Abstract
Purpose: The success of solid organ transplantation and the consequent increase in the patients on the waiting list has led to an increased utilization of donor kidneys with a high kidney donor profile index (KDPI)/expanded criteria. In our study, patients who underwent transplantation based on the standard and expanded donor criteria were compared in terms of factors affecting graft survival. Data of patients who underwent transplantation from cadavers with standard and extended criteria (SCD, ECD) between 01 July 2011 and 30 June 2016 were evaluated retrospectively. Donor characteristics, treatment type, response and graft characteristics, 1st-, 3rd-, and 5th-year graft survival, and acute rejection rates were analyzed retrospectively. Recent findings: In terms of the causes of death, cerebrovascular accidents were more common in the ECD group (p < 0.001). Hypertension and diabetes were more common in both donor groups and were detected more frequently in recipients in the ECD group (p < 0.001). The absence of mycophenolate mofetil (MMF) use and the presence of an acute rejection attack adversely affected graft survival at the end of the 1st, 3rd, and 5th years. Summary: The utilization of expanded criteria donors is widespread. Appropriate monitoring of patients undergoing immunosuppressive therapy, especially using mycophenolate mofetil (MMF) and the presence of acute rejection, affect graft survival.
1. Introduction
The incidence of chronic kidney disease (CKD) has been increasing worldwide with the rise in comorbid diseases (such as hypertension and diabetes), and kidney transplantation remains the priority for patient survival receiving renal replacement therapy (RRT) in CKD. In previous studies, survival after transplantation from a suitable cadaver seems to be superior to other RRTs [1,2,3].
In recent years, the difficulties in finding donors and the decrease in the cadaver pool has increased the waiting time in dialysis and the interest in utilizing expanded-criteria donors. In the USA, before 2001, the rate of cadaveric age ≥60 years was 50%. The rate of cadavers over 60 years of age in France was 26.8% in 2005. In 2002, The Organ Procurement and Transplantation Network (OPTN) defined expanded-criteria donors (ECDs). ECDs were defined as older kidney donors (≥60 years old) or donors who are aged 50–59 years old and have the following two characteristics: hypertension, baseline serum creatinine >1.5 mg/dL, or death from cerebrovascular accidents [2,3,4,5,6,7]. Scoring systems such as the kidney donor profile index (KDPI) and kidney donor risk index (KDRI) are used to assess cadaver quality [8,9].
In our study, we evaluated graft and patient survival and outcomes of cadaveric kidney transplantation according to the ECD and standard-criteria donor (SCD) definitions in our unit, retrospectively.
2. Methods
2.1. Patients
The data, donor characteristics, recipient treatments, graft characteristics, and clinical and laboratory values of patients who underwent transplantation in our center between 01 July 2011 and 30 June 2016 utilizing ECD (63 patients) and SCD (92 patients) were compared randomly and retrospectively from hospital records. ECDs were described as older kidney donors (> or =60 yr) or donors who are aged 50 to 59 years old and have two of the following three features: hypertension, terminal serum creatinine >1.5 mg/dL, or death from cerebrovascular accident.
The kidney donor risk index (KDRI) score was calculated by evaluating data on age, weight, height, race, creatinine, blood pressure, diabetes, and hepatitis C history. KDRI values were converted to KDPI values using OPTN data [10]. In our study, calcineurin inhibitor (CNI)-based regimens [Kidney Disease: Improving Global Outcomes (KDIGO transplantation guidelines)] were mostly used. As recommended by the guidelines, we attempted to keep the cyclosporine level around 200 ng/mL in the first 3 months, 150–200 ng/mL in the next 6 months, and then 100–150ng/mL until the 2nd year, and 100 ng/mL after the 2nd year. We tried to keep the tacrolimus level in the range of 12–15 ng/mL for the first 3 months, then 10–12 ng/mL for the first 6 months, then 8–10 ng/mL until the 2nd year, and then 5.2–8 ng/mL after the 2nd year. We tried to give as full as possible a dose of mycophenolate mofetil (2000 mg/day for MMF, 1440 mg/day for mycophenolate sodium). The dose was reduced in patients with diarrhea, severe infection, risk of sepsis, thrombocytopenia, severe leukopenia, etc. (according to the Elite-Symphony Trial).
