Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Osteopenia, Osteoporosis, and Risk of Fractures in IBD: Focus on UC
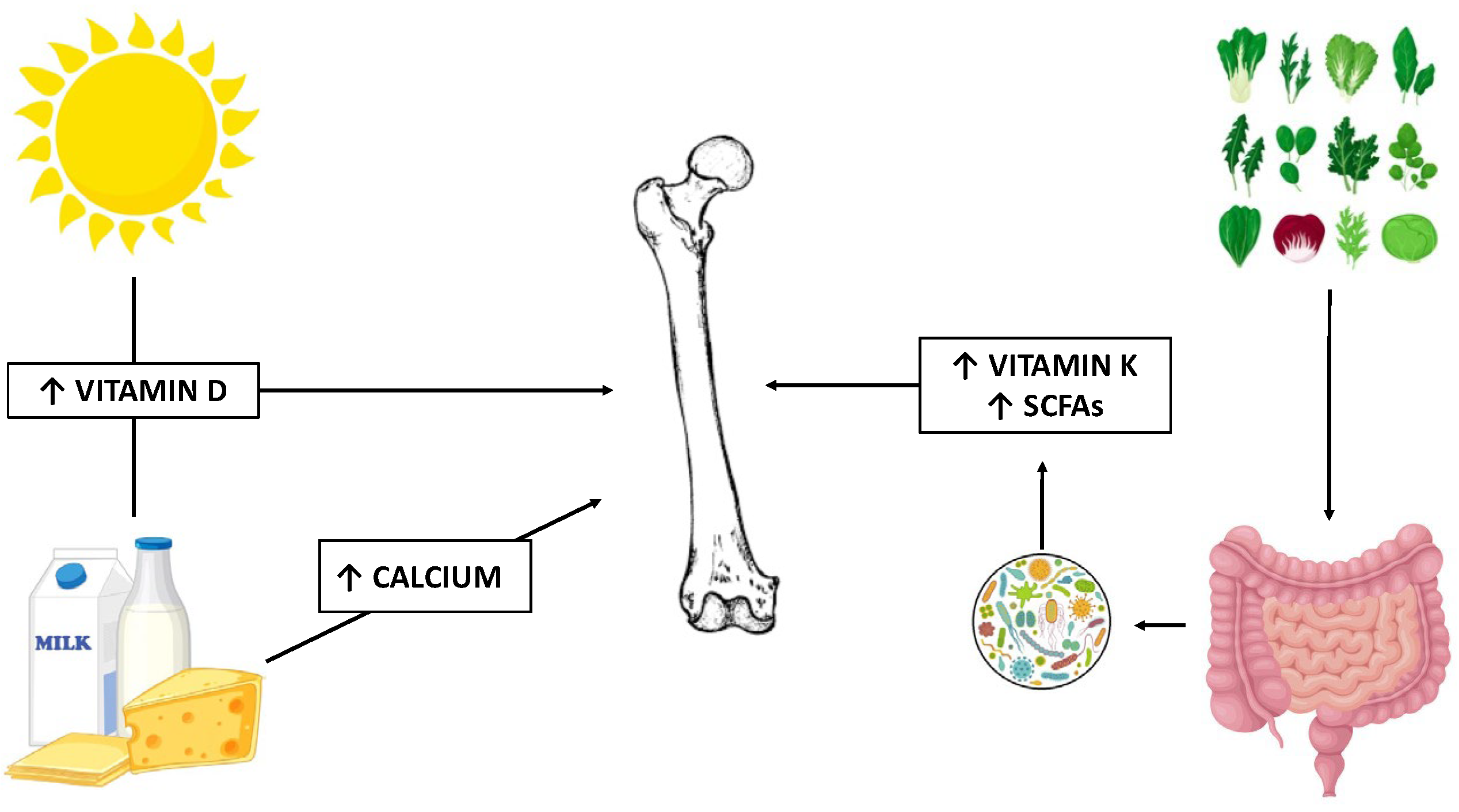
3.2. Calcium
3.3. Vitamin D
3.4. Vitamin K
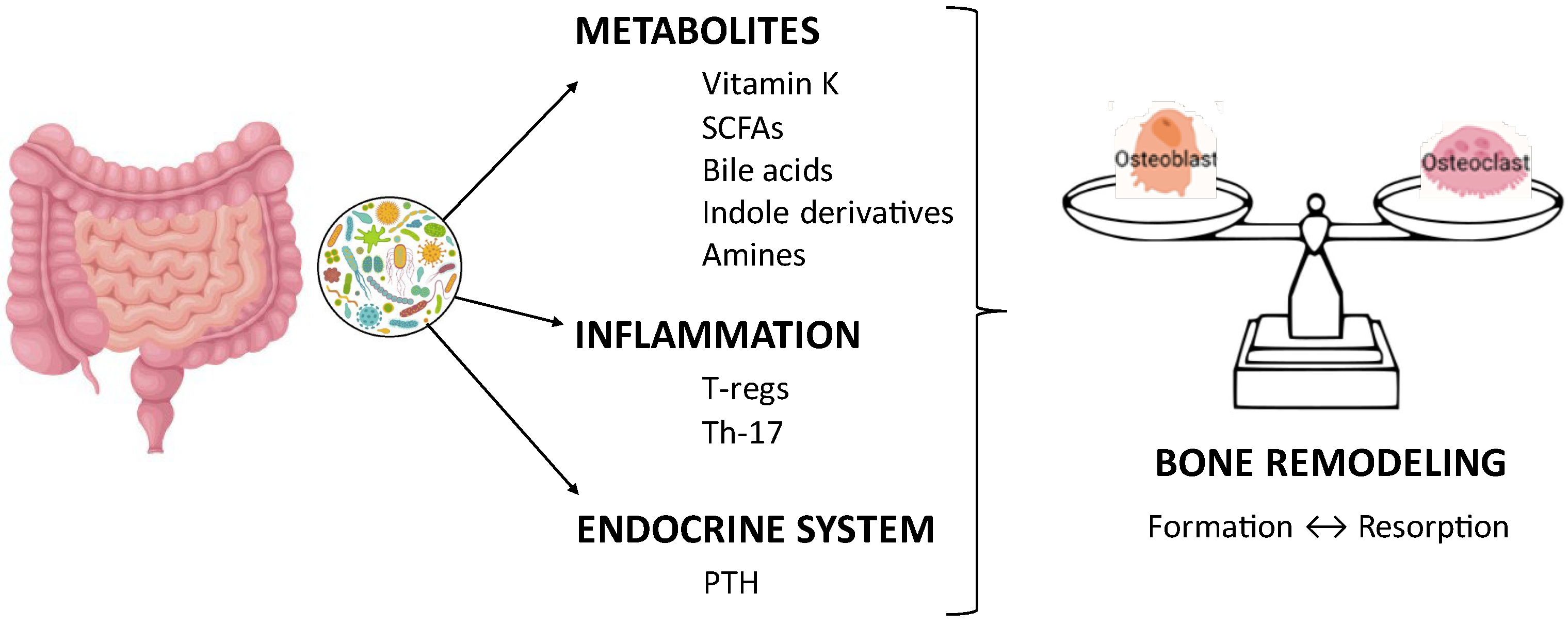
3.5. Microbiota, Diet, and Bone Health
3.6. Dietary Intervention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| UC | ulcerative colitis |
| BMD | bone mineral density |
| MeSH | Medical Subject Headings |
| CD | Crohn’s disease |
| RANKL | receptor activator of nuclear factor kB ligand |
| OPG | osteoprotegerin |
| TNF-α | tumor necrosis factor α |
| IPAA | ileal pouch anal anastomosis |
| RDA | recommended daily allowance |
| PTH | parathyroid hormone |
| VDR | vitamin D receptor |
| PT | prothrombin time |
| PIVKA-II | protein induced by vitamin K absence-II |
| ucOC | undercarboxylated osteocalcin |
| SCFA | short-chain fatty acid |
| BA | secondary bile acid |
References
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef]
- Bardella, M.T.; Bianchi, M.L.; Teti, A. Chronic inflammatory intestinal diseases and bone loss. Gut 2005, 54, 1508. [Google Scholar]
- Ghishan, F.K.; Kiela, P.R. Vitamins and minerals in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Oh, H.J.; Ryu, K.H.; Park, B.J.; Yoon, B.H. Osteoporosis and osteoporotic fractures in gastrointestinal disease. J. Bone Metab. 2018, 25, 213–217. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef]
- Grüner, N.; Ortlepp, A.L.; Mattner, J. Pivotal role of intestinal microbiota and intraluminal metabolites for the maintenance of gut–bone physiology. Int. J. Mol. Sci. 2023, 24, 5161. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Imperio, G.; Ventura, A.; Cipullo, M.; Coppola, A.; Federico, A. Profiling the patient with inflammatory bowel disease in the relationship between physical activity and partner/social network status: A post hoc patient-tailored analysis of the “BE-FIT-IBD” study. Gastroenterol. Hepatol. 2025, 48, 502203. [Google Scholar] [CrossRef]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in inflammatory bowel disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Macpherson, A.; Mackintosh, C.; Buxton-Thomas, M.; Forgacs, I.; Moniz, C. Reduced bone density in patients with inflammatory bowel disease. Gut 1997, 40, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Pollak, R.D.; Karmeli, F.; Eliakim, R.; Ackerman, Z.; Tabb, K.; Rachmilewitz, D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1998, 93, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Crowson, C.S.; Sandborn, W.J.; Tremaine, W.J.; O’Fallon, W.M.; Melton, L.J., 3rd. Long-term fracture risk in patients with Crohn’s disease: A population-based study in Olmsted County, Minnesota. Gastroenterology 2002, 123, 468–475. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr.; Achenbach, S.J.; Sandborn, W.J.; Tremaine, W.J.; Oberg, A.L.; Melton, L.J., 3rd. Risk of fracture in ulcerative colitis: A population-based study from Olmsted County, Minnesota. Clin. Gastroenterol. Hepatol. 2003, 1, 465–473. [Google Scholar] [CrossRef]
- Shim, J.H.; Stavre, Z.; Gravallese, E.M. Bone loss in rheumatoid arthritis: Basic mechanisms and clinical implications. Calcif. Tissue Int. 2018, 102, 533–546. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Leslie, W.; Wajda, A.; Yu, B.N. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann. Intern. Med. 2000, 133, 795–799. [Google Scholar] [CrossRef]
