Preterm Cesarean Delivery and Safety of Subsequent Delivery: Risk of Uterine Rupture and Other Maternal and Neonatal Outcomes—Multicenter Retrospective Cohort Study
Abstract
1. Introduction
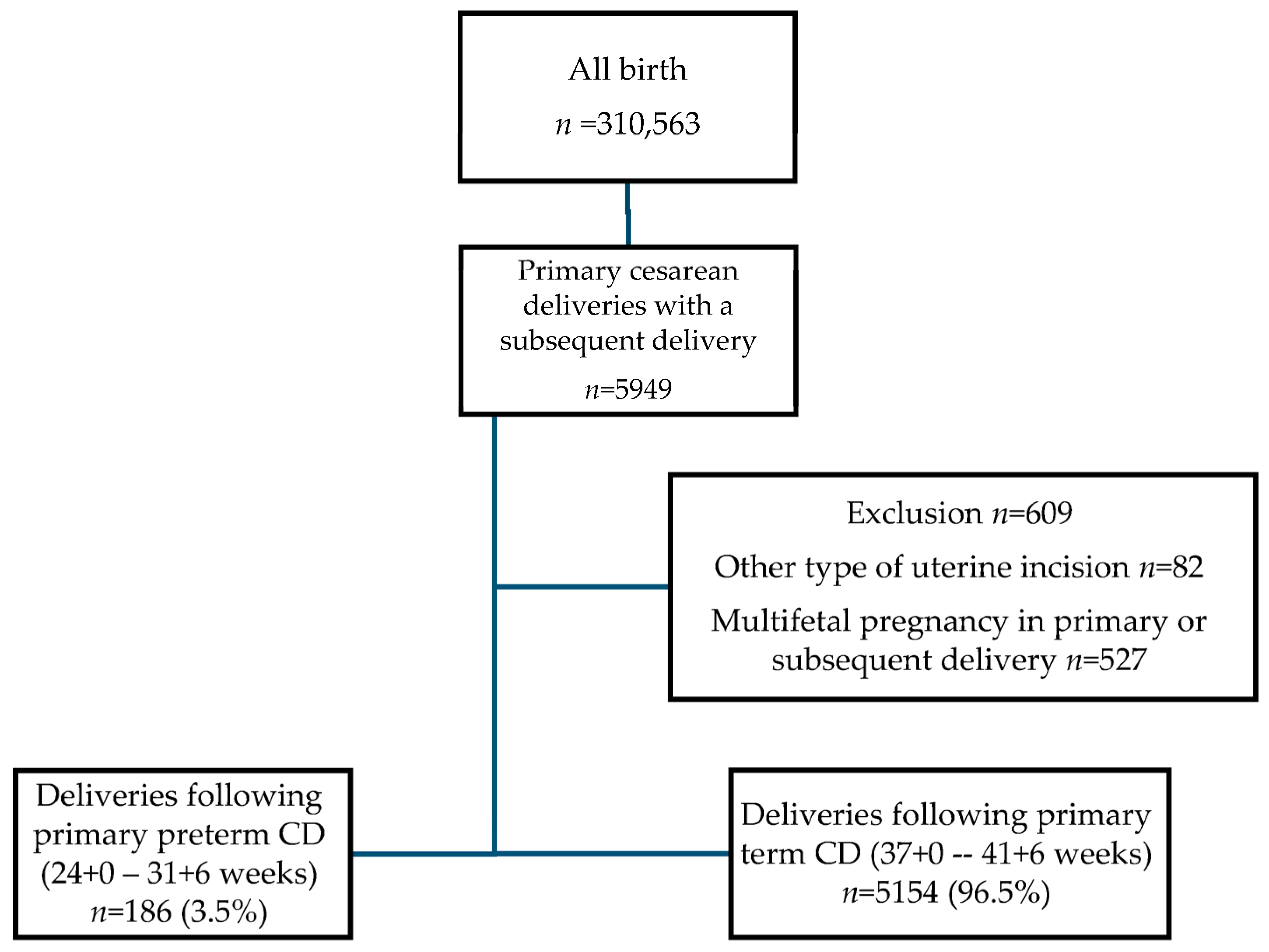
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Results in the Context of What Is Known
4.3. Mechanisms and Clinical Interpretation
4.4. Clinical Implications
4.5. Research Implications
4.6. Strengths and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSTCS | Low-segment transverse cesarean section |
| ACOG | American College of Obstetrics and Gynecology |
| aOD | Adjusted Odds ratio |
| ART | Assisted reproductive technologies |
| BCH | Bikur Cholim Hospital |
| BMI | Body mass index |
| CI | Confidence interval |
| EMR | Electronic medical record |
| GDM | Gestational diabetes mellitus |
| ICD | International Classification of Diseases |
| IQR | Interquartile range |
| IUFD | Intrauterine fetal death |
| NICU | Neonatal intensive care unit |
| pCD | Preterm cesarean delivery |
| PPH | Postpartum hemorrhage |
| RDS | Respiratory distress syndrome |
| SD | Standard deviations |
| tCD | Term cesarean delivery |
| TOLAC | Trial of labor after cesarean |
| UR | Uterine rupture |
| VBAC | Vaginal birth after cesarean |
References
- Keag, O.E.; Norman, J.E.; Stock, S.J. Long-Term Risks and Benefits Associated with Cesarean Delivery for Mother, Baby, and Subsequent Pregnancies: Systematic Review and Meta-Analysis. PLoS Med. 2018, 15, e1002494. [Google Scholar] [CrossRef] [PubMed]
- Tanos, V.; Toney, Z.A. Uterine Scar Rupture—Prediction, Prevention, Diagnosis, and Management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 115–131. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin No. 115: Vaginal Birth after Previous Cesarean Delivery. Obstet. Gynecol. 2010, 116, 450–463. [CrossRef]
- ACOG Practice Bulletin No. 205: Vaginal Birth After Cesarean Delivery. Obstet. Gynecol. 2019, 133, e110–e127. [CrossRef]
- Durnwald, C.P.; Mercer, B.M. Myometrial Thickness According to Uterine Site, Gestational Age and Prior Cesarean Delivery. J. Matern. Fetal Neonatal Med. 2008, 21, 247–250. [Google Scholar] [CrossRef]
- Mantel, Ä.; Ajne, G.; Lindblad Wollmann, C.; Stephansson, O. Previous Preterm Cesarean Delivery and Risk of Uterine Rupture in Subsequent Trial of Labor-a National Cohort Study. Am. J. Obstet. Gynecol. 2021, 224, 380.e1–380.e13. [Google Scholar] [CrossRef]