The corticosteroid dose (prednisolone) was reduced to 5 mg/day after 6 months. Switching to mTOR was performed in patients who developed calcineurin toxicity (nephrotoxicity) [11].
Donors who did not meet the condition of being cadavers, such as those with a history of malignancy, high risk of transmission, and active infection, etc., were excluded from the study.
The diagnosis of rejection was made based on clinical findings and kidney biopsy.
CNI toxicity was detected by drug level and kidney biopsy results when kidney function values increased.
Patients who did not have regular follow-ups during the specified period and were transferred to another center were also excluded from the study. We de-identified all patient details [12].
Ethics committee approval was obtained from another center (15–21 January 2019). We allow fellow researchers to reproduce our methodology. Our study was retrospective, and the ethics committee allowed us to use the data in an anonymized form. Since this study was retrospective, patient consent was not obtained following the recommendation of the ethics committee.
2.2. Biostatistical Analysis
Data are expressed as means ± SD and median (min–max). Percentage change values were calculated as follows: (postoperative value-preoperative value)/preoperative value for time-dependent values, and these values were used in intergroup comparisons. The Mann–Whitney U test, the independent sample t-test, Pearson’s chi-square test, Fisher’s exact test, and the Fisher–Freeman–Halton test were used for comparisons. Survival times were calculated using Kaplan–Meier analysis, and factors were examined using the log-rank test. Cox’s regression analysis was used to determine the hazard ratios and risk factors. Statistical analyses were performed using the Statistical Package for Social Sciences for Windows [version 22.0; (SPSS, Chicago, IL, USA)]. Statistical significance was set at p < 0.05.
3. Results
As expected, the mean age of the SCD group was lower than that of the ECD group. The last and 24 h urine volumes were lower in the ECD group. The body mass index (BMI) was higher in the ECD group. The number of cadavers with a history of diabetes and hypertension was higher in the ECD group. Additionally, the cause of death due to trauma was more prominent in the SCD group. The cold ischemia time was longer in patients with ECD. The rates of HT and DM in recipients and the KDPI and KDRI scores were higher in the ECD group. When the recipients were evaluated, the history of HT was higher in transplant recipients from the ECD group. In terms of hospitalization due to infection, acute rejection, and graft and patient survival after transplantation, there was no change in the recipients according to the type of cadaver (Table 1 and Table 2).

Table 1.
Comparisons about donor type.

Table 2.
Recipient profile/demographics.
The incidence of primary non-functioning kidneys was higher in the ECD group. The first-month creatinine level was higher and the GFR level was lower in the ECD group (Table 3).

Table 3.
Comparisons about primer treatments.
No difference was observed between the two groups in terms of the surgical and medical complications observed after transplantation (Table 4 and Table 5).

Table 4.
Comparisons about serum creatinine and GFR.

Table 5.
Post-transplant surgical and medical complications.
According to the 1-, 3-, and 5-year survival results (for graft), renal survival was longer in the mycophenolate mofetil (MMF) users, and renal survival was adversely affected in patients with acute rejection (Figure 1, Figure 2 and Figure 3).
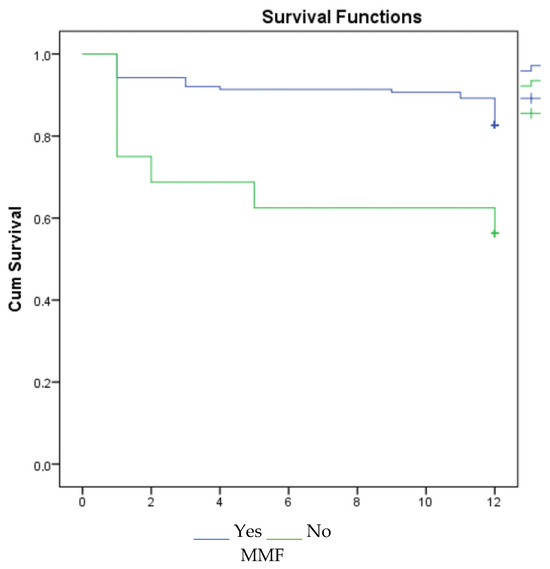
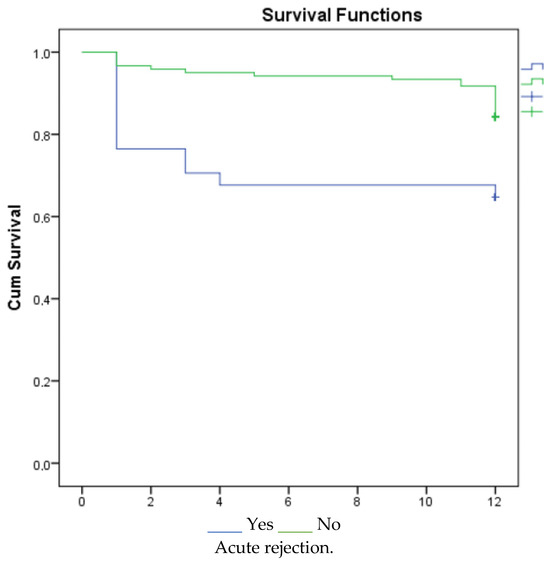
Figure 1.