- Lo, B.; Holm, J.P.; Vester-Andersen, M.K.; Bendtsen, F.; Vind, I.; Burisch, J. Incidence, risk factors and evaluation of osteoporosis in patients with inflammatory bowel disease a Danish population-based inception cohort with 10 years of follow up. J. Crohn’s Colitis 2020, 14, 904–914. [Google Scholar] [CrossRef]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berni Canani, R.; O’Mahony, L.; Peroni, D.; Sokolowska, M.; Vassilopoulou, E.; Venter, C. Nutrition in chronic inflammatory conditions: Bypassing the mucosal block for micronutrients. Allergy 2024, 79, 353–383. [Google Scholar] [CrossRef]
- Miranda-Bautista, J.; Verdejo, C.; Díaz-Redondo, A.; Bretón, I.; Bellón, J.M.; Pérez-Valderas, M.D.; Caballero-Marcos, A.; de Dios-Lascuevas, M.; González-Río, E.; García-Sánchez, C.; et al. Metabolic bone disease in patients diagnosed with inflammatory bowel disease from Spain. Therap Adv. Gastroenterol. 2019, 12, 1756284819862152. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.; Fournier, N.; Zeitz, J.; Scharl, M.; Morell, B.; Greuter, T.; Schreiner, P.; Misselwitz, B.; Safroneeva, E.; Schoepfer, A.M.; et al. Early Initiation of Anti-TNF is Associated with Favourable Long-term Outcome in Crohn’s Disease: 10-Year-Follow-up Data from the Swiss IBD Cohort Study. J. Crohn’s Colitis 2019, 13, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Kuisma, J.; Luukkonen, P.; Jarvinen, H.; Kahri, A.; Farkkila, M. Risk of osteopenia after proctocolectomy and ileal pouch–anal anastomosis for ulcerative colitis. Scand. J. Gastroenterol. 2002, 37, 171–176. [Google Scholar] [CrossRef]
- Abitbol, V.; Roux, C.; Guillemant, S.; Valleur, P.; Hautefeuille, P.; Dougados, M.; Couturier, D.; Chaussade, S. Bone assessment in patients with ileal pouch–anal anastomosis for inflammatory bowel disease. Br. J. Surg. 1997, 84, 1551–1554. [Google Scholar]
- McLaughlin, S.D.; Perry-Woodford, Z.L.; Clark, S.K.; Johnson, M.W.; Tekkis, P.P.; Ciclitira, P.J.; Nicholls, R.J. Osteoporosis in patients over 50 years of age following restorative proctocolectomy for ulcerative colitis: Is DXA screening warranted? Inflamm. Bowel Dis. 2010, 16, 250–255. [Google Scholar] [CrossRef]
- Navaneethan, U.; Shen, L.; Venkatesh, P.G.; Hammel, J.; Patel, V.; Remzi, F.H.; Kiran, R.P. Influence of ileal pouch anal anastomosis on bone loss in ulcerative colitis patients. J. Crohn’s Colitis 2011, 5, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Houborg, K.B.; Vestergaard, P.; Kissmeyer-Nielsen, P.; Mosekilde, L.; Laurberg, S. Improved physical performance and increased lean tissue and fat mass in patients with ulcerative colitis four to six years after ileo-anal anastomosis with a J-pouch. Dis. Colon. Rectum 2002, 45, 1601–1607. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Gado, M.; Baschant, U.; Hofbauer, L.C.; Henneicke, H. Bad to the bone: The effects of therapeutic glucocorticoids on osteoblasts and osteocytes. Front. Endocrinol. 2022, 13, 835720. [Google Scholar] [CrossRef]
- Tome, J.; Sehgal, K.; Kamboj, A.K.; Comstock, B.; Harmsen, W.S.; Khanna, S.; Pardi, D.S. Budesonide maintenance in microscopic colitis: Clinical outcomes and safety profile from a population-based study. Am. J. Gastroenterol. 2022, 117, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Sokal-Dembowska, A.; Filip, R. Nutritional support: The use of antioxidants in inflammatory bowel disease. Int. J. Mol. Sci. 2024, 25, 4390. [Google Scholar] [CrossRef]
- Zou, M.; Liang, Q.; Zhang, W.; Liang, J.; Zhu, Y.; Xu, Y. Diet-derived circulating antioxidants and risk of inflammatory bowel disease: A Mendelian randomization study and meta-analysis. Front. Immunol. 2024, 15, 1334395. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Sokal-Dembowska, A.; Helma, K.; Motyka, E.; Jarmakiewicz-Czaja, S.; Filip, R. Modulation of the gut microbiota by nutrition and its relationship to epigenetics. Int. J. Mol. Sci. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Vernia, F.; Burrelli Scotti, G.; Bertetti, N.S.; Donato, G.; Necozione, S.; Vernia, P.; Pallotta, N. Low Vitamin K and Vitamin D Dietary Intake in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1678. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef]
