Systematic Review on Upper Urinary Tract Carcinoma in Kidney Transplant Recipients
Abstract
1. Introduction
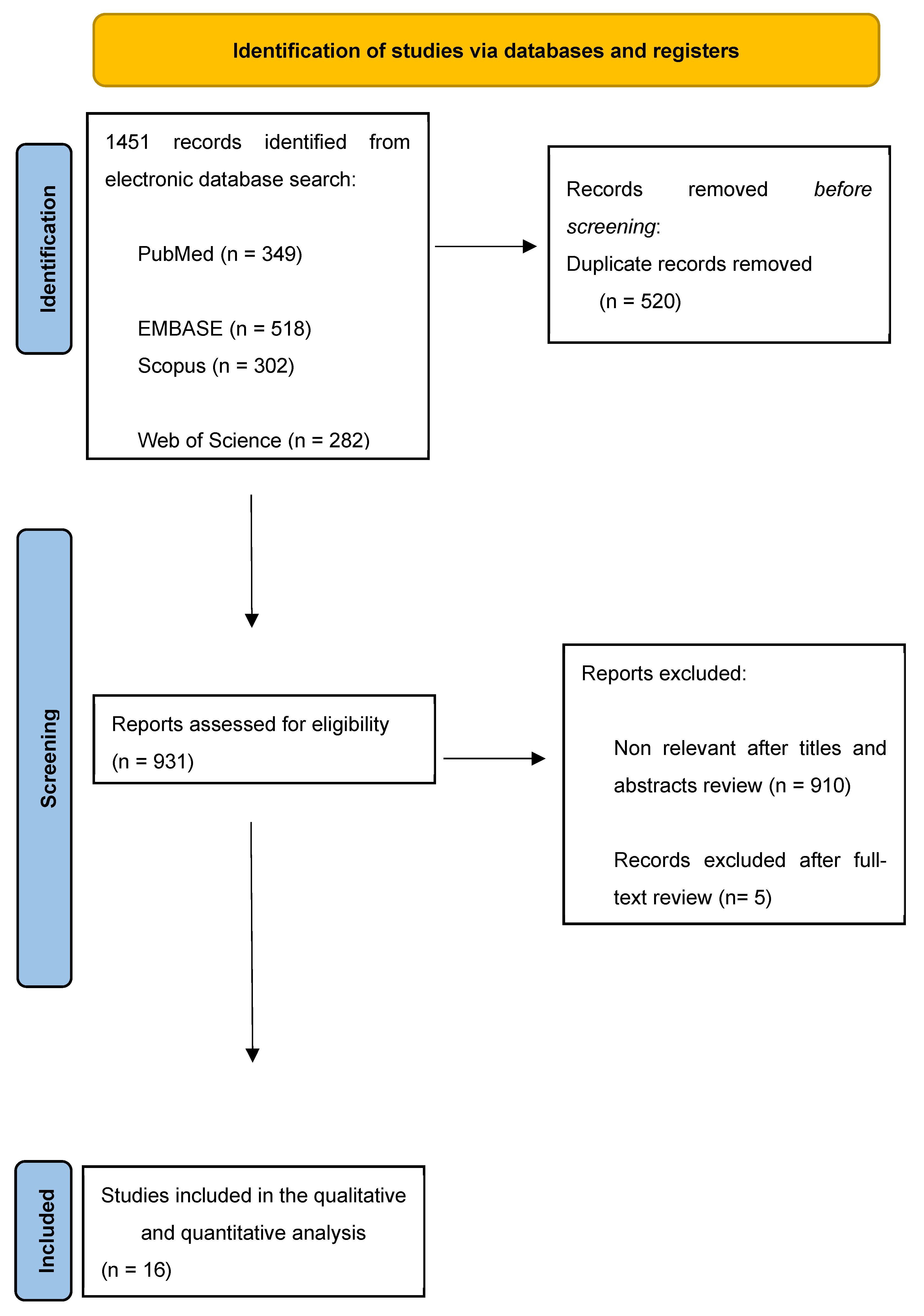
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
- (P): adults (age > 18 yrs) previously submitted to KT, diagnosed with de novo UTUC after KT;
- (I): radical nephroureterectomy or endoscopic treatment
- (C): either comparative or noncomparative studies;
- (O): overall survival, cancer-specific survival;
- (S): prospective or retrospective studies.