Survival graph for 1 year by MMF and acute rejection.
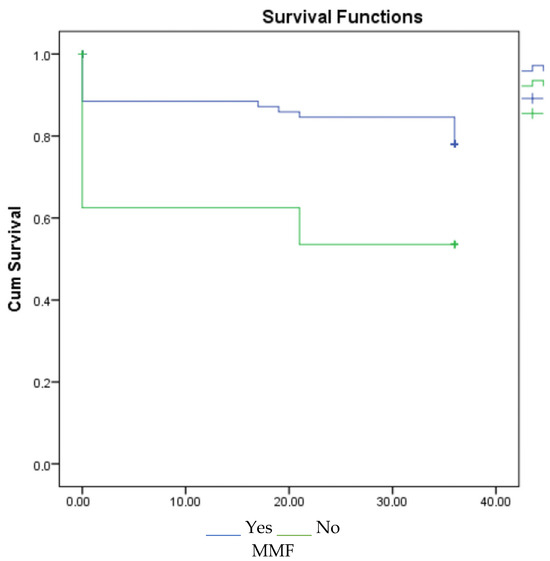
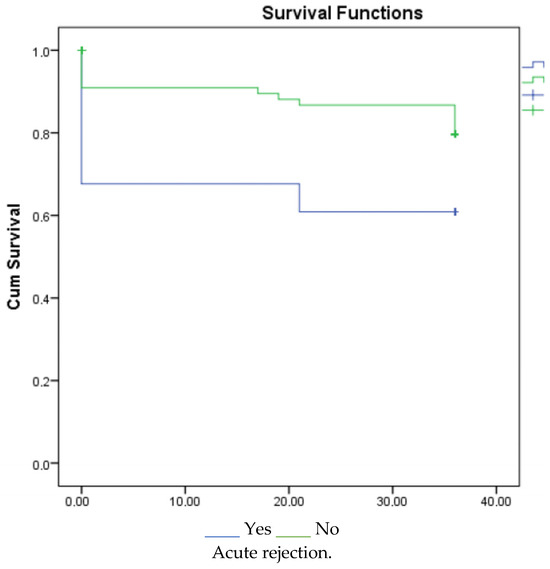
Figure 2.
Survival graph for 3 years by MMF and acute rejection.
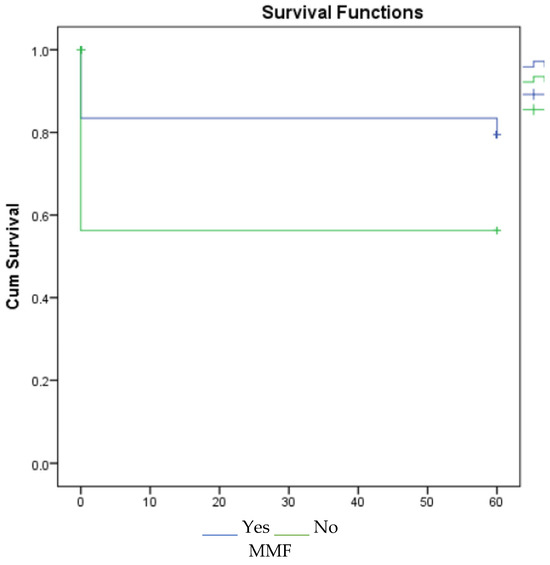
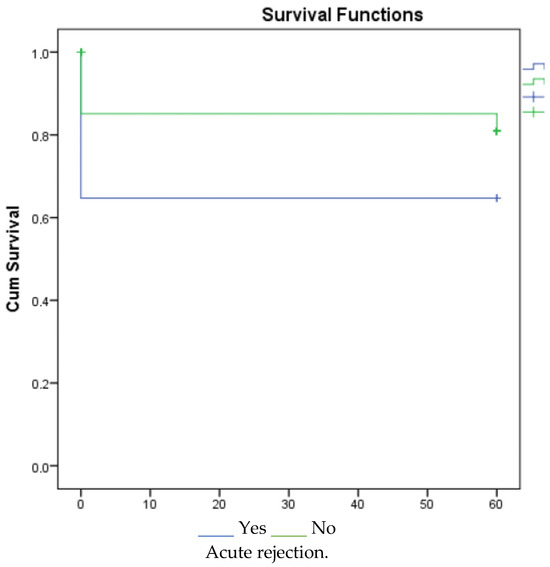
Figure 3.