- Larussa, T.; Suraci, E.; Marasco, R.; Imeneo, M.; Abenavoli, L.; Luzza, F. Self-Prescribed Dietary Restrictions are Common in Inflammatory Bowel Disease Patients and Are Associated with Low Bone Mineralization. Medicina 2019, 55, 507. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Loizos, P.; Di Giuseppantonio, I.; Amore, B.; Chiappini, A.; Cannizzaro, S. Dietary calcium intake in patients with inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 312–317. [Google Scholar] [CrossRef]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A cross-sectional study on nutrient intake and -status in inflammatory bowel disease patients. Nutr. J. 2016, 15, 61. [Google Scholar] [CrossRef]
- Rizzello, F.; Gionchetti, P.; Spisni, E.; Saracino, I.M.; Bellocchio, I.; Spigarelli, R.; Collini, N.; Imbesi, V.; Dervieux, T.; Alvisi, P.; et al. Dietary Habits and Nutrient Deficiencies in a Cohort of European Crohn’s Disease Adult Patients. Int. J. Mol. Sci. 2023, 24, 1494. [Google Scholar] [CrossRef] [PubMed]
- Pierote, N.R.; Braz, A.F.; Barros, S.L.; Moita Neto, J.M.; Parente, J.M.L.; Silva, M.D.C.M.; Beserra, M.S.; Soares, N.R.M.; Marreiro, D.N.; do Noscimento Nogueira, N. Effect of mineral status and glucocorticoid use on bone mineral density in patients with Crohn’s disease. Nutrition 2018, 48, 13–17. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.J.; Hong, S.J.; Kim, S. Nutrient intake and bone mineral density by nutritional status in patients with inflammatory bowel disease. J. Bone Metab. 2014, 21, 195–203. [Google Scholar] [CrossRef] [PubMed]
- UUsi-Rasi, K.; Kärkkäinen, M.; Lamberg-Allardt, C. Calcium intake in health maintenance—A systematic review. Food Nutr. Res. 2013, 57, 21082. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Fedorak, R.N.; Siminoski, K.; Jen, H.; Vaudan, E.; Abraham, N.; Steinhart, H.; Greenberg, G. Randomized trial of etidronate plus calcium and vitamin D for treatment of low bone mineral density in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Ward, L.M.; Gallagher, J.C.; Rauch, F.; Barrowman, N.; Warren, J.; Beedle, S.; Mack, D.R. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 538–545. [Google Scholar] [CrossRef]
- Melek, J.; Sakuraba, A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: A meta-analysis and systematic review. Clin. Gastroenterol. Hepatol. 2014, 12, 32–44. [Google Scholar] [CrossRef]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An overview of gene regulation, ranging from metabolism to genomic effects. Genes. 2023, 14, 1691. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and Bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Christakos, S.; Li, S.; DeLa Cruz, J.; Verlinden, L.; Carmeliet, G. Vitamin D and Bone. Handb. Exp. Pharmacol. 2020, 262, 47–63. [Google Scholar] [PubMed]
- Wongdee, K.; Charoenphandhu, N. Vitamin D-enhanced duodenal calcium transport. Vitam. Horm. 2015, 98, 407–440. [Google Scholar]
- Krela-Kaźmierczak, I.; Szymczak, A.; Łykowska-Szuber, L.; Eder, P.; Stawczyk-Eder, K.; Klimczak, K.; Linke, K.; Horst-Sikorska, W. The importance of vitamin D in the pathology of bone metabolism in inflammatory bowel diseases. Arch. Med. Sci. 2015, 11, 1028–1032. [Google Scholar] [PubMed]