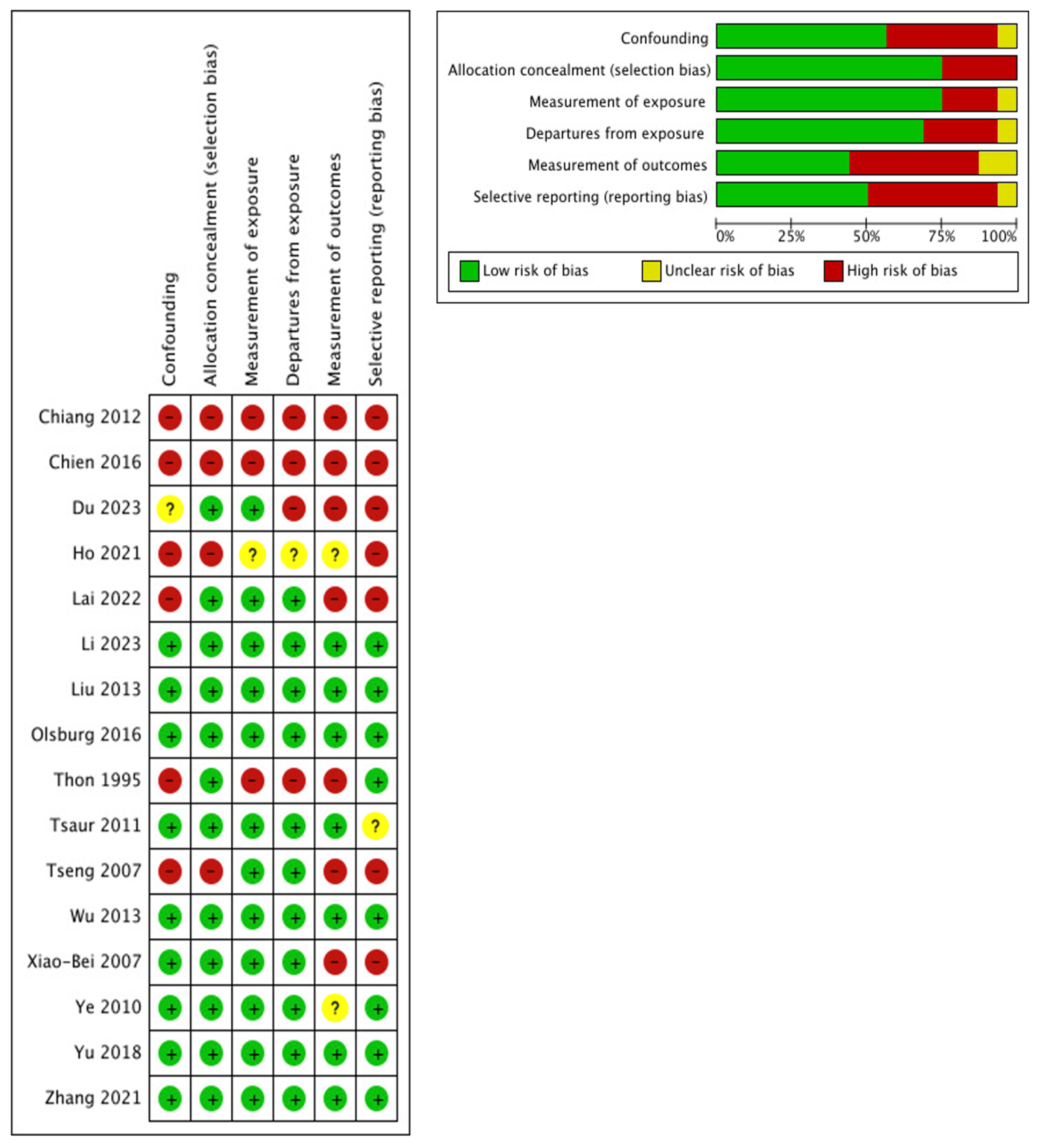
2.3. Risk-of-Bias Assessment
2.4. Data Extraction and Analysis
3. Evidence Synthesis
3.1. Risk of Bias and Confounding Assessment
3.2. Study Characteristics
3.3. Tumor Characteristics
3.4. Treatment Approaches
3.5. Follow-Up and Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hickman, L.A.; Sawinski, D.; Guzzo, T.; Locke, J.E. Urologic malignancies in kidney transplantation. Am. J. Transplant. 2018, 18, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zheng, M.; Wang, Z.; Zhang, J.; Lin, J.; Zhang, L.; Tian, Y.; Zhu, Y. Clinical characteristics and treatment outcomes of kidney transplant recipients with de novo urothelial carcinoma: Thirty years of experience from a single center. BMC Urol. 2023, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liu, G.M.; Fang, Q.; Ma, H.S.; Sun, G.; Wang, X.C. Distinguishing characteristics of urothelial carcinoma in kidney transplant recipients between China and Western countries. Transplant. Proc. 2013, 45, 2197–2202. [Google Scholar] [CrossRef]
- Thon, W.F.; Kliem, V.; Truss, M.C.; Anton, P.; Kuczyk, M.; Stief, C.G.; Brunkhorst, R. Denovo urothelial carcinoma of the upper and lower urinary tract in kidney--transplant patients with end-stage analgesic nephropathy. World J. Urol. 1995, 13, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Xing, N.-Z.; Wang, Y.; Hu, X.-P.; Yin, H.; Zhang, X.-D. Transitional cell carcinoma in renal transplant recipients: A single center experience. Int. J. Urol. 2008, 15, 53–57. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Tian, Y.; Zhu, Y.; Guo, Y.; Wang, Z.; Yang, Y.; Ding, G.; Lin, J. De novo upper tract urothelial carcinoma after renal transplantation: A single-center experience in China. BMC Urol. 2023, 23, 23. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Wu, L.-C.; Kong, P.-H.; Tsai, H.-H.; Chen, Y.-T.; Cheng, Y.-T.; Luo, H.-L.; Li, C.-F. High Level of Aristolochic Acid Detected With a Unique Genomic Landscape Predicts Early UTUC Onset After Renal Transplantation in Taiwan. Front. Oncol. 2022, 11, 828314. [Google Scholar] [CrossRef]
- Ho, C.-J.; Huang, Y.-H.; Hsieh, T.-Y.; Yang, M.-H.; Wang, S.-C.; Chen, W.-J.; Lee, T.-H.; Sung, W.-W.; Chen, S.-L. Native Kidney Hydronephrosis Is Associated with Upper Urinary Tract Urothelial Carcinoma in Post-Kidney Transplantation Patients. J. Clin. Med. 2021, 10, 4474. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Li, Y.; Lu, M.; Zhang, H.; Qiu, M.; Zhao, L.; Zhang, S.; Huang, Y.; Hou, X.; et al. Bilateral Nephroureterectomy Versus Unilateral Nephroureterectomy for Treating De Novo Upper Tract Urothelial Carcinoma After Renal Transplantation: A Comparison of Surgical and Oncological outcomes. Clin. Med. Insights Oncol. 