Survival graph for 5 years by MMF and acute rejection.
When patient survival time was examined for 1-, 3- and 5-year survival for the patients, it was not statistically significant. No significant results were found in the multivariate analysis.
4. Discussion
The need for RRT has been increasing due to the increase in the incidence and prevalence of CKD and end-stage renal disease. In kidney transplantation, the need to find donors and cadavers and the length of the waiting time in dialysis have increased. Paired kidney exchange, dual kidney transplantation, and ECD are utilized to meet patient needs.
Considering the global increase in the elderly population, an increase in ECD is expected in the future. In our country’s database, the donor rate between the ages of 45–64 was 36.05%, and it was 5.36% over the age of 65 years. These rates have increased compared to those in the previous year (29.72% and 4.04, respectively) [9,13]. The utility of ECD affects both patient and graft survival [14]. Graft and patient loss were higher in ECD at the end of the 1st and 5th years compared to those transplanted with standard criteria. In a study that evaluated 1554 recipients from Switzerland, patient and graft survival were negatively affected when donor age increased [15,16]. As expected, in our study, age was higher in the ECD group compared to the SCD group.
The incidences of rejection and primary non-functional grafts were higher after the utility of ECD. The incidence of a primary non-functioning kidney was approximately 2% in patients with a KDPI ≥ 80. The rejection rate was 7% by the end of the 6th month. In a study in which 720 cadaveric donors were evaluated, acute rejection rates were higher in patients with a KDPI > 85. In this study, the contribution of the acute rejection rate to graft function at the end of the first year was not significant. In another study, 663 transplants were analyzed, and age-related acute rejections after three months had a negative effect on graft survival at the end of the 1st and 5th years. In addition, rejection rates were similar between the SCD and ECD groups in some studies. Acute rejection rates were high in patients with delayed graft (DGF) function [9,17,18,19,20]. In our study, no change was detected in the rejection rates in both groups. In the regression analysis, the 1st-, 3rd-, and 5th-year graft survivals were low in those who had an acute rejection attack. Rejection is thought to affect graft survival due to the effects of the primary disease and immunosuppressive therapies.
Drug pharmacokinetics in elderly patients should also be considered, particularly with regard to the effectiveness of immunosuppressive therapies. Considering the patient’s clinical and side-effect profiles in terms of immunosuppressive therapies, it was determined that the pharmacokinetics of CNI and steroids changed but MMF did not [21].
However, CNI-free regimens based on reduced CNI exposure and mammalian target of rapamycin inhibitors (mTOR inhs) have shown acceptable results in appropriately selected ECD transplant recipients. CNI-free mTOR inhs-based therapy compared with MMF-based treatment in kidney transplantation with advanced-age donors was associated with an acceptable outcome, but increased proteinuria and acute rejection in mTOR inhs-treated patients was detected more. The best results with mTOR inhs have been found in patients without delayed graft function [22]. In a multicenter study including 3143 patients, the combination of CNI and MMF increased graft survival and decreased acute rejection rates [23]. Studies have shown that MMF plays a key role in immunosuppressive treatment regimens, and rejection and graft survival are adversely affected by dose reduction and discontinuation of the drug [24]. In our study, MMF had a positive effect on 1st-, 3rd-, and 5th-year graft survival.