- Atkins, G.J.; Anderson, P.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.; O’Loughlin, P.D.; Morris, H.A. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef]
- van den Bemd, G.J.; Pols, H.A.; Birkenhäger, J.C.; Kleinekoort, W.M.; van Leeuwen, J.P. Differential effects of 1,25-dihydroxyvitamin D3-analogs on osteoblast-like cells and on in vitro bone resorption. J. Steroid Biochem. Mol. Biol. 1995, 55, 337–346. [Google Scholar] [CrossRef]
- Shi, Y.C.; Worton, L.; Esteban, L.; Baldock, P.; Fong, C.; Eisman, J.A.; Gardiner, E.M. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone 2007, 41, 87–96. [Google Scholar] [CrossRef]
- Thompson, L.; Wang, S.; Tawfik, O.; Templeton, K.; Tancabelic, J.; Pinson, D.; Anderson, H.C.; Keighley, J.; Garimella, R. Effect of 25-hydroxyvitamin D3 and 1 α,25 dihydroxyvitamin D3 on differentiation and apoptosis of human osteosarcoma cell lines. J. Orthop. Res. 2011, 30, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Piek, E.; Sleumer, L.S.; van Someren, E.P.; Heuver, L.; de Haan, J.R.; de Grijs, I.; Gilissen, C.; Hendriks, J.M.; van Ravestein-van Os, R.I.; Bauerschmidt, S.; et al. Osteo-transcriptomics of human mesenchymal stem cells: Accelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone 2010, 46, 613–627. [Google Scholar] [CrossRef]
- Kato, H.; Ochiai-Shino, H.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Promoting effect of 1,25(OH)2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015, 5, 140201. [Google Scholar] [CrossRef]
- van der Meijden, K.; Lips, P.; van Driel, M.; Heijboer, A.C.; Schulten, E.A.J.M.; Heijer, M.D.; Bravenboer, N. Primary Human Osteoblasts in Response to 25-Hydroxyvitamin D3, 1,25-Dihydroxyvitamin D3 and 24R,25-Dihydroxyvitamin D3. PLoS ONE 2014, 9, e110283. [Google Scholar] [CrossRef]
- Kitazawa, R.; Mori, K.; Yamaguchi, A.; Kondo, T.; Kitazawa, S. Modulation of mouse RANKL gene expression by Runx2 and vitamin D3. J. Cell. Biochem. 2008, 105, 1289–1297. [Google Scholar] [CrossRef]
- Kim, S.; Yamazaki, S.; Zella, L.A.; Shevde, N.K.; Pike, J.W. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol. Cell Biol. 2006, 26, 6469–6486. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.; Nakamichi, Y.; et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.M.; Baldock, P.A.; Thomas, G.P.; Sims, N.A.; Henderson, N.K.; Hollis, B.; White, C.P.; Sunn, K.L.; Morrison, N.A.; Walsh, W.R.; et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000, 14, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Eisman, J.A.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. Bonekey Rep. 2014, 3, 499. [Google Scholar] [CrossRef]
- Lin, Y.D.; Arora, J.; Diehl, K.; Bora, S.A.; Cantorna, M.T. Vitamin D is required for ILC3 derived IL-22 and protection from Citrobacter rodentium infection. Front. Immunol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Mathieu, C.; Adorini, L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol. Med. 2002, 8, 174–179. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient nuclear receptor VDR with new functions: Microbiome and inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The role of vitamin D in the immune system as a pro-survival molecule. Clin. Ther. 2017, 39, 894–916. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Bacchetta, J.; Edouard, T.; Laverny, G.; Bernardor, J.; Bertholet-Thomas, A.; Castanet, M.; Garnier, C.; Gennero, I.; Harambat, J.; Lapillonne, A.; et al. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch. Pediatr. 2022, 29, 312–325. [Google Scholar] [CrossRef]
- ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of Postmenopausal Osteoporosis: ACOG Clinical Practice Guideline No 2. Obstet. Gynecol. 2022, 139, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.T.; Chatur, N.; Cheong-Lee, C.; Salh, B. Hypovitaminosis D in adults with inflam-matory bowel disease: Potential role of ethnicity. Dig. Dis. Sci. 2012, 57, 2144–2148. [Google Scholar] [CrossRef] [PubMed]
- Burrelli Scotti, G.; Afferri, M.T.; De Carolis, A.; Vaiarello, V.; Fassino, V.; Ferrone, F.; Minisola, S.; Nieddu, L.; Vernia, P. Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig. Liver Dis. 2019, 51, 657–662. [Google Scholar] [CrossRef]
- Yang, C.T.; Yen, H.H.; Su, P.Y.; Chen, Y.Y.; Huang, S.P. High prevalence of vitamin D deficiency in Taiwanese patients with inflammatory bowel disease. Sci. Rep. 2024, 14, 14091. [Google Scholar] [CrossRef]
- Memel, Z.; Thiemann, A.; Dort, C.; Mahadevan, U.; Beck, K.R. Prevalence of Malnutrition and Micronutrient Deficiencies in Older Adults with Ulcerative Colitis. Dig. Dis. Sci. 2024, 69, 4203–4213. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dragoni, G.; Alpigiano, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. Nutritional adequacy in surgical IBD patients. Clin. Nutr. ESPEN 2021, 41, 198–207. [Google Scholar] [CrossRef]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A prospective analysis of micronutrient status in quiescent inflammatory bowel disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef]
- Garg, M.; Lubel, J.S.; Sparrow, M.P.; Holt, S.G.; Gibson, P.R. Review article: Vitamin D and inflammatory bowel disease--established concepts and future directions. Aliment. Pharmacol. Ther. 2012, 36, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.P.; Sassaki, L.Y.; Dorna, M.S.; Carvalhaes, M.A.; Martini, L.A.; Ferreira, A.L. Nutritional intake according to injury extent in ulcerative colitis patients. J. Hum. Nutr. Diet. 2013, 26, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Burrelli Scotti, G.; Dei Giudici, A.; Chiappini, A.; Cannizzaro, S.; Afferri, M.T.; de Carolis, A. Inadequate sunlight exposure in patients with inflammatory bowel disease. J. Dig. Dis. 2018, 19, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, N.H.; Manaf, Z.A.; Mokhtar, N.M.; Raja Ali, R.A. An assessment of dietary intake, food avoidance and food beliefs in patients with ulcerative colitis of different disease status. Intest. Res. 2020, 18, 447–458. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.G.; et al. Association of Vitamin D level with clinical status in inflammatory bowel disease: A 5-year longitudinal study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble vitamin deficiencies and inflammatory bowel disease: Systematic review and meta-analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Godala, M.; Gaszyńska, E.; Walczak, K.; Małecka-Wojciesko, E. Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers. Nutrients 2023, 15, 3479. [Google Scholar] [CrossRef]
- Zator, Z.A.; Cantu, S.M.; Konijeti, G.G.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Ananthakrishnan, A.N. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J. Parenter. Enteral Nutr. 2014, 38, 385–391. [Google Scholar] [CrossRef]
- Xia, S.L.; Min, Q.J.; Shao, X.X.; Lin, D.P.; Ma, G.L.; Wu, H.; Cao, S.G.; Jiang, Y. Influence of Vitamin D3 Supplementation on Infliximab Effectiveness in Chinese Patients With Crohn’s Disease: A Retrospective Cohort Study. Front. Nutr. 2021, 8, 739285. [Google Scholar] [CrossRef]