- Rochelson, B.; Pagano, M.; Conetta, L.; Goldman, B.; Vohra, N.; Frey, M.; Day, C. Previous Preterm Cesarean Delivery: Identification of a New Risk Factor for Uterine Rupture in VBAC Candidates. J. Matern. Fetal Neonatal Med. 2005, 18, 339–342. [Google Scholar] [CrossRef]
- Harper, L.M.; Cahill, A.G.; Stamilio, D.M.; Odibo, A.O.; Peipert, J.F.; Macones, G.A. Effect of Gestational Age at the Prior Cesarean Delivery on Maternal Morbidity in Subsequent VBAC Attempt. Am. J. Obstet. Gynecol. 2009, 200, 276.e1–276.e6. [Google Scholar] [CrossRef]
- Sciscione, A.C.; Landon, M.B.; Leveno, K.J.; Spong, C.Y.; Macpherson, C.; Varner, M.W.; Rouse, D.J.; Moawad, A.H.; Caritis, S.N.; Harper, M.; et al. Previous Preterm Cesarean Delivery and Risk of Subsequent Uterine Rupture. Obstet. Gynecol. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Lannon, S.M.R.; Guthrie, K.A.; Vanderhoeven, J.P.; Gammill, H.S. Uterine Rupture Risk after Periviable Cesarean Delivery. Obstet. Gynecol. 2015, 125, 1095–1100. [Google Scholar] [CrossRef]
- Kwee, A.; Smink, M.; Van Der Laar, R.; Bruinse, H.W. Outcome of Subsequent Delivery after a Previous Early Preterm Cesarean Section. J. Matern. Fetal Neonatal Med. 2007, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.; Mahajna, M.; Ehrlich, Z.; Ratner, M.; Grisaru-Granovsky, S.; Reichman, O. Risk of Preterm Birth among Secundiparas with a Previous Cesarean Due to a Failed Vacuum Delivery. J. Clin. Med. 2023, 12, 7358. [Google Scholar] [CrossRef] [PubMed]
- Atia, O.; Rotem, R.; Reichman, O.; Jaffe, A.; Grisaru-Granovsky, S.; Sela, H.Y.; Rottenstreich, M. Number of Prior Vaginal Deliveries and Trial of Labor after Cesarean Success. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 189–193. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health National Institutes of Health Consensus Development Conference Statement Vaginal Birth after Cesarean: New Insights March 8–10, 2010. Semin. Perinatol. 2010, 34, 351–365. [CrossRef]
- Landon, M.B.; Hauth, J.C.; Leveno, K.J.; Spong, C.Y.; Leindecker, S.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Maternal and Perinatal Outcomes Associated with a Trial of Labor after Prior Cesarean Delivery. N. Engl. J. Med. 2004, 351, 2581–2589. [Google Scholar] [CrossRef]
