The Impact of Maternal Obesity on the Duration of Labor Stages in Dinoprostone-Induced Vaginal Delivery
Abstract
1. Introduction
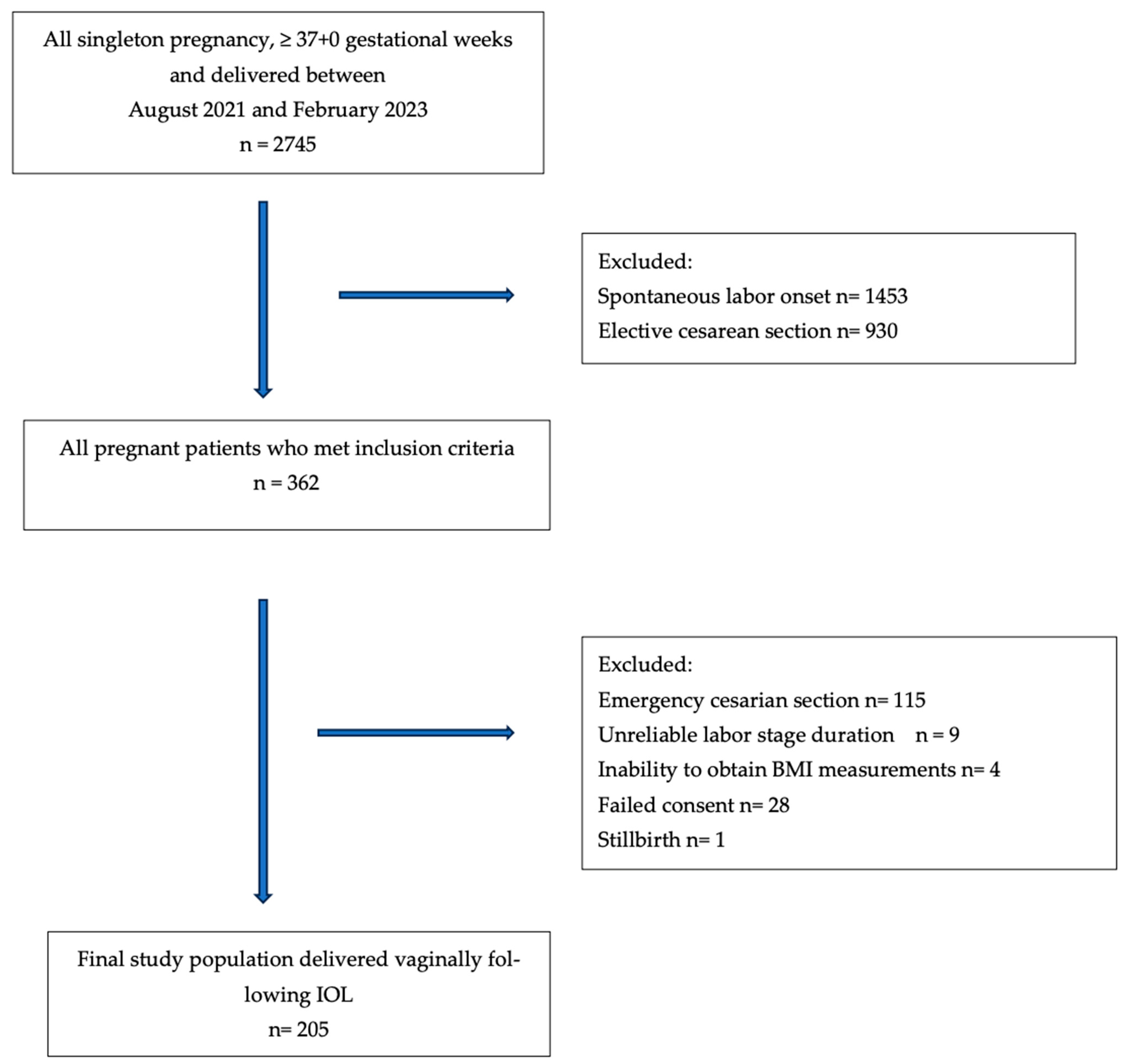
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keirse, M.J. Prostaglandins in preinduction cervical ripening. Meta-analysis of world-wide clinical experience. J. Reprod. Med. 1993, 38 (Suppl. S1), 69–98. [Google Scholar]
- Middleton, P.; Shepherd, E.; Crowther, C.A. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst. Rev. 2018, 5, CD004945. [Google Scholar] [CrossRef]
- Altman, M.R.; Lydon-Rochelle, M.T. Prolonged second stage of labor and risk of adverse maternal and perinatal outcomes: A systematic review. Birth 2006, 33, 315–322. [Google Scholar] [CrossRef]
- Bakker, R.; Pierce, S.; Myers, D. The role of prostaglandins E1 and E2, dinoprostone, and misoprostol in cervical ripening and the induction of labor: A mechanistic approach. Arch. Gynecol. Obstet. 2017, 296, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Word, R.A.; Li, X.H.; Hnat, M.; Carrick, K. Dynamics of cervical remodeling during pregnancy and parturition: Mechanisms and current concepts. Semin. Reprod. Med. 2007, 25, 69–79. [Google Scholar] [CrossRef]
- Shirley, M. Dinoprostone vaginal insert: A review in cervical ripening. Drugs 2018, 78, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Brown, C.M.; Barger, B.; Carlson, N.S. Influence of Maternal Obesity on Labor Induction: A Systematic Review and Meta-Analysis. J. Midwifery Women’s Health 2019, 64, 55–67. [Google Scholar] [CrossRef]
- Pevzner, L.; Powers, B.L.; Rayburn, W.F.; Rumney, P.; Wing, D.A. Effects of maternal obesity on duration and outcomes of prostaglandin cervical ripening and labor induction. Obs. Gynecol. 2009, 114, 1315–1321. [Google Scholar] [CrossRef]
- Carlhäll, S.; Källén, K.; Blomberg, M. The effect of maternal body mass index on duration of induced labor. Acta Obs. Gynecol. Scand. 2020, 99, 669–678. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Zhang, J.; Vanveldhuisen, P.; Troendle, J.; Beaver, J.; Hibbard, J.U. Contemporary labor patterns: The impact of maternal body mass index. Am. J. Obs. Gynecol. 2011, 205, 244.e1–244.e8. [Google Scholar] [CrossRef]
- Lassiter, J.R.; Holliday, N.; Lewis, D.F.; Mulekar, M.; Abshire, J.; Brocato, B. Induction of labor with an unfavorable cervix: How does BMI affect success? (double dagger). J. Matern. Fetal Neonatal Med. 2016, 29, 3000–3002. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; El-Semary, A.M.; Marie, H.M.; Belal, D.S.; Hany, A.; Taymour, M.A.; Omran, E.F.; Elbaradie, S.M.Y.; Mohamed, M.A.K. Effect of maternal obesity on labor induction in postdate pregnancy. Arch. Gynecol. Obstet. 2018, 298, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, E.G.; Plachouras, N.; Drougia, A.; Andronikou, S.; Vlachou, C.; Stefos, T.; Paraskevaidis, E.; Zikopoulos, K. Comparison of misoprostol and dinoprostone for elective induction of labour in nulliparous women at full term: A randomized prospectivestudy. Reprod. Biol. Endocrinol. 2004, 2, 70. [Google Scholar] [CrossRef]