2021, 15, 11795549211035541. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lee, C.U.; Kang, M.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Sung, H.H. Incidences and oncological outcomes of urothelial carcinoma in kidney transplant recipients. Cancer Manag. Res. 2019, 11, 157–166. [Google Scholar] [CrossRef]
- Chien, C.-S.; Luo, H.L.; Ling, C.S.; Chiang, P.-H.; Chen, Y.-T.; Cheng, Y.T. Upper urinary tract urothelial carcinoma behaviors in patients with end-stage renal disease after kidney transplantation in Taiwan. Int. Urol. Nephrol. 2016, 48, 1261–1265. [Google Scholar] [CrossRef]
- Olsburgh, J.; Zakri, R.H.; Horsfield, C.; Collins, R.; Fairweather, J.; O’Donnell, P.; Koffman, G. TCC in Transplant Ureter--When and When Not to Preserve the Transplant Kidney. Am. J. Transplant. 2016, 16, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-T.; Wan, F.-C.; Gao, Z.-L.; Wang, J.-M.; Yang, D.-D. Transperitoneal laparoscopic nephroureterectomy for native upper tract urothelial carcinoma in renal transplant recipients. World J. Urol. 2013, 31, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-J.; Yang, P.-S.; Wang, H.-H.; Lin, K.-J.; Liu, K.-L.; Chu, S.-H.; Hsieh, C.-Y. Urothelial cancer after renal transplantation: An update. Transplant. Proc. 2012, 44, 744–745. [Google Scholar] [CrossRef]
- Tsaur, I.; Karalis, A.; Blaheta, R.; Juengel, E.; Vallo, S.; Scheuermann, E.-H.; Kachel, H.-G.; Waaga-Gasser, A.-M.; Chandraker, A.; Obermüller, N.; et al. Transitional cell carcinoma of the native urinary tract after kidney transplantation: Recommendations following a long-term retrospective analysis. Am. J. Med. Sci. 2011, 341, 478–483. [Google Scholar] [CrossRef]
- Ye, J.; Ma, L.; Huang, Y.; Hou, X.; Xiao, C.; Zhao, L.; Wang, G.; Hong, K.; Lu, J. Retroperitoneal laparoscopic nephroureterectomy with bladder cuff excision for native upper tract transitional cell carcinoma ipsilateral to a transplanted kidney. Urology 2010, 76, 1395–1399. [Google Scholar] [CrossRef]
- Tseng, S.-F.; Chen, Y.-T.; Cheng, Y.-T.; Hsieh, H.-H. Method and outcome of transvesical ureterectomy of the distal ureter in nephroureterectomy of native kidney upper tract urothelial carcinoma ipsilateral to a transplanted kidney. Urology 2007, 69, 1045–1048. [Google Scholar] [CrossRef]