- Eden, K.B.; McDonagh, M.; Denman, M.A.; Marshall, N.; Emeis, C.; Fu, R.; Janik, R.; Walker, M.; Guise, J.-M. New Insights on Vaginal Birth after Cesarean: Can It Be Predicted? Obstet. Gynecol. 2010, 116, 967–981. [Google Scholar] [CrossRef]
- Ananth, C.V.; Getahun, D.; Peltier, M.R.; Salihu, H.M.; Vintzileos, A.M. Recurrence of spontaneous versus medically indicated preterm birth. Am. J. Obstet. Gynecol. 2006, 195, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Gimovsky, A.C.; Levine, L.D.; Vink, J. Cesarean in the second stage: A possible risk factor for subsequent spontaneous preterm birth. Am. J. Obstet. Gynecol. 2017, 217, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silver, R.M.; Landon, M.B.; Rouse, D.J.; Leveno, K.J.; Spong, C.Y.; Thom, E.A.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006, 107, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.A.; Silver, R.M. Long-term maternal morbidity associated with repeat cesarean delivery. Am. J. Obstet. Gynecol. 2011, 205 (Suppl. S6), S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Saá, L.E.; Sendra, R.; Carriles, I.; Sousa, M.; Turiel, M.; Ruiz-Zambrana, Á.; Chiva, L. Maternal and Fetal Outcomes after Multiple Cesarean Deliveries. J. Clin. Med. 2024, 13, 4425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hook, B.; Kiwi, R.; Amini, S.B.; Fanaroff, A.; Hack, M. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics 1997, 100 Pt 1, 348–353. [Google Scholar] [CrossRef] [PubMed]
| Delivery Characteristics | pCD n = 186 | tCD n = 5154 | p Value |
|---|---|---|---|
| Gestational age, median (range) | 29 (27–30) | 39 (38–40) | <0.001 @ |
| Nulliparity n (%) | 97 (52.2) | 3107 (60.3) | 0.026 ^ |
| Maternal age, years mean ± SD | 28.9 ± 5.5 | 30.6 ± 5.6 | <0.001 # |
| Advanced maternal age (>35 years) n (%) | 26 (14%) | 1021 (19.8%) | 0.049 ^ |
| Pregnancy Comorbidities n (%) * | |||
| GDM | 4 (2.2%) | 347 (6.7%) | 0.100 ^ |
| Hypertension | 47 (25.3%) | 238 (4.6%) | <0.001^ |
| ART | 12 (6.5%) | 423 (8.2%) | 0.400 ^ |
| Neonatal birthweight, grams mean ±SD | 1151 ± 385 | 3236 ±538 | <0.001 @ |
| 1’ Apgar score < 7 n (%) | 119 (64%) | 594 (11.5%) | <0.001 ^ |
| 5’ Apgar score < 7 n (%) | 74 (39.8%) | 129 (2.5%) | <0.001 ^ |
| NICU admission n (%) | 180 (96.8%) | 420 (8.1%) | <0.001 ^ |
| History of pCD n = 186 | History of tCD n = 5154 | p Value | |
|---|---|---|---|
