Longitudinal Systolic Excursion of the Mitral Annular Plane and Left Ventricular Rotational Mechanics Are Associated in Healthy Adults—Three-Dimensional Speckle-Tracking Echocardiography-Derived Insights from the MAGYAR-Healthy Study
Abstract
1. Introduction
2. Subjects and Methods
3. Results
4. Discussion
- One of the most important technical limitations is the low (30 ± 2 fps) frame rate of 3DSTE, which may limit the ability of 3DSTE in all measurements. Moreover, it should be taken into account that the 3DSTE-capable transducers are larger than those used for 2D Doppler echocardiography, limiting their positioning on the chest. It should also be considered that six subvolumes during six cardiac cycles are necessary for optimal image quality, potentially resulting in stitching/motion artifacts during data analysis [2,3,4,5,6].
- The 3DSTE method offers a complex volumetric and functional assessment of the LV, including strain measurements. In a recent study, the associations between 3DSTE-derived LV strains and MAPSE have been clarified; therefore, such comparisons were not performed in this study [27].
- Moreover, 3D casts can be created for all of the other chambers, and volumetric and strain analyses of both atria and the right ventricle can be performed using the same acquired 3D echocardiographic dataset. However, this study did not aim to make such evaluations.
- A more complex 3DSTE evaluation of certain valves and their annuli is an option, like ‘en-face’ measurement of the annular dimensions and the calculation of ‘sphincter-like’ functional properties. However, this study did not aim to make such assessments [30].
- Moreover, comparisons of 3DSTE with 2D Doppler echocardiography or other imaging techniques for valvular studies were not purposed, either. However, this sort of comparison would be a topic for future investigations.
- All subjects involved were considered to be healthy; however, it cannot be stated that subclinical abnormalities were excluded with 100% certainty in these subjects. Further clinical tests could have better confirmed the absence of abnormalities, although performing these tests without clinical indications could have raised ethical questions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caro, C.C.; Pedley, T.J.; Schroter, R.C.; Seed, W.A. The Mechanics of Circulation; Oxford University Press: Oxford, UK, 1978. [Google Scholar]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bloechlinger, S.; Grander, W.; Bryner, J.; Dünser, M.W. Left ventricular rotation: A neglected aspect of the cardiac cycle. Intensive Care Med. 2011, 37, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.S.; Vallabhajosyula, S.; Sengupta, P.P. Left Ventricular Twist and Torsion. Research Observations and Clinical Applications. Circ. Cardiovasc. Imaging 2015, 8, e003029. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Silbiger, J.J. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am. Heart J. 2012, 164, 163–176. [Google Scholar] [CrossRef]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Wiedemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef]
- Cirin, L.; Crișan, S.; Luca, C.T.; Buzaș, R.; Lighezan, D.F.; Văcărescu, C.; Cozgarea, A.; Tudoran, C.; Cozma, D. Mitral Annular Plane Systolic Excursion (MAPSE): A Review of a Simple and Forgotten Parameter for Assessing Left Ventricle Function. J. Clin. Med. 2024, 13, 5265. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Forster, T. Recent echocardiographic examination of the left ventricle–from M-mode to 3D speckle-tracking imaging. Orv Hetil. 2015, 156, 1723–1740. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- Nemes, A.; Kormanyos, A.; Kalapos, A.; Domsik, P.; Gyenes, N.; Ambrus, N.; Lengyel, C. Normal reference values of left ventricular strain parameters in healthy adults: Real-life experience from the single-center three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 368–377. [Google Scholar] [CrossRef]
- Hensel, K.O.; Roskopf, M.; Wilke, L.; Heusch, A. Intraobserver and interobserver reproducibility of M-mode and B-mode acquired mitral annular plane systolic excursion (MAPSE) and its dependency on echocardiographic image quality in children. PLoS ONE 2018, 13, e0196614. [Google Scholar] [CrossRef]
- Matos, J.D.; Balachandran, I.; Heidinger, B.H.; Mohebali, D.; Feldman, S.A.; McCormick, I.; Litmanovich, D.; Manning, W.J.; Carroll, B.J. Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion for risk stratification of acute pulmonary embolism. Echocardiography 2020, 37, 1008–1013. [Google Scholar] [CrossRef]