- Grossmann, N.C.; Soria, F.; Juvet, T.; Potretzke, A.M.; Djaladat, H.; Ghoreifi, A.; Kikuchi, E.; Mari, A.; Khene, Z.-E.; Fujita, K.; et al. Comparing Oncological and Perioperative Outcomes of Open versus Laparoscopic versus Robotic Radical Nephroureterectomy for the Treatment of Upper Tract Urothelial Carcinoma: A Multicenter, Multinational, Propensity Score-Matched Analysis. Cancers 2023, 15, 1409. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-M.; Kung, L.-F.; Kuo, M.-C.; Huang, A.-M.; Kuo, H.-T. Timing of mTORI usage and outcomes in kidney transplant recipients. Int. J. Med. Sci. 2021, 18, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Nortier, J.L.; Martinez, M.C.; Schmeiser, H.H.; Arlt, V.M.; Bieler, C.A.; Petein, M.; Depierreux, M.F.; De Pauw, L.; Abramowicz, D.; Vereerstraeten, P.; et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N. Engl. J. Med. 2000, 342, 1686–1692. [Google Scholar] [CrossRef]
- Milojevic, B.; Dzamic, Z.; Grozdic Milojevic, I.; Bumbasirevic, U.; Santric, V.; Kajmakovic, B.; Janicic, A.; Durutovic, O.; Dragicevic, D.; Bojanic, N.; et al. Prognostic value of Balkan endemic nephropathy and gender on upper tract urothelial carcinoma outcomes after radical nephroureterectomy: A cohort study. Urol. Oncol. 2021, 39, e9–e786. [Google Scholar] [CrossRef]
- Territo, A.; Gallioli, A.; Meneghetti, I.; Fontana, M.; Huguet, J.; Palou, J.; Breda, A. Diagnostic ureteroscopy for upper tract urothelial carcinoma: Friend or foe? Arab J. Urol. 2021, 19, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gallioli, A.; Territo, A.; Mercadé, A.; Fontana, M.; Boissier, R.; Gaya, J.M.; Emiliani, E.; Sánchez-Puy, A.; Martínez, M.J.; Palou, J.; et al. The Impact of Ureteroscopy following Computerized Tomography Urography in the Management of Upper Tract Urothelial Carcinoma. J. Urol. 2021, 205, 392–399. [Google Scholar] [CrossRef]
- Gallioli, A.; Uleri, A.; Verri, P.; Tedde, A.; Mertens, L.S.; Moschini, M.; Del Giudice, F.; Soria, F.; Laukhtina, E.; Subiela, J.D.; et al. Oncologic Outcomes of Endoscopic Management of Upper Tract Urothelial Carcinoma: A Systematic Review and Pooled Analysis from the EAU-YAU Urothelial Working Group. Eur. Urol. Focus 2025. ahead of print. [Google Scholar] [CrossRef]
- Rai, B.P.; Parmar, K.; Pradere, B.; Capoun, O.; Soukup, V.; Gontero, P.; Soria, F.; Birtle, A.; Compérat, E.M.; Dominguez-Escrig, J.-L.; et al. Benefit and Harms of Radical Nephroureterectomy as Part of a Multimodal Treatment Strategy for Upper Tract Urothelial Carcinoma Patients Presenting with Clinical Evidence of Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis by the European Association of Urology Guidelines. Eur. Urol. Oncol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Territo, A.; Foerster, B.; Shariat, S.F.; Rouprêt, M.; Gaya, J.M.; Palou, J.; Breda, A. Diagnosis and kidney-sparing treatments for upper tract urothelial carcinoma: State of the art. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2018, 70, 242–251. [Google Scholar] [CrossRef]