| Gestational age, median (range) | 39 (37–40) | 39 (38–40) | <0.010 @ |
| Preterm delivery < 37 weeks n (%) | 37 (19.9%) | 240 (4.7%) | <0.001 ^ |
| Onset of labor n (%) | |||
| Induction of labor | 11 (5.9%) | 396 (7.7%) | 0.230 ^ |
| Planned CD | 49 (26.3%) | 1676 (32.5%) | 0.050 ^ |
| Spontaneous onset of labor | 137 (73.7%) | 3478 (67.5%) | reference |
| Comorbidities n (%) * | |||
| GDM | 10 (5.4%) | 382 (7.4%) | 0.296 ^ |
| Hypertension | 38 (20.4%) | 130 (2.5%) | <0.001 ^ |
| ART n (%) | 10 (5.4%) | 316 (6.1%) | 0.673 ^ |
| History of pCD n = 186 | History of tCD n = 5154 | p Value | |
|---|---|---|---|
| Uterine Rupture n (%) | 0 (0%) | 32 (0.6%) | 0.321 ^ |
| Mode of delivery n (%) | |||
| Spontaneous vaginal | 106 (57%) | 2290 (44%) | reference |
| Instrumental | 13 (7%) | 424 (8.2%) | 0.170 ^ |
| Emergent CD | 18 (10%) | 764 (14.8%) | 0.080 ^ |
| Planned CD | 49 (26%) | 1676 (33%) | 0.090 ^ |
| PPH n (%) | 1 (0.5%) | 47 (0.9%) | 0.595 ^ |
| Blood transfusion n (%) | 1 (0.5%) | 19 (0.4%) | 0.711 ^ |
| Placenta accreta spectrum n (%) | 1 (0.5%) | 6 (0.11%) | 0.119 ^ |
| Peripartum hysterectomy n (%) | 1 (0.5%) | 4 (0.08%) | 0.044 ^ |
| Uterine dehiscence n (%) | 1 (0.5%) | 82 (1.6%) | 0.254 ^ |
| IUFD n (%) | 1 (0.5%) | 24 (0.5%) | 0.972 ^ |
| Puerperal fever n (%) | 1 (0.6%) | 25 (0.6%) | 0.631 ^ |
| Birth weight (mean ± SD) | 2939 ± 691 | 3275 ± 526 | <0.001 @ |
| 1’ Apgar score < 7 n (%) | 21 (11.3%) | 265 (5.1%) | <0.001 ^ |
| 5’ Apgar score < 7 n (%) | 8 (4.3%) | 96 (1.9%) | 0.018 ^ |
| NICU admission n (%) | 30 (16.1%) | 291 (5.6%) | <0.001 ^ |
| Jaundice n (%) | 19 (10.9%) | 327 (6.7%) | 0.031 ^ |
| Sepsis n (%) | 4 (2.3%) | 45 (0.92%) | 0.087 ^ |
| RDS n (%) | 15 (8.6%) | 192 (3.2%) | 0.002 ^ |
| Neonatal hypoglycemia n (%) | 11 (6.3%) | 348 (7.1) | 0.678 ^ |
| Neonatal blood transfusion n (%) | 3 (1.6%) | 12 (0.24%) | 0.014 ^ |
| Composite of neonatal adverse outcomes n (%) | 40 (24.5%) | 634 (15.1%) | 0.001 ^ |
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|
| Study Group history of pCD (Reference = history of tCD) | 1.826 (1.265–2.634) | 0.794 (0.486–1.298) | 0.358 |
| Nulliparity at index delivery | 0.989 (0.836–1.171) | 0.946 (0.780–1.147) | 0.571 |
| Preterm Birth (<37 weeks) | 20.854 (15.640–27.808) | 12.684 (9.059–17.760) | <0.001 |
| Mode of Delivery | |||
| —Vaginal (Reference) | |||
| —Instrumental | 1.220 (0.916–1.624) | 1.939 (1.397–2.691) | <0.001 |