The most important shortcomings of our study are that it was retrospective, the number of patients was small, and HLA- and donor-specific antibodies could not be tested. In conclusion, acute rejection attacks negatively affect graft survival, and MMF plays an important role in the RRT treatment protocol. Therefore, attention should be given to immunosuppressive treatment and rejection in ECD transplant recipients.
Author Contributions
Conceptualization, Y.A. and A.E.; methodology, Y.A.; software, A.Y.; validation, A.O.; formal analysis, F.E.C.; investigation, Y.A. and E.I.S.; resources, A.Y., E.I.S. and A.O.; data curation, Y.A.; writing—original draft preparation, Y.A.; writing—review and editing, Y.A. and A.E.; visualization, Y.A., E.I.S. and A.E.; supervision, A.E.; project administration, A.E., A.O. and A.Y.; funding acquisition, Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Bursa Uludag University Ethics Committee 15 January 2019-2019-1/21.
Informed Consent Statement
Since this study was retrospective, patient consent was not obtained following the recommendation of the ethics committee.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. 2015, 5, 2–7. [Google Scholar]
- Noble, J.; Jouve, T.; Malvezzi, P.; Süsal, C.; Rostaing, L. Transplantation of Marginal Organs: Immunological Aspects and Therapeutic Perspectives in Kidney Transplantation. Front. Immunol. 2020, 10, 3142. [Google Scholar]
- Wang, Z.; Durai, P.; Tiong, H.Y. Expanded criteria donors in deceased donor kidney transplantation—An Asian perspective. Indian J. Urol. 2020, 36, 89–94. [Google Scholar] [PubMed]
- Ko, K.J.; Kim, Y.H.; Kwon, K.H.; Kim, M.H.; Jun, K.W.; Hwang, J.K.; Kim, S.D.; Park, S.C.; Kim, J.I.; Yun, S.S.; et al. Kidney Transplantation Using Expanded-Criteria Deceased Donors: A Comparison With Ideal Deceased Donors and Non-Expanded-Criteria Deceased Donors. Transplant. Proc. 2018, 50, 3222–3227. [Google Scholar]
- Salguero, J.; Chamorro, L.; Gomez-Gomez, E.; Robles, J.E.; Campos, J.P. Midterm Outcomes of Kidney Transplantation from Expanded Criteria Donors After Circulatory Death: A Single-Center Retrospective Cohort Study. Exp. Clin. Transplant. 2023, 21, 481–486. [Google Scholar] [CrossRef]
- Oruç, A.; Ersoy, A.; Ayar, Y.; Akgür, S.; Yildiz, A. Exclusion Reasons of Cadaveric Kidney Transplantation Candidates. Turk. J. Nephrol. 2018, 27, 82–86. [Google Scholar]
- Çoban, Ş.; Yıldız, S.; Bozkaya, E.; Derici, Z.S.; Ünlü, M.; Çelik, A.; Sifil, A.; Cavdar, C.; Camsari, T. Evaluation of Morbidity and Patient and Graft Survival in Kidney Transplant Recipients: Experience of Dokuz Eylul University Hospital. Turk. J. Nephrol. 2017, 26, 41–47. [Google Scholar]
- Sexton, D.J.; O’Kelly, P.; Kennedy, C.; Denton, M.; de Freitas, D.G.; Magee, C.; O’Seaghdha, C.M.; Conlon, P.J. Assessing the discrimination of the Kidney Donor Risk Index/Kidney Donor Profile Index scores for allograft failure and estimated glomerular filtration rate in Ireland’s National Kidney Transplant Programme. Clin. Kidney J. 2019, 12, 569–573. [Google Scholar]
- Goussous, N.; De Leon, F.; Alghannam, K.; Howard, B.C.; Than, P.A.; Wang, A.X.; Sageshima, J.; Perez, R.V. Outcomes Using High KDPI Kidneys in Recipients Over 65 y of Age. Transpl. Direct. 2024, 10, e1738. [Google Scholar] [CrossRef]
- Lentine, K.L.; Smith, J.M.; Lyden, G.R.; Miller, J.M.; Dolan, T.G.; Bradbrook, K.; Larkin, L.; Temple, K.; Handarova, D.K.; Weiss, S.; et al. OPTN/SRTR 2022 Annual Data Report: Kidney. Am. J. Transpl. 2024, 24 (Suppl. S1), S19–S118. [Google Scholar] [CrossRef]