- Hanley, G.E.; Munro, S.; Greyson, D.; Gross, M.M.; Hundley, V.; Spiby, H.; Janssen, P.A. Diagnosing onset of labor: A systematic review of definitions in the research literature. BMC Pregnancy Childbirth 2016, 16, 71. [Google Scholar] [CrossRef]
- López Jiménez, N.; García Sánchez, F.; Pailos, R.H.; Rodrigo Álvaro, V.; Pascual Pedreño, A.; Moreno Cid, M.; Hernández Martínez, A.; Molina Alarcón, M. Prediction of an effective cervical ripenning in the induction of labour using vaginal dinoprostone. Sci. Rep. 2023, 13, 6855. [Google Scholar] [CrossRef]
- Hirshberg, A.; Levine, L.D.; Srinivas, S. Labor length among overweight and obese women undergoing induction of labor. J. Matern. Fetal Neonatal Med. 2014, 27, 1771–1775. [Google Scholar] [CrossRef]
- Vahratian, A.; Zhang, J.; Troendle, J.F.; Savitz, D.A.; Siega-Riz, A.M. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obs. Gynecol. 2004, 104, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Verdiales, M.; Pacheco, C.; Cohen, W.R. The effect of maternal obesity on the course of labor. J. Perinat. Med. 2009, 37, 651–655. [Google Scholar] [CrossRef]
- Wing, D.A.; Brown, R.; Plante, L.A.; Miller, H.; Rugarn, O.; Powers, B.L. Misoprostol vaginal insert and time to vaginal delivery: A randomized controlled trial. Obs. Gynecol. 2013, 122 Pt 1, 201–209. [Google Scholar] [CrossRef]
- Zhang, J.; Bricker, L.; Wray, S.; Quenby, S. Poor uterine contractility in obese women. BJOG 2007, 114, 343–348. [Google Scholar] [CrossRef]
- Adams, A.D.; Coviello, E.M.; Drassinower, D. The Effect of Maternal Obesity on Oxytocin Requirements to Achieve Vaginal Delivery. Am. J. Perinatol. 2020, 37, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Garabedian, M.J.; Hansen, W.F.; McCord, L.A.; Manning, M.A.; O’Brien, J.M.; Curry, T.E., Jr. Up-regulation of oxytocin receptor expression at term is related to maternal body mass index. Am. J. Perinatol. 2013, 30, 491–497. [Google Scholar] [PubMed]
- Cheymol, G. Clinical pharmacokinetics of drugs in obesity. An update. Clin. Pharmacokinet. 1993, 25, 103–114. [Google Scholar] [CrossRef] [PubMed]
| Normal Weight (n = 28) | Overweight (n = 76) | Obese (n = 101) | p-Value a | |
|---|---|---|---|---|
| Maternal age (years) (SD) | 23.5 (20–26) | 28 (24–31) | 27 (25–32) | <0.01 |
| Parity, n (%) | ||||
| Nulliparous | 20 (71.4) | 54 (71.1) | 62 (61.4) | 0.38 |
| Parous | 8 (28.6) | 22 (28.9) | 39 (37.6) | |
