 (4636.1–29,994.3) | EUR 3104.12 | <0.001 |
| EUR 3965.8 (3965.8–3965.8) | EUR 3965.8 (3965.8–3965.8) | EUR 0 | 1 |
| EUR 3830 (3064–3830) | EUR 6894 (4596–29,874) | EUR 3064 | <0.001 |
| EUR 80.24 (40.12–120.36) | EUR 120.36 (40.12–361.08) | EUR 40.12 | <0.001 |
| EUR 0 (0–508.32) | EUR 0 (0–508.32) | EUR 0 | 0.293 |
| Costs incurred after hospital admission for KT within one year of KT | EUR 228.72 (92.88–20,737.36) | EUR 163.96 (30.96–46,275.28) | EUR −64.76 | 0.207 |
| EUR 0 (0–3947.8) | EUR 0 (0–3947.8) | EUR 0 | 0.381 |
| EUR 0 (0–19,916) | EUR 0 (0–45,960) | EUR 0 | 0.886 |
| EUR 0 (0–641.92) | EUR 0 (320.96) | EUR 0 | 0.076 |
| EUR 40.12 (0–361.08) | EUR 40.12 (481.44)) | EUR 0 | 0.451 |
| EUR 0 (0–1485.12) | EUR 0 (0–2473.32) | EUR 0 | 0.414 |
| EUR 123.8 (46.4–232.2) | EUR 108.36 (30.9–247.6) | EUR −16 | 0.359 |
| Total costs of the first year after KT | EUR 8104.76 (7218.4–28,573.2) | EUR 12,620.52 (8725.7–58,061.5) | EUR 4515.76 | <0.001 |
| EUR 3965.8 (3965.8–7913.8) | EUR 3965.8 (3965.8–7913.8) | EUR 0 | 0.381 |
| EUR 3830 (3064–23,746) | EUR 8426 (4596–53,620) | EUR 4596 | <0.001 |
| EUR 80.24 (40.12–682.04) | EUR 120.36 (40.12–481.44) | EUR 40.12 | 0.003 |
| EUR 40.12 (0–361.08) | EUR 40.12 (0–481.44) | EUR 0 | 0.451 |
| EUR 0 (0–1485.12) | EUR 0 (0–2473.32) | EUR 0 | 0.243 |
| EUR 40.12 (0–361.08) | EUR 40.12 (0–481.44) | EUR 0 | 0.451 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, F.; Angelico, R.; Toti, L.; Orsi, M.; Marsella, V.E.; Manzia, T.M.; Emberti Gialloreti, L.; Tisone, G. The Enhanced Recovery After Surgery Pathway Is Safe, Feasible and Cost-Effective in Delayed Graft Function After Kidney Transplant. J. Clin. Med. 2025, 14, 2387. https://doi.org/10.3390/jcm14072387
Romano F, Angelico R, Toti L, Orsi M, Marsella VE, Manzia TM, Emberti Gialloreti L, Tisone G. The Enhanced Recovery After Surgery Pathway Is Safe, Feasible and Cost-Effective in Delayed Graft Function After Kidney Transplant. Journal of Clinical Medicine. 2025; 14(7):2387. https://doi.org/10.3390/jcm14072387
Chicago/Turabian StyleRomano, Francesca, Roberta Angelico, Luca Toti, Michela Orsi, Valentina Enrica Marsella, Tommaso Maria Manzia, Leonardo Emberti Gialloreti, and Giuseppe Tisone. 2025. "The Enhanced Recovery After Surgery Pathway Is Safe, Feasible and Cost-Effective in Delayed Graft Function After Kidney Transplant" Journal of Clinical Medicine 14, no. 7: 2387. https://doi.org/10.3390/jcm14072387
APA StyleRomano, F., Angelico, R., Toti, L., Orsi, M., Marsella, V. E., Manzia, T. M., Emberti Gialloreti, L., & Tisone, G. (2025). The Enhanced Recovery After Surgery Pathway Is Safe, Feasible and Cost-Effective in Delayed Graft Function After Kidney Transplant. Journal of Clinical Medicine, 14(7), 2387. https://doi.org/10.3390/jcm14072387