- Winter, R.W.; Collins, E.; Cao, B.; Carrellas, M.; Crowell, A.M.; Korzenik, J.R. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-α medications among patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Rubin, S.J.S.; Bai, L.; Haileselassie, Y.; Levitte, S.; Balabanis, T.; Patel, A.; Sharma, A.; Sinha, S.R.; Habtezion, A. Vitamin D Is Associated with α4β7+ Immunophenotypes and Predicts Vedolizumab Therapy Failure in Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Chanchlani, N.; Lin, S.; Smith, R.; Roberts, C.; Nice, R.; McDonald, T.J.; Hamilton, B.; Bishara, M.; Bewshea, C.; Kennedy, N.A.; et al. Pretreatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients With Active Luminal Crohn’s Disease. Crohn’s Colitis 360 2023, 5, otad026. [Google Scholar] [CrossRef]
- Rodríguez-Olleros Rodríguez, C.; Díaz Curiel, M. Vitamin K and bone health: A review on the effects of vitamin K deficiency and supplementation and the effect of non-vitamin K antagonist oral anticoagulants on different bone parameters. J. Osteoporos. 2019, 2019, 2069176. [Google Scholar] [CrossRef]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin K and osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef]
- Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am. J. Gastroenterol. 2004, 99, 2178–2185. [Google Scholar] [CrossRef]
- Schoon, E.J.; Muller, M.C.; Vermeer, C.; Schurgers, L.J.; Brummer, R.J.; Stockbrügger, R.W. Low serum and bone vitamin K status in patients with longstanding Crohn’s disease: Another pathogenetic factor of osteoporosis in Crohn’s disease? Gut 2001, 48, 473–477. [Google Scholar] [CrossRef]
- Hao, G.; Zhang, B.; Gu, M.; Chen, C.; Zhang, Q.; Zhang, G.; Cao, X. Vitamin K intake and the risk of fractures: A meta-analysis. Medicine 2017, 96, e6725. [Google Scholar] [CrossRef]
- Feskanich, D.; Weber, P.; Willett, W.C.; Rockett, H.; Booth, S.L.; Colditz, G.A. Vitamin K intake and hip fractures in women: A prospective study. Am. J. Clin. Nutr. 1999, 69, 74–79. [Google Scholar] [CrossRef]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Pagano, M.C.; Santarpia, L.; Pellegrini, L.; Testa, A.; Marra, M.; Contaldo, F.; Castiglione, F.; et al. Evaluation of nutritional adequacy in adult patients with Crohn’s disease: A cross-sectional study. Eur. J. Nutr. 2020, 59, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Grzybowska-Chlebowczyk, U.; Landowski, P.; Szaflarska-Poplawska, A.; Klincewicz, B.; Adamczak, D.; Banasiewicz, T.; Plawski, A.; Walkowiak, J. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci. Rep. 2014, 4, 4768. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moysés, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef]
- Kuwabara, A.; Tanaka, K.; Tsugawa, N.; Nakase, H.; Tsuji, H.; Shide, K.; Kamao, M.; Chiba, T.; Inagaki, N.; Okano, T.; et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 2009, 20, 935–942. [Google Scholar] [CrossRef]
- Nakajima, S.; Iijima, H.; Egawa, S.; Shinzaki, S.; Kondo, J.; Inoue, T.; Hayashi, Y.; Ying, J.; Mukai, A.; Akasaka, T.; et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition 2011, 27, 1023–1028. [Google Scholar] [CrossRef]
- O’Connor, E.M.; Grealy, G.; McCarthy, J.; Desmond, A.; Craig, O.; Shanahan, F.; Cashman, K.D. Effect of phylloquinone (vitamin K1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn’s disease. Br. J. Nutr. 2014, 112, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Emaus, N.; Gjesdal, C.G.; Almås, B.; Christensen, M.; Grimsgaard, A.S.; Berntsen, G.K.R.; Salomonsen, L.; Fønnebø, V. Vitamin K2 supplementation does not influence bone loss in early menopausal women: A randomised double-blind placebo-controlled trial. Osteoporos. Int. 2010, 21, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Inaba, N.; Sato, T.; Yamashita, T. Low-Dose Daily Intake of Vitamin K2 (Menaquinone-7) Improves Osteocalcin γ-Carboxylation: A Double-Blind, Randomized Controlled Trials. J. Nutr. Sci. Vitaminol. 2015, 61, 471–480. [Google Scholar] [CrossRef]