- Heemann, U.; Abramowicz, D.; Spasovski, G.; Vanholder, R.; European Renal Best Practice Work Group on Kidney Transplantation. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: A European Renal Best Practice (ERBP) position statement. Nephrol. Dial. Transpl. 2011, 26, 2099–2106. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern Med. 2007, 147, 573–577. [Google Scholar] [PubMed]
- Regıstry of the Nephrology, Dıalysıs and Transplantatıon in Turkey, Regıstry 2019 and 2020. In Mınıstry of Health and Turkısh Socıety of Nephrology Joınt Report; Suleymanlar, G., Ates, K., Seyahi, N., Kocyigit, I., Eds.; Turkish Society of Nephrology Registry Committee: Ankara, Turkey, 2020; pp. 52–53. [Google Scholar]
- Monteoliva, P.B.; Redondo-Pachón, D.; García, E.M.; Calabia, E.R. Kidney transplant outcome of expanded criteria donors after circulatory death. Nefrol. Engl. Ed. 2021, 42, 135–144. [Google Scholar]
- Ma, M.K.; Lim, W.H.; Craig, J.C.; Russ, G.R.; Chapman, J.R.; Wong, G. Mortality among Younger and Older Recipients of Kidney Transplants from Expanded Criteria Donors Compared with Standard Criteria Donors. Clin. J. Am. Soc. Nephrol. 2016, 11, 128–136. [Google Scholar] [PubMed]
- Kuhn, C.; Lang, B.M.; Lörcher, S.; Karolin, A.; Binet, I.; Beldi, G.; Golshayan, D.; Hadaya, K.; Mueller, T.F.; Schaub, S.; et al. Outcome of kidney transplantation from very senior donors in Switzerland—A national cohort study. Transpl. Int. 2021, 34, 689–699. [Google Scholar]
- Molinari, M.; Kaltenmeier, C.; Liu, H.; Ashwat, E.; Jorgensen, D.; Puttarajappa, C.; Wu, C.M.; Mehta, R.; Sood, P.; Shah, N.; et al. Function and longevity of renal grafts from high-KDPI donors. Clin. Transpl. 2022, 36, e14759. [Google Scholar]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am. J. Transpl. 2019, 19, 425–433. [Google Scholar]
- Arias-Cabrales, C.E.; Pérez-Sáez, M.J.; Redondo-Pachón, D.; Buxeda, A.; Burballa, C.; Duran, X.; Mir, M.; Crespo, M.; Pascual, J. Relevance of KDPI value and acute rejection on kidney transplant outcomes in recipients with delayed graft function—A retrospective study. Transpl. Int. 2020, 33, 1071–1077. [Google Scholar]
- Jalalzadeh, M.; Mousavinasab, N.; Peyrovi, S.; Ghadiani, M.H. The impact of acute rejection in kidney transplantation on long-term allograft and patient outcome. Nephro-Urol. Mon. 2015, 7, e24439. [Google Scholar]
- Peeters, L.E.J.; Andrews, L.M.; Hesselink, D.A.; de Winter, B.C.M.; van Gelder, T. Personalized immunosuppression in elderly renal transplant recipients. Pharmacol. Res. 2018, 130, 303–307. [Google Scholar]
- Diekmann, F.; Gutiérrez-Dalmau, A.; López, S.; Cofán, F.; Esforzado, N.; Ricart, M.J.; Rossich, E.; Saval, N.; Torregrosa, J.V.; Oppenheimer, F.; et al. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: Comparison of two CNI-free protocols. Nephrol. Dial. Transpl. 2007, 22, 2316–2321. [Google Scholar]
- Knight, S.R.; Russell, N.K.; Barcena, L.; Morris, P.J. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: A systematic review. Transplantation 2009, 87, 785–794. [Google Scholar] [PubMed]
- Su, V.C.H.; Greanya, E.D.; Ensom, M.H. Impact of Mycophenolate Mofetil Dose Reduction on Allograft Outcomes in Kidney Transplant Recipients on Tacrolimus-Based Regimens: A Systematic Review. Ann. Pharmacother. 2011, 45, 248–257. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).