- Tsuboi, I.; Matsukawa, A.; Kardoust Parizi, M.; Klemm, J.; Schulz, R.J.; Cadenar, A.; Mancon, S.; Chiujdea, S.; Fazekas, T.; Miszczyk, M.; et al. Differential effect of surgical technique on intravesical recurrence after radical nephroureterectomy in patients with upper tract urothelial cancer: A systematic review and Meta-analysis. World J. Urol. 2024, 42, 488. [Google Scholar] [CrossRef]
- Territo, A.; Gallioli, A.; Diana, P.; Boissier, R.; Fontana, M.; Gaya, J.M.; Sanguedolce, F.; Calderón, J.; Piana, A.; Fontanet, S.; et al. DNA Methylation Urine Biomarkers Test in the Diagnosis of Upper Tract Urothelial Carcinoma: Results from a Single-Center Prospective Clinical Trial. J. Urol. 2022, 208, 570–579. [Google Scholar] [CrossRef]
| Study | Number of Patients | Sex, n (%) | Age at UTUC Diagnosis, Years | Time from KT to UTUC Diagnosis, Months, Mean (SD) | Synchronous Bilateral UTUC, n (%) | UTUC Pathological Stage (TNM Classification), n (%) | Concomitant BC | UTUC Grade, n (%) | Treatment | Median FU, Months (Range) | OS, Timepoint (%) | PFS (%) | RFS (%) | Bladder Recurrence, n (%) | Local Recurrences/Metastasis, n (%) | Death for UTUC, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Du 2023 [2] | 97 | F: 77 (79.4) M: 20 (20.6) | NA | 98.1 (66.4) | 5 (5.15) | Ta: 2 (2.1) Tis: 0 (0) T1: 25 (25.8) T2: 14 (14.4) T3: 9 (9.3) T4: 1 (1.03) | 29 (29.9) | 1: 15 (15.5) 2: 40 (41.2) 3: 25 (25.8) | Radical nephroureterectomy ± TURB | NA | UTUC Stage ≤ T1, 5 yrs 88.2 Stage ≥ T2, 5 yrs 90.2 UTUC + BC Stage ≤ T1, 5 yrs 57.7 Stage ≥ T2, 5 yrs 48.2 | NA | NA | NA | NA | NA |
| Li 2023 [9] | 106 | F: 89 (83.9) M: 17 (16.0) | 57 (51–62) | 91.5 (48–143.75) | 7 (6.6) | Ta: 6 (5.7) Tis: 0 (0) T1: 46 (43.4) T2: 17 (16) T3: 28 (26.4) T4: 9 (8.5) | 33 (31.13) | 1: 3 (2.83) 2: 53 (50) 3: 50 (47.2) | Radical nephroureterectomy ± TURB | 96 (55–148) months | 1 year: 88.3 5 yrs: 66.1 10 yrs: 49.7 | NA | 1 year: 80.4 3 yrs: 68.5 5 yrs: 50.9 | 24 (22.6) | 35 (33.0) (controlateral recurrence) | 41 (38.7) |
| Lai 2022 [10] | 61 | F: 38 (62.3) M: 23 (37.7) | NA | 79.7 (NA) | NA | Renal pelvis: Ta: 6 (9.8) T1: 9 (14.8) T2–T4: 19 (31.1) Ureter tumor: Ta: 10 (16.4) Tis: 41 (67.2) T1: 9 (14.8) T2–T4: 18 (29.5) | NA | NA | Radical nephroureterectomy | 58.8 (NA) | NA | NA | NA | NA | NA | NA |