- Tanaka, S.; Miyazaki, T.; Uemura, Y.; Miyakawa, N.; Gorai, I.; Nakamura, T.; Fukunaga, M.; Ohashi, Y.; Ohta, H.; Mori, S.; et al. Comparison of concurrent treatment with vitamin K2 and risedronate compared with treatment with risedronate alone in patients with osteoporosis: Japanese Osteoporosis Intervention Trial-03. J. Bone Miner. Metab. 2017, 35, 385–395. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Zhang, J.; Liu, M.; Liu, M.; Faber, K.N.; Weersma, R.K. Untargeted faecal metabolomics for the discovery of biomarkers and treatment targets for inflammatory bowel diseases. Gut 2024, 73, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of histone post-translational modifications in inflammatory diseases. Front. Immunol. 2022, 13, 852272. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Roediger, W.E.W. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Trotter, A.; Rodriguez-Palacios, A.; Cominelli, F. Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front. Immunol. 2016, 7, 290. [Google Scholar] [CrossRef]
- Pacifici, R. Role of gut microbiota in the skeletal response to PTH. J. Clin. Endocrinol. Metab. 2021, 106, 636–645. [Google Scholar] [CrossRef]
- Uchida, Y.; Irie, K.; Fukuhara, D.; Kataoka, K.; Hattori, T.; Ono, M.; Ekuni, D.; Kubota, S.; Morita, M. Commensal microbiota enhance both osteoclast and osteoblast activities. Molecules 2018, 23, 1517. [Google Scholar] [CrossRef]
- Lee, M.H.; Nuccio, S.P.; Mohanty, I.; Hagey, L.R.; Dorrestein, P.C.; Chu, H.; Raffatellu, M. How bile acids and the microbiota interact to shape host immunity. Nat. Rev. Immunol. 2024, 24, 798–809. [Google Scholar] [CrossRef]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 2020, 579, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional roles of B-vitamins in the gut and gut microbiome. Mol. Nutr. Food Res. 2020, 64, e2000426. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Exploring the Role of Vitamin D and the Vitamin D Receptor in the Composition of the Gut Microbiota. Front. Biosci. 2023, 28, 116. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, C.; Di Paolo, M.C.; Graziani, M.G.; Delle Fave, G. Probiotics and vitamin D/vitamin D receptor pathway interaction: Potential therapeutic implications in inflammatory bowel disease. Front. Pharmacol. 2021, 12, 747856. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Y.; Wen, Q.; Zhang, S.; Li, P.; Marcella, C.; Hu, B.; Liu, H.; Zhang, F.; Cui, B. Washed microbiota transplantation improved the level of serum vitamin D in ulcerative colitis. J. Gastroenterol. Hepatol. 2024, 39, 2394–2401. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Yamada, S.; Ao, M.; Matsuura, M.; Tsuji, H.; Iida, T.; Miyamoto, K.; Oka, K.; Takahashi, M.; Tanaka, K.; et al. Diversity of Gut Microbiota Affecting Serum Level of Undercarboxylated Osteocalcin in Patients with Crohn’s Disease. Nutrients 2019, 11, 1541. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, A.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus Reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef]
- Islam, B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.W.; Song, P.R.; Wangb, S.C.; Liu, H.; Shie, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients 2023, 15, 3224. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Blanco, E.; Burrows, R.; Reyes, M.; Lozoff, B.; Gahagan, S.; Albala, C. Breastfeeding as the sole source of milk for 6 months and adolescent bone mineral density. Osteoporos. Int. 2017, 28, 2823–2830. [Google Scholar] [CrossRef]
