Kuttner’s Tumour: A Case Series and Narrative Review on Diagnosis, Management, and Outcomes
Abstract
1. Introduction
2. Methods
2.1. Case Series
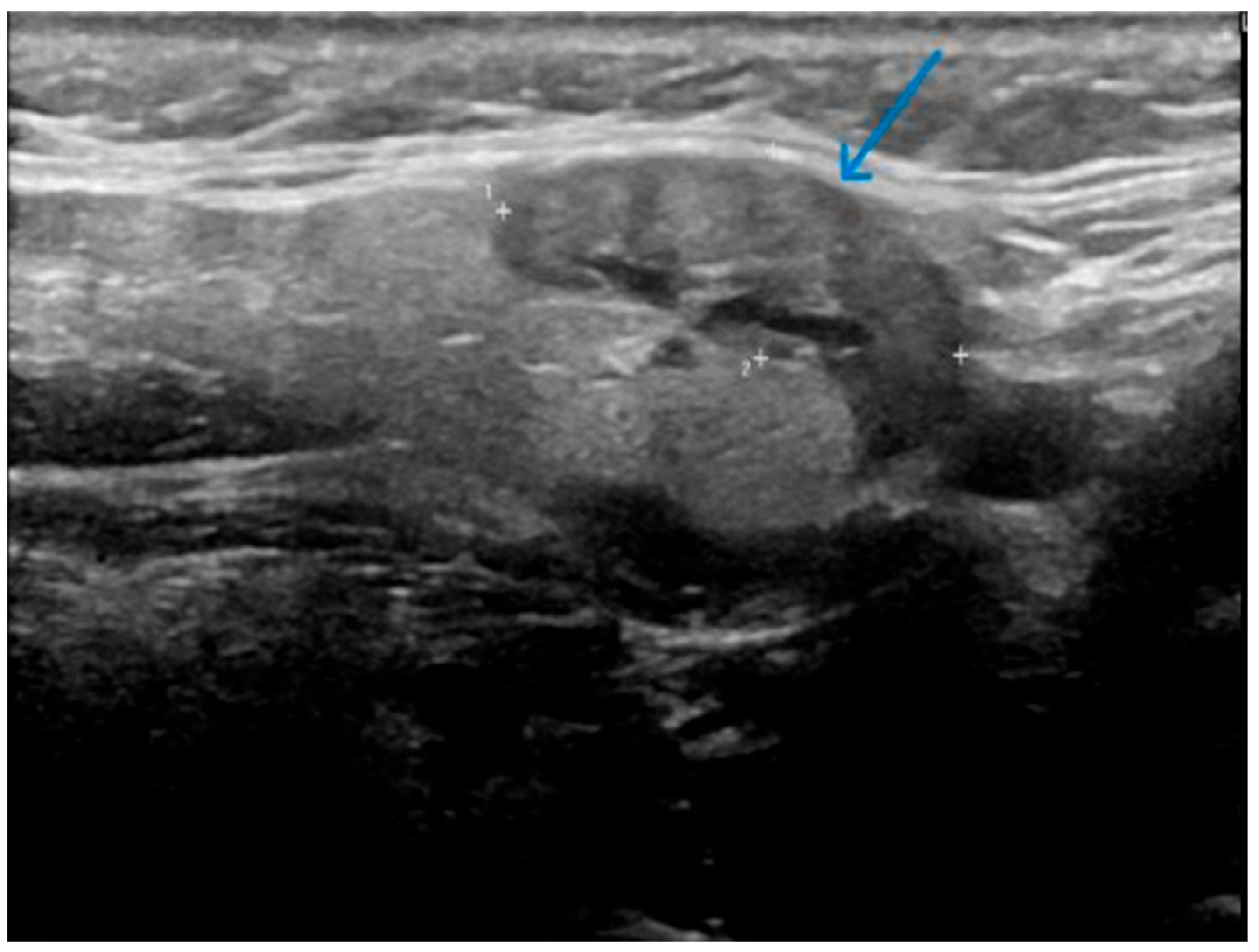
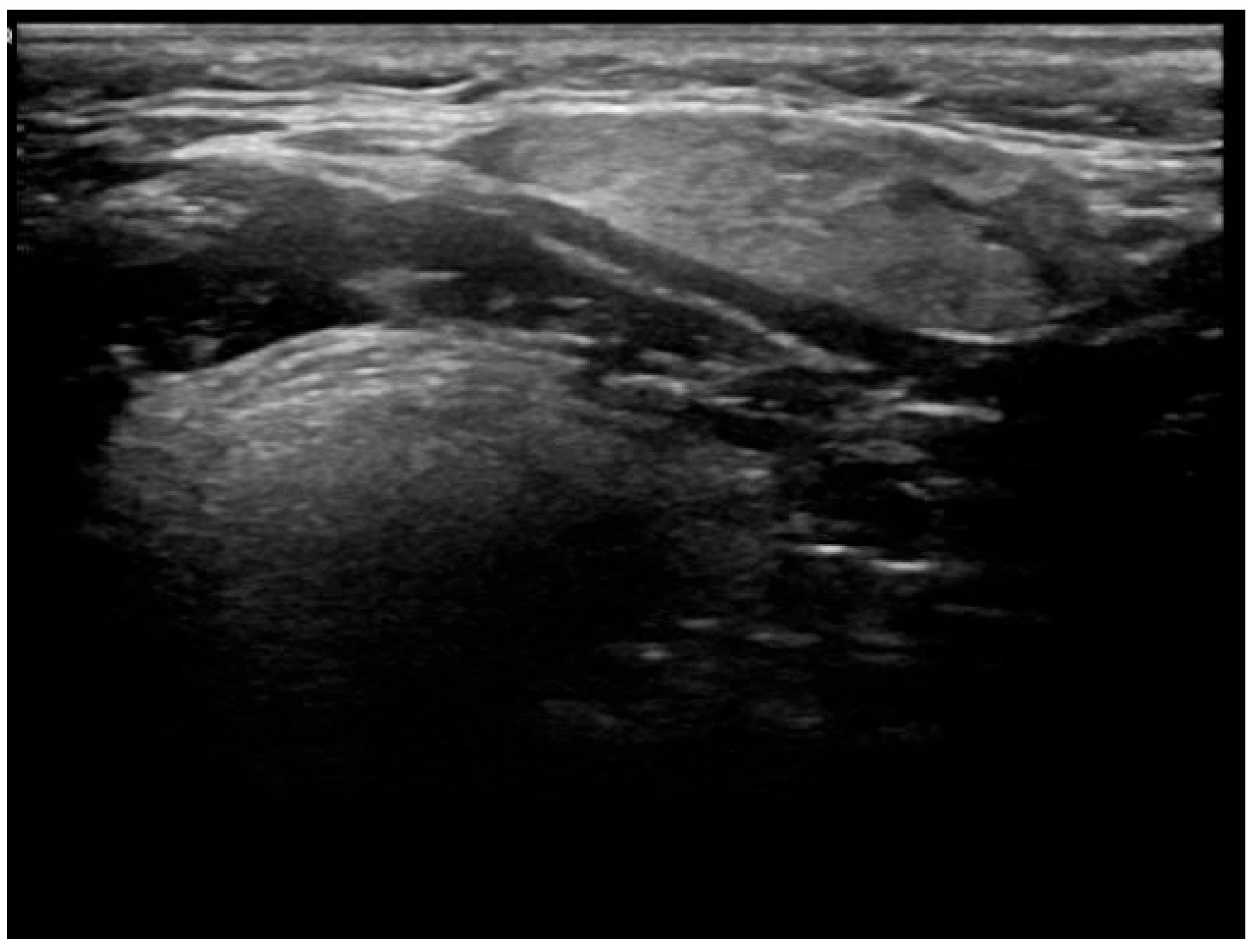
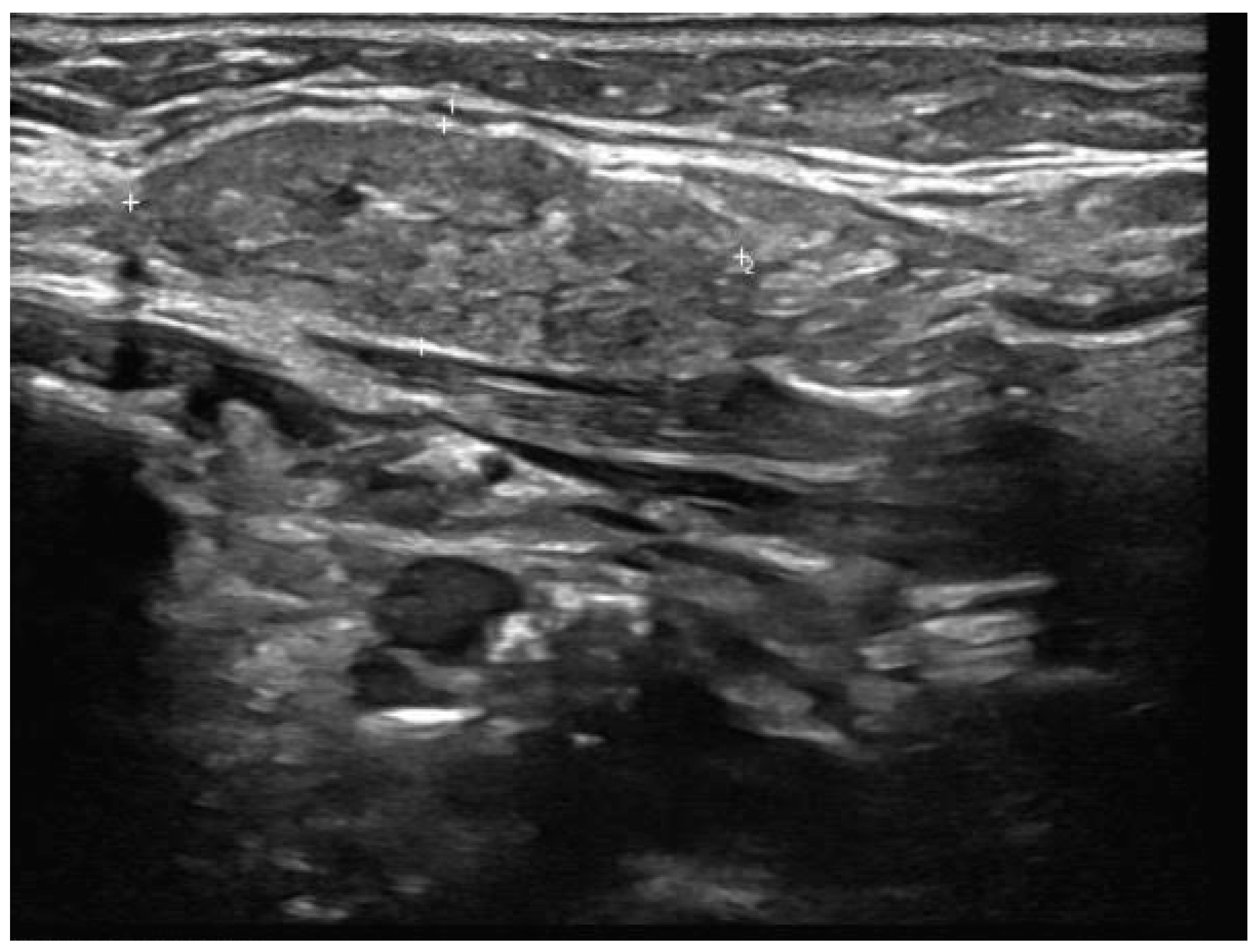
- Well-demarcated geographical area of low echogenicity with increased vascularity;
- Mild focal intrinsic duct dilatation within the SMG;
- No suggestion of a neoplasm or space-occupying lesion.