| Ho 2021 [11] | 67 | F:49 (73.1) M:18 (26.9) | NA | 7.53 (NA) | 14 (15.9) | Ta/is: 7 (10.4) T1: 16 (23.9) T2: 13 (19.4) T3: 27 (40.3) T4: 0 (0) | NA | 1: 4 (5.9) 2/3: 56 (83.6) Unknown: 7 (10.4) | NA | 118.6 (70.2) | NA | NA | NA | 41 (61.2) | NA | NA |
| Zhang 2021 [12] | 48 | F:44 (91.6) M:4 (8.3) | 58.5 (52–64) | 7 (4–10) | 8 (16.6) | Ta/1: 14 (29.2) T2: 15 (31.2) T3: 13 (27.1) T4: 2 (4.2) | 9 (18.7) | Low-grade: 5 (10.4) High-grade: 43 (89.6) | Unilateral radical nephroureterectomy: 27 (56.2) Simultaneous bilateral radical nephroureterectomy: 21 (43.8) | 65 (33.8– 90.5) | End of FU: 86 | NA | End of FU: 68.8 | 9 (18.8) | 15 (33.3) | 12 (25) |
| Yu 2018 [13] | 10 (9 in native UT 1 in graft UT) | F: 8 (80) M: 1 (10) | 45.9 (8.5) | 181.3 (73) | 1 (10) | T1: 2 (20) T2: 2 (20) T3: 5 (50) (1 in the graft) T4: 1 (10) | NA | High-grade: 10 (100) | Radical nephroureterectomy | 71.8 (47.9) | End of FU: 80 | End of FU: 50 | End of FU: 50 | 3 (30) | Recurrence: 5 (50) Progression: 5 (50) | 2 (20) |
| Chien 2016 [14] | 25 | F:17 (68) M:8 (32) | 54.1 (5.6) | NA | NA | Ta/Tis/T0: 5 (20) T1: 6 (24) T2: 7 (28) T3: 7 (28) T4: 0 (0) | 12 (48) | Low-grade: 1 (4) High-grade: 24 (96) | NA | NA | NA | NA | NA | 12 (48) | 6 (24) | NA |
| Olsburgh 2016 [15] | 4 (graft tumor) | M: 4 (100%) | 45 (14.4) | 21.8 (9.4) | 0 on native UTs | T1: 1 (25) T2: 1 (25) T3: 2 (50) | 0 (0) | 3: 2 (50) 3 + cis: 2 (50) | - Attempted transplant Ureterectomy but converted to TNU - Transplant ureterectomy + Pyelovesicostomy - Initially ureteric resection + pyelovesicostomy - Transplant pyelo-ureterectomy + partial cystectomy. Subsequent transplant pyelovesicostomy and excision of right native ureter | 34 (21) | End of FU: 50 | End of FU: 50 | End of FU: 50 | NA | 2 (50) | 2 (50) |
| Liu 2013 [6] | 24 | F:14 (58.3) M:10 (41.6) | 53.5 (9) | NA | 5 (20.8) | Ta/1: 4 (16.7) T2: 6 (25) T3/4: 14 (58.3) | 13 (54.2) | NA | Radical nephroureterectomy | 31 (range 4–94) | End of FU: 80 | End of FU: 33.3 | NA | 7 (29.2) | 8 (33.3) | 4 (16.7) |
| Wu 2013 [16] | 11 | F:10 (90.9) M:1 (9.09) | 54–74 | 38.7 ± 16.0 | 2 (18.2) | T1: 5 (45.4) T2: 5 (45.5) T2 + N1: 1 T3: 2 | 1 (9.1) | NA | Radical nephroureterectomy | 21.7 (3–48) | End of FU: 90.9 | End of FU:90.9 | NA | 2 (18.2) | 1 (9.1) | 1 (9.1) |
| Chiang 2012 [17] | 45 | F:22 (48.9) M:23 (51.1) | NA | 67.4 | NA | NA | 22 (48.9) | LG: 1 (2.2) HG: 44 (97.8) | Radical nephroureterectomy | NA | End of FU: 26.7 | NA | NA | NA | NA | 12 (26.7) |
| Tsaur 2011 [18] | 6 | F:3 (50) M:3 (50) | 61.0 (6.6) | 66.0 (70.7) | 0 (0) | Ta/1: 0 (0) T2: 1 (16.7) T3: 4 (66.7) T4: 1 (16.7) | 4 (66.7) | G1: 0 (0) G2: 1 (16.7) G3: 5 (83.3) | Radical nephroureterectomy +/- TURBT | 115 (range NA) | End of FU: 66.7 | End of FU: 66.6 | NA | NA | 2 (33.3) | 1 (16.7) |