- Wang, F.; Wei, W.; Liu, P.J. Effects of probiotic supplementation on bone health in postmenopausal women: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1487998. [Google Scholar] [CrossRef]
- Wong, S.; Walker, J.; Carr, R.; Graff, L.A.; Clara, I.; Promislow, S.; Rogala, L.; Miller, N.; Rawsthorne, P.; Bernstein, C.N. The information needs and preferences of persons with longstanding inflammatory bowel disease. Can. J. Gastroenterol. Hepatol. 2012, 26, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Yan, X.; Wang, X.; Zhang, J.; Ming, Z.; Zhang, C.; Ma, P.; Liu, Q.; Xu, Y.; Cheng, L.; Pang, X.; et al. National trends in nine key minerals intake (quantity and source) among U.S. adults, 1999 to march 2020. Nutr. J. 2024, 23, 52. [Google Scholar] [CrossRef]
- Vernia, P.; Di Camillo, M.; Foglietta, T.; Avallone, V.E.; De Carolis, A. Diagnosis of lactose intolerance and the “nocebo” effect: The role of negative expectations. Dig. Liver Dis. 2010, 42, 616–619. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar]
- Vesa, T.H.; Korpela, R.A.; Sahi, T. Tolerance to small amounts of lactose. Am. J. Clin. Nutr. 1996, 64, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Shrier, I.; Heilpern, D.; Je, J.; Park, S.; Chong, G.; Lalonde, C.; Cote, L.; Lee, B. Differential impact of lactose/lactase phenotype on colonic microflora. Can. J. Gastroenterol. Hepatol. 2010, 24, 373–379. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose intolerance, dairy avoidance, and treatment options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef]
- Kalkwarf, H.J.; Khoury, J.C.; Lanphear, B.P. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am. J. Clin. Nutr. 2003, 77, 257–265. [Google Scholar] [CrossRef]
- Homann, H.H.; Kemen, M.; Fuessenich, C.; Senkal, M.; Zumtobel, V. Reduction in diarrhea incidence by soluble fiber in patients receiving total or supplemental enteral nutrition. JPEN J. Parenter. Enteral Nutr. 1994, 18, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, C.; Altomare, A.; Imperia, E.; Spiezia, C.; Khazrai, Y.M.; Guarino, M.P.L. The Role of dietary fibers in the management of IBD symptoms. Nutrients 2022, 14, 4775. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, S.; Wu, D.; Wang, Y.; Chen, J.; Duan, L.; Li, S.; Li, Y. Vitamin K: Infection, inflammation, and auto-immunity. J. Inflamm. Res. 2024, 17, 1147–1160. [Google Scholar] [CrossRef]
- Homik, J.; Suarez-Almazor, M.E.; Shea, B.; Cranney, A.; Wells, G.; Tugwell, P. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database Syst. Rev. 2000, 1998, CD000952. [Google Scholar] [CrossRef]
- Gordon, H.; Burisch, J.; Ellul, P.; Karmiris, K.; Katsanos, K.; Allocca, M.; Bamias, G.; Barreiro-de Acosta, M.; Braithwaite, T.; Greuter, T.; et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J. Crohn’s Colitis 2024, 18, 1–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernia, F.; Ribichini, E.; Burrelli Scotti, G.; Latella, G. Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis. J. Clin. Med. 2025, 14, 3202. https://doi.org/10.3390/jcm14093202
Vernia F, Ribichini E, Burrelli Scotti G, Latella G. Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis. Journal of Clinical Medicine. 2025; 14(9):3202. https://doi.org/10.3390/jcm14093202
Chicago/Turabian StyleVernia, Filippo, Emanuela Ribichini, Giorgia Burrelli Scotti, and Giovanni Latella. 2025. "Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis" Journal of Clinical Medicine 14, no. 9: 3202. https://doi.org/10.3390/jcm14093202
APA StyleVernia, F., Ribichini, E., Burrelli Scotti, G., & Latella, G. (2025). Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis. Journal of Clinical Medicine, 14(9), 3202. https://doi.org/10.3390/jcm14093202