2.2. Literature Review
3. Results
4. Discussion
4.1. Overview and Contribution to the Literature
4.2. Diagnostic Approach and Imaging
4.3. Management Strategies and Follow-Up
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computer Tomography |
| FNAC | Fine-Needle Aspiration Cytology |
| IgG4-RD | Immunoglobulin-Related Disease |
| KT | Kuttner’s Tumour |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MRI | Magnetic Resonance Imaging |
| RCD | Revised Comprehensive Diagnostic |
| SMG | Submandibular Gland |
| UK | United Kingdom |
| N/A | Not Applicable |
References
- Radiopaedia. Küttner Tumour. Available online: https://radiopaedia.org/articles/kuttner-tumour?lang=gb (accessed on 3 March 2025).
- Westergaard-Nielsen, M.; Godballe, C.; Eriksen, J.G.; Larsen, S.R.; Kiss, K.; Agander, T.; Ulhøi, B.P.; Charabi, B.W.; Klug, T.E.; Jacobsen, H.; et al. Epidemiology, Outcomes, and Prognostic Factors in Submandibular Gland Carcinomas: A National DAHANCA Study. Eur. Arch. Otorhinolaryngol. 2023, 280, 3405–3413. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.T.; Ferry, J.A.; Harris, N.L.; Stone, J.H.; Zukerberg, L.R.; Lauwers, G.Y.; Pilch, B.Z.; Deshpande, V. Chronic Sclerosing Sialadenitis (Küttner Tumor) Is an IgG4-Associated Disease. Am. J. Surg. Pathol. 2010, 34, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Marcus, K.S.; Hoffman, H.T.; Rajan Kd, A. Not All Küttner Tumors Are IgG4-Related Disease (IgG4-RD). Head Neck Pathol. 2021, 15, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, M.; Mancuso, G.; Della-Torre, E. Advances in the Diagnosis and Management of IgG4 Related Disease. BMJ 2020, 369, m1067. [Google Scholar] [CrossRef] [PubMed]
- Muniz, V.R.V.M.; Altemani, A.; Freitas, V.S.; Pires, B.C.; De Santana, D.A.; Couto, L.A.; Cangussu, M.C.T.; Gomez, R.S.; De Souza, S.C.O.M.; Vargas, P.A.; et al. Chronic Sclerosing Sialadenitis of the Submandibular Gland and Its Histopathological Spectrum in the IgG4-Related Disease: A Series of 17 Cases. Head Neck Pathol. 2024, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Peuraharju, E.; Saarinen, R.; Aro, K.; Mäkinen, L.K.; Tarkkanen, J.; Mäkitie, A.; Haglund, C.; Hagström, J.; Atula, T. Sclerosing Sialadenitis of the Submandibular Gland Is Rarely an Immunoglobulin G4-Related Disease in the Finnish Population. Mod. Pathol. 2020, 33, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Zen, Y.; Harada, K.; Sasaki, M.; Sato, Y.; Minato, H.; Watanabe, K.; Kurumaya, H.; Katayanagi, K.; Masuda, S.; et al. Abundant IgG4-Positive Plasma Cell Infiltration Characterizes Chronic Sclerosing Sialadenitis (Küttner’s Tumor). Am. J. Surg. Pathol. 2005, 29, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.L.; Chan, T.T.F.; Choi, C.Y.; Lam, S.H. Kuttner’s Tumour (Chronic Sclerosing Sialadenitis) of the Submandibular Gland: A Clinical Perspective. Hong Kong Med. J. 2008, 14, 46–49. [Google Scholar] [PubMed]