| Ye 2010 [19] | 13 | F: 12 (92,3%) M: 1 (7.69%) | 56.3 (9.4) | 51.3 (32.6) | 2 (15.4) | T1: 7 (53.8) T1+ is: 1 (7.7) T2: 5 (38.5) | 4 (30.8) | 1: 1 (7.7) 2: 6 (46.1) 3: 9 (69.2) | Radical nephroureterectomy + TURBT + chemotherapy | 30 (10–43) | 100 | NA | NA | 2 (15.4) | 2 (15.4) | 0 (0) |
| Tseng 2007 [20] | 9 | F: 6 (66.67) M: 3 (33.33) | 48.3 (range 41–63) | 11–99.6 | NA | Ta: 2 (22.2) Tis/T1–T4: 7 (77.7) | NA | NA | transvesical ureterectomy + radical nephroureterectomy | NA | NA | NA | NA | NA | 1 (11.1) | NA |
| Li 2008 [8] | 11 | F: 9 (81.8) M: 3 (27.3) | 57.3 years (range 44–76) | 44.1 (range 16–89) | 1 (9.1) | Ta: 1 (9.1) T1: 9 (81.8) T2: 1 (9.1) | 2 (18.2) | G1: 1 (9.1) G2: 9 (81.8) G3: 1 (9.1) | Radical nephroureterectomy ± TURB | 26 (8–65). | End of FU: 81.8 | NA | NA | NA | 1 (9.1) | 3 (27.3) |
| Thon 1995 [7] | 6 | NA | 56.1 (51–66) | 72 (42–108) | 1 (16.7) | Tis: 1 (16.7) Ta: 2 (33.3) T1: 1 (16.7) T2: 1 (16.7) T3: 1 (16.7) | 2 (2) | G1: 4 (66.7) G2: 1 (16.7) | Nephroureterectomy ± TURB | 16.9 (4–33) | End of FU: 16.7 | End of FU: 83.3 | NA | NA | NA | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piana, A.; López-Abad, A.; Lanzillotta, B.; Pecoraro, A.; Prudhomme, T.; Haberal, H.B.; Di Dio, M.; Bañuelos Marco, B.; Dönmez, M.I.; Breda, A.; et al. Systematic Review on Upper Urinary Tract Carcinoma in Kidney Transplant Recipients. J. Clin. Med. 2025, 14, 3927. https://doi.org/10.3390/jcm14113927
Piana A, López-Abad A, Lanzillotta B, Pecoraro A, Prudhomme T, Haberal HB, Di Dio M, Bañuelos Marco B, Dönmez MI, Breda A, et al. Systematic Review on Upper Urinary Tract Carcinoma in Kidney Transplant Recipients. Journal of Clinical Medicine. 2025; 14(11):3927. https://doi.org/10.3390/jcm14113927
Chicago/Turabian StylePiana, Alberto, Alicia López-Abad, Battista Lanzillotta, Alessio Pecoraro, Thomas Prudhomme, Hakan Bahadır Haberal, Michele Di Dio, Beatriz Bañuelos Marco, Muhammet Irfan Dönmez, Alberto Breda, and et al. 2025. "Systematic Review on Upper Urinary Tract Carcinoma in Kidney Transplant Recipients" Journal of Clinical Medicine 14, no. 11: 3927. https://doi.org/10.3390/jcm14113927
APA StylePiana, A., López-Abad, A., Lanzillotta, B., Pecoraro, A., Prudhomme, T., Haberal, H. B., Di Dio, M., Bañuelos Marco, B., Dönmez, M. I., Breda, A., & Territo, A., on behalf of YAU Kidney Transplantation Working Group . (2025). Systematic Review on Upper Urinary Tract Carcinoma in Kidney Transplant Recipients. Journal of Clinical Medicine, 14(11), 3927. https://doi.org/10.3390/jcm14113927