| Gestational age (week), median (IQR) | 39 (38–40) | 40 (38–41) | 40 (38–41) | 0.42 |
| Gestational age (n, %) | ||||
| <39 weeks | 8 (28.6) | 20 (26.3) | 28 (27.7) | |
| 39–41 weeks | 14 (50) | 32 (42.1) | 32 (31.7) | 0.31 |
| 41 weeks | 6 (21.4) | 24 (31.6) | 41 (40.6) | |
| BMI, kg/m2, median (IQR) | 23.1 (21.6–24.2) | 27.9 (26.7–29.2) | 32.55 (31.2–35.2) | <0.01 |
| Oxytocin augmentation (n,%) | 8 (28.6) | 30 (38.5) | 58 (58.6) | 0.022 |
| Oxytocin time, h, median (IQR) | 7 (2–9) | 6 (3–7) | 6 (3.75–7.35) | 0.017 |
| Baseline Bishop score, median (IQR) | 3 (1–4) | 3 (1–4) | 3 (1–4) | 0.178 |
| Infant weight, gram, median (IQR) | 2955 (2800–3140) | 3220 (3000–3420) | 3325 (3030–3600) | <0.01 |
| Birth weight (n,%) | ||||
| <2500 g | 6 (21.4) | 4 (5.3) | 8 (7.9) | 0.029 |
| 2500–4000 g | 20 (71.4) | 70 (9.2) | 82 (81.2) | |
| >4000 g | 2 (7.1) | 2 (2.6) | 11 (10.9) | |
| Indications for induction, n (%) | ||||
| Oligohydramnios | 6 (21.4) | 26 (34.2) | 24 (23.8) | |
| IUGR | 6 (21.4) | 2 (2.6) | 8 (7.9) | 0.059 |
| Postmaturity | 10 (35.7) | 26 (34.2) | 44 (43.6) | |
| Fetal reasons | 3 (10.7) | 12 (15.8) | 12 (11.9) | |
| Other | 3 (10.7) | 10 (13.2) | 13 (12.9) |
| Phases a | Normal Weight (n = 28) | Overweight (n = 76) | Obese (n = 101) | p-Value b |
|---|---|---|---|---|
| Latent labor time, hours | 7 (4–9) | 8 (5.5–10) | 8.50 (6.5–12) | 0.048 |
| Active labor time, hours | 4.5 (3–7) | 6 (4–13) | 9 (4–16.5) | 0.02 |
| Total labor time, hours | 11.25 (8–14.38) | 14 (8.5–20.5) | 17 (10.63–22.75) | 0.009 |
| B Coefficient a | 95% CI | p Value b | |
|---|---|---|---|
| Duration of latent labor (LL) | 0.36 | (0.012–0.855) | 0.856 |
| Duration of active labor (AL) | 3.14 | (1.205–6.332) | <0.01 |
| Duration of total induced labor c | 3.54 | (1.457–7.057) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezirganoglu Altuntas, N.; Bayoglu Tekin, Y. The Impact of Maternal Obesity on the Duration of Labor Stages in Dinoprostone-Induced Vaginal Delivery. J. Clin. Med. 2025, 14, 3209. https://doi.org/10.3390/jcm14093209
Bezirganoglu Altuntas N, Bayoglu Tekin Y. The Impact of Maternal Obesity on the Duration of Labor Stages in Dinoprostone-Induced Vaginal Delivery. Journal of Clinical Medicine. 2025; 14(9):3209. https://doi.org/10.3390/jcm14093209
Chicago/Turabian StyleBezirganoglu Altuntas, Neslihan, and Yesim Bayoglu Tekin. 2025. "The Impact of Maternal Obesity on the Duration of Labor Stages in Dinoprostone-Induced Vaginal Delivery" Journal of Clinical Medicine 14, no. 9: 3209. https://doi.org/10.3390/jcm14093209
APA StyleBezirganoglu Altuntas, N., & Bayoglu Tekin, Y. (2025). The Impact of Maternal Obesity on the Duration of Labor Stages in Dinoprostone-Induced Vaginal Delivery. Journal of Clinical Medicine, 14(9), 3209. https://doi.org/10.3390/jcm14093209