- Machado De Sousa, S.O.; Linhares Ferrazzo, K.; Mota Loyola, A.; Dos Santos, J.N.; De Araújo, V.C. Immunoprofile of Kuttner Tumor (Chronic Sclerosing Sialadenitis). Int. J. Surg. Pathol. 2008, 16, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Culver, E.L.; Hunt, A.; Crewe, E.; Shah, K.A.; Martinez-Devesa, P. Immunoglobulin G4 Related Chronic Sclerosing Sialadenitis. J. Laryngol. Otol. 2015, 129, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Moriyama, M.; Kawano, S.; Tanaka, A.; Maehara, T.; Hayashida, J.; Goto, Y.; Kiyoshima, T.; Shiratsuchi, H.; Ohyama, Y.; et al. Clinical Relevance of K Üttner Tumour and I g G 4-related Dacryoadenitis and Sialoadenitis. Oral Dis. 2015, 21, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Uhliarova, B.; Svec, M. Kuttner Tumor. Bratisl. Med. J. 2012, 114, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Shalley, S.; Chand, N.; Aggarwal, A.; Garg, L.N.; Yadav, V.; Yadav, A. Diagnostic Accuracy of Fine Needle Aspiration Cytology in Lesions of Oral Cavity and Salivary Glands: A Clinico-Pathological Study. Open Dent. J. 2018, 12, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Klöppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus Statement on the Pathology of IgG4-Related Disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Okazaki, K.; Masaki, Y.; Kawano, M.; Yamamoto, M.; Saeki, T.; Matsui, S.; Yoshino, T.; Nakamura, S.; Kawa, S.; et al. Comprehensive Diagnostic Criteria for IgG4-Related Disease (IgG4-RD), 2011. Mod. Rheumatol. 2012, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Ishizaka, N.; Akamizu, T.; Sato, Y.; Kawano, M.; et al. The 2020 Revised Comprehensive Diagnostic (RCD) Criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Fiorella, R.; Di Nicola, V.; Fiorella, M.L.; Spinelli, D.A.; Coppola, F.; Luperto, P.; Madami, L. Major Salivary Gland Diseases. Multicentre Study. Acta Otorhinolaryngol. Ital. 2005, 25, 182–190. [Google Scholar]
- Ahuja, A.T.; Richards, P.S.; Wong, K.T.; King, A.D.; Yuen, H.Y.; Ching, A.S.C.; To, E.W.H.; To, K.F. Kuttner Tumour (Chronic Sclerosing Sialadenitis) of the Submandibular Gland: Sonographic Appearances. Ultrasound Med. Biol. 2003, 29, 913–919. [Google Scholar] [CrossRef] [PubMed]




| Case | Sex | SMG Affected | Symptoms of Dry Eye and Dry Mouth at Initial Presentation | Respiratory Symptoms at Initial Presentation (Cough or Shortness of Breath) | Past Medical History of Inflammatory Joint Disease at Initial Presentation | IgG4 Concentrations Tested | Number of Follow-Up Ultrasounds | Outcome | Time from Diagnosis Until Recovery (Years) | Immunosuppressive Therapy Required |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | Left | No | No | No | No | 3 | Resolved | 1.5 | No |
| 2 | Female | Left | No | No | No | No | 4 | Resolved | 2 | No |
| 3 | Male | Left | No | No | No | No | 4 | Resolved | 2 | No |
| 4 | Female | Right | No | No | No | No | 3 | Resolved | 1.5 | No |
| 5 | Male | Right | No | No | No | No | 3 | Resolved | 1.5 | No |
| 6 | Female | Left | No | No | No | No | 3 | Resolved | 1.5 | No |
| 7 | Female | Left | No | No | No | No | 3 | Resolved | 1.5 | No |
| 8 | Female | Left | No | No | No | No | 3 | Resolved | 1.5 | No |
| 9 | Male | Left | No | No | No | No | 3 | Resolved | 1.5 | Yes |
| 10 | Female | Left | No | No | No | No | 4 | Resolved | 2 | |
| 11 | Male | Right | No | No | No | No | 4 | Resolved | 2 | |
| 12 | Female | Right then left | No | No | No | Yes | 4 | IgG4-RD | Change to IgG4-RD |
| Article | Date of Publication | Country of Publication | Sample Size | Cases with Presence of IGg4 Related Disease | Investigation | Igg4 Definition Criteria | Management of Kuttner’s Tumour With IgG4-Related Disease | Surveillance Method and Frequency |