| —Cesarean Delivery | 1.399 (1.186–1.651) | 1.259 (1.032–1.536) | 0.023 |
| Macrosomia | 0.809 (0.576–1.135) | 1.131 (0.797–1.604) | 0.491 |
| Low Birth Weight (LBW) | 8.987 (6.984–11.566) | 2.700 (1.922–3.792) | <0.001 |
| Design | Sample Size | Primary Outcome | OR (95% CI) | |
|---|---|---|---|---|
| Sciscione et al., 2008 [9] | Prospective observational <37 weeks | 5839 | Uterine rupture | 1.62 (1.01–2.50) |
| Harper et al., 2009 [8] | Retrospective cohort <34 weeks | 508 | Maternal morbidity, including uterine rupture | No difference |
| Rochelson et al., 2009 [7] | Case-control <36 weeks | 25 | Uterine rupture | 5.39 (2.3–12.4) |
| Lannon et al., 2015 [10] | Retrospective longitudinal 20–27 weeks | 456 | Uterine rupture | 4.7 (2.3–10.6) |
| Mantel et al., 2021 [6] | Prospective <32 weeks, 32–36 weeks | 9300 | Uterine rupture | No difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helman, S.; Fridman Lev, S.; Solnica, A.; Reichman, O.; Farkash, R.; Grisaru-Granovsky, S.; Bas Lando, M. Preterm Cesarean Delivery and Safety of Subsequent Delivery: Risk of Uterine Rupture and Other Maternal and Neonatal Outcomes—Multicenter Retrospective Cohort Study. J. Clin. Med. 2025, 14, 1522. https://doi.org/10.3390/jcm14051522
Helman S, Fridman Lev S, Solnica A, Reichman O, Farkash R, Grisaru-Granovsky S, Bas Lando M. Preterm Cesarean Delivery and Safety of Subsequent Delivery: Risk of Uterine Rupture and Other Maternal and Neonatal Outcomes—Multicenter Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(5):1522. https://doi.org/10.3390/jcm14051522
Chicago/Turabian StyleHelman, Sarit, Shira Fridman Lev, Amy Solnica, Orna Reichman, Rivka Farkash, Sorina Grisaru-Granovsky, and Maayan Bas Lando. 2025. "Preterm Cesarean Delivery and Safety of Subsequent Delivery: Risk of Uterine Rupture and Other Maternal and Neonatal Outcomes—Multicenter Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 5: 1522. https://doi.org/10.3390/jcm14051522
APA StyleHelman, S., Fridman Lev, S., Solnica, A., Reichman, O., Farkash, R., Grisaru-Granovsky, S., & Bas Lando, M. (2025). Preterm Cesarean Delivery and Safety of Subsequent Delivery: Risk of Uterine Rupture and Other Maternal and Neonatal Outcomes—Multicenter Retrospective Cohort Study. Journal of Clinical Medicine, 14(5), 1522. https://doi.org/10.3390/jcm14051522