|---|---|---|---|---|---|---|---|---|
| Muniz et al. [6]. | 2024 | Brazil | 17 | 3 | Excisional biopsy Immunohistochemistry Histopathology | Umehara et al. | Not reported | Not reported |
| Peuraharju et al. [7]. | 2020 | Finland | 51 | 2 (both classified as non-sclerosing chronic sialadenitis. One of these cases had a preceding IgG4 related dacryoadenitis) | Excisional biopsy Immunohistochemistry Histopathology Preoperative ultrasound (n = 49) MRI (n = 9) CT (n = 2) Serum IgG4 level (n = 2) | Boston consensus statement | Oral prednisolone 7 months (n = 1) Oral prednisolone and azathioprine (n = 1) | Symptom questionnaire |
| Furukawa et al. [12]. | 2015 | Japan | 54 | 1 | Excisional biopsy Immunohistochemistry Histopathology Serum IgG4 level (n = 6) | Umehara et al. | Oral prednisolone (n = 1) | Recurrence documented, method of monitoring not reported |
| Culver et al. [11]. | 2015 | UK | N/A | N/A | Serum IgG4 level USS head and neck Fine-needle aspiration Ultrasound guided biopsy Excisional biopsy Immunohistochemistry Histopathology | Boston consensus statement | N/A | Recognised risk of recurrence, no method of monitoring suggested |
| Uhliarova et al. [13]. | 2013 | Slovakia | 7 | 0 | Pre-operative ultrasound (n = 7) CT (n = 2) Fine-needle aspiration (n = 5) | Not applicable | Not applicable | Mention of annual USS to monitor recurrence of CSS, nothing specific to IgG4 related disease |
| Geyer et al. [3]. | 2010 | USA | 13 | 3 | Serum IgG4 levels (n = 11) Biopsy [unspecified] (n = 2) CT (n = 1) | Clinical/pathological evidence | Glucocorticoids (n = 1) | Follow-up method not specified (mean follow-up of 6 years) |
| Machado De Sousa et al. [10]. | 2008 | Brazil | 2 | 0 | Sialogram (n = 2) CT (n = 1) | Not reported | Not applicable | Not reported |
| Chow et al. [9]. | 2008 | Hong Kong | 9 | 0 | USS (n = 9) CT (n = 2) MRI (n = 1) Fine-needle aspiration and cytology (n = 6) | Not applicable | Not applicable | Not reported |
| Kitagawa et al. [8]. | 2005 | Japan | 12 | Not formally identified: 45% of IgG-positive plasma cells were of IgG4-type in all CSS cases | Not reported | Not applicable | Not applicable (all samples underwent excisional biopsy) | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Deerawi, Z.; El-Badawi, K.; Shrivastava, A.; Barrak, H. Kuttner’s Tumour: A Case Series and Narrative Review on Diagnosis, Management, and Outcomes. J. Clin. Med. 2025, 14, 3208. https://doi.org/10.3390/jcm14093208
Al-Deerawi Z, El-Badawi K, Shrivastava A, Barrak H. Kuttner’s Tumour: A Case Series and Narrative Review on Diagnosis, Management, and Outcomes. Journal of Clinical Medicine. 2025; 14(9):3208. https://doi.org/10.3390/jcm14093208
Chicago/Turabian StyleAl-Deerawi, Zaid, Kamal El-Badawi, Arpan Shrivastava, and Husham Barrak. 2025. "Kuttner’s Tumour: A Case Series and Narrative Review on Diagnosis, Management, and Outcomes" Journal of Clinical Medicine 14, no. 9: 3208. https://doi.org/10.3390/jcm14093208
APA StyleAl-Deerawi, Z., El-Badawi, K., Shrivastava, A., & Barrak, H. (2025). Kuttner’s Tumour: A Case Series and Narrative Review on Diagnosis, Management, and Outcomes. Journal of Clinical Medicine, 14(9), 3208. https://doi.org/10.3390/jcm14093208