- Ashraf, M.; Myronenko, A.; Nguyen, T.; Inage, A.; Smith, W.; Lowe, R.I.; Thiele, K.; Gibbons Kroeker, C.A.; Tyberg, J.V.; Smallhorn, J.F.; et al. Defining left ventricular apex-to-base twist mechanics computed from high-resolution 3D echocardiography: Validation against sonomicrometry. JACC Cardiovasc. Imaging 2010, 3, 227–234. [Google Scholar] [CrossRef]
- Zhou, Z.; Ashraf, M.; Hu, D.; Dai, X.; Xu, Y.; Kenny, B.; Cameron, B.; Nguyen, T.; Xiong, L.; Sahn, D.J. Three-dimensional speckle-tracking imaging for left ventricular rotation measurement: An in vitro validation study. J. Ultrasound. Med. 2010, 29, 903–909. [Google Scholar] [CrossRef]
- Andrade, J.; Cortez, L.D.; Campos, O.; Arruda, A.L.; Pinheiro, J.; Vulcanis, L.; Shiratsuchi, T.S.; Kalil-Filho, R.; Cerri, G.G. Left ventricular twist: Comparison between two- and three-dimensional speckle-tracking echocardiography in healthy volunteers. Eur. J. Echocardiogr. 2011, 12, 76–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kormányos, Á.; Kalapos, A.; Domsik, P.; Lengyel, C.; Forster, T.; Nemes, A. Normal values of left ventricular rotational parameters in healthy adults-Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Healthy Study. Echocardiography 2019, 36, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults-Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. J. Clin. Med. 2023, 12, 7389. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Left ventricular rotational mechanics and left ventricular volumes: Is there a relationship in healthy adults?-three-dimensional speckle-tracking echocardiography-derived insights from the MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2023, 13, 6583–6589. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Complexity of left ventricular strains in response to elevated volumes in healthy adults-Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Int. J. Cardiol Heart. Vasc. 2023, 47, 101236. [Google Scholar] [CrossRef]
- Nemes, A.; Ambrus, N.; Lengyel, C. Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study. Biomedicines 2025, 13, 625. [Google Scholar] [CrossRef]
- Støylen, A.; Dalen, H.; Molmen, H.E. Left ventricular longitudinal shortening: Relation to stroke volume and ejection fraction in ageing, blood pressure, body size and gender in the HUNT3 study. Open Heart 2020, 7, e001243. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Right Ventricular Longitudinal Shortening is not Associated with Left Ventricular Rotational Mechanics in Healthy Adults-Insights from the Three-dimensional Speckle-tracking Echocardiographic MAGYAR-Healthy Study. Rev. Cardiovasc. Med. 2024, 25, 53. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Lengyel, C. Comparison of dimensions and functional features of mitral and tricuspid annuli in the same healthy adults: Insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2024, 14, 6780–6791. [Google Scholar] [CrossRef]
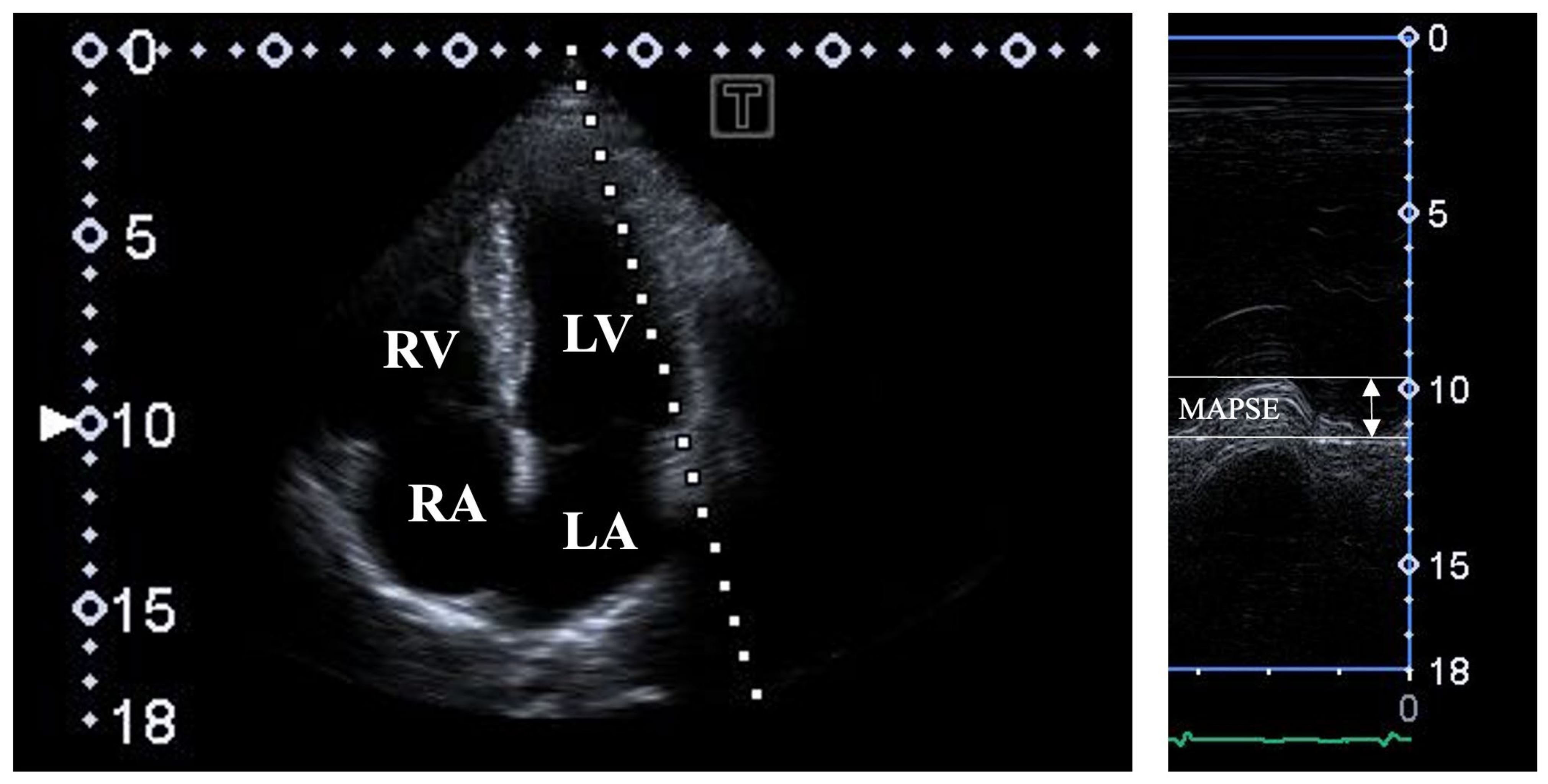
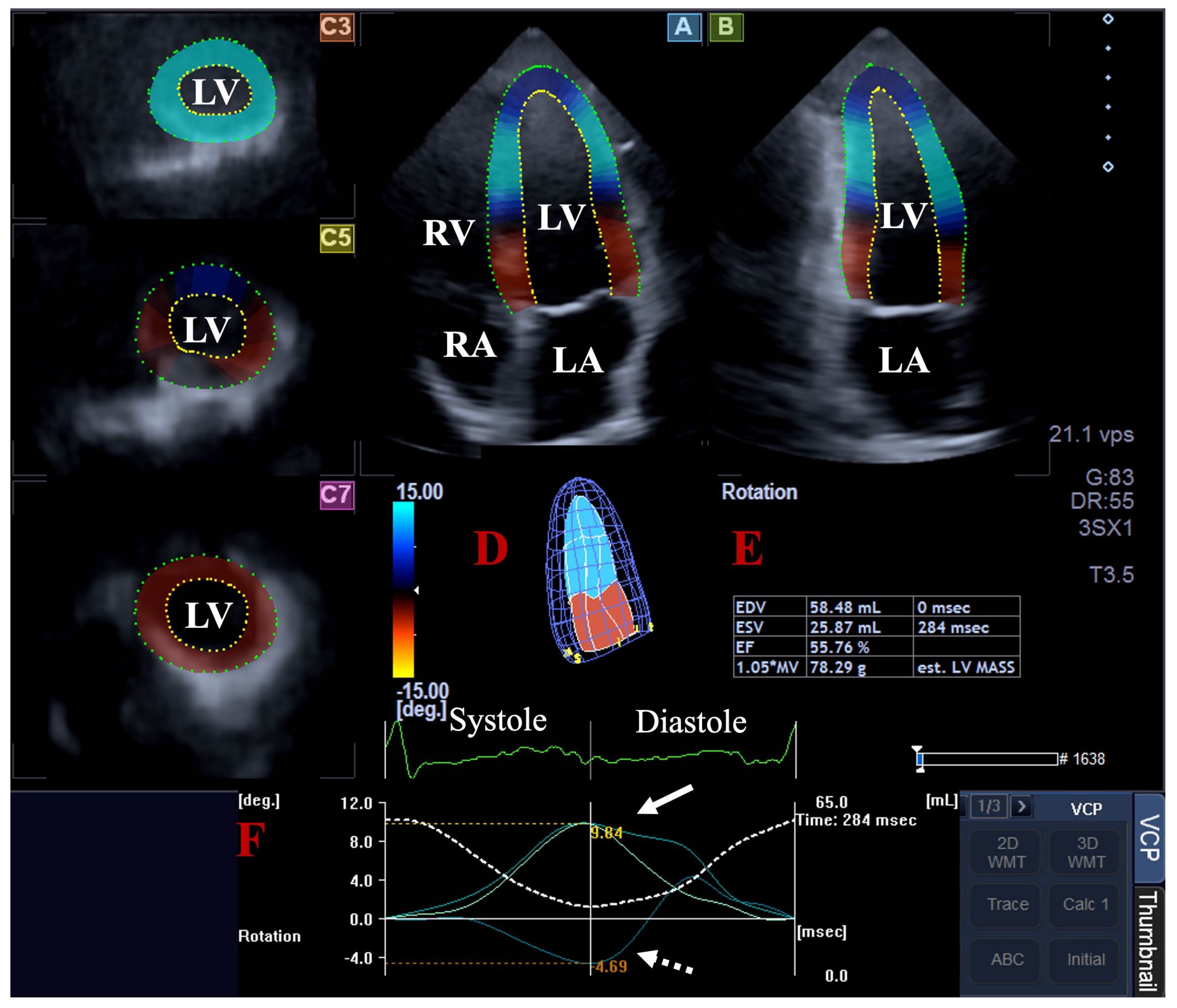

| All Subjects (n = 108) | MAPSE ≤ 11 mm (n = 14) | 11 mm < MAPSE < 17 mm (n = 72) | 17 mm ≤ MAPSE (n = 22) | |
|---|---|---|---|---|
| Two-dimensional echocardiography | ||||
| LA (mm) | 37.5 ± 3.7 | 38.2 ± 4.5 | 37.2 ± 3.6 | 37.9 ± 3.4 |
| LV-EDD (mm) | 47.9 ± 3.5 | 48.5 ± 3.8 | 47.4 ± 3.2 | 49.0 ± 4.0 |
| LV-EDV (mL) | 105.3 ± 23.2 | 110.0 ± 23.9 | 102.1 ± 19.5 | 116.0 ± 26.1 † |
| LV-ESD (mm) | 31.9 ± 3.1 | 31.6 ± 3.2 | 31.4 ± 2.9 | 33.3 ± 3.4 †† |
| LV-ESV (mL) | 37.7 ± 9.0 | 37.5 ± 9.0 | 36.5 ± 7.5 | 41.6 ± 12.1 ††† |
| IVS (mm) | 9.2 ± 1.3 | 9.9 ± 1.6 | 9.1 ± 1.2 * | 9.1 ± 1.3 |
| LV-PW (mm) | 9.4 ± 1.5 | 10.0 ± 1.8 | 9.2 ± 1.4 | 9.7 ± 1.4 |
| LV-EF (%) | 64.5 ± 3.7 | 66.2 ± 3.0 | 64.3 ± 3.8 | 64.3 ± 3.3 |
| MAPSE (mm) | 14.4 ± 3.2 | 9.2 ± 1.8 | 14.2 ± 1.6 ** | 18.7 ± 1.4 ***/†††† |
| Three-dimensional speckle-tracking echocardiography | ||||
| apical LV rotation (degrees) | 10.1 ± 3.6 | 9.3 ± 2.8 | 10.0 ± 3.7 | 10.9 ± 3.5 |
| basal LV rotation (degrees) | -4.1 ± 2.1 | -3.8 ± 2.1 | -4.0 ± 2.0 | -4.9 ± 2.5 |
| LV twist (degrees) | 14.2 ± 4.1 | 13.1 ± 2.7 | 14.0 ± 4.0 | 15.8 ± 4.6 **** |
| time-to-LV twist (ms) | 341.9 ± 128.5 | 328.3 ± 112.8 | 331.9 ± 132.8 | 384.8 ± 116 |
| Basal LV Rotation ≤ −2 Degrees (n = 13) | −2 Degrees < Basal LV Rotation < −6.2 Degrees (n = 76) | Basal LV Rotation ≥ −6.2 Degrees (n = 19) | Apical LV Rotation ≤ 6.5 Degrees (n = 15) | 6.5 Degrees < Apical LV Rotation < 13.7 Degrees (n = 75) | Apical LV Rotation ≥ 13.7 Degrees (n = 18) | |
|---|---|---|---|---|---|---|
| Two-dimensional echocardiography | ||||||
| LA (mm) | 37.2 ± 4.8 | 37.3 ± 3.0 | 38.5 ± 5.0 | 37.2 ± 3.0 | 37.4 ± 3.8 | 37.7 ± 3.6 |
| LV-EDD (mm) | 47.2 ± 3.8 | 48.2 ± 3.6 | 47.6 ± 3.5 | 47.0 ± 3.1 | 48.2 ± 3.5 | 47.5 ± 3.4 |
| LV-EDV (mL) | 95.8 ± 32.5 | 107.3 ± 21.7 | 105.0 ± 19.8 | 100.4 ± 19.9 | 105.9 ± 24.2 | 105.0 ± 19.7 |
| LV-ESD (mm) | 31.0 ± 3.1 | 32.1 ± 3.2 | 31.2 ± 2.7 | 30.5 ± 3.0 | 32.2 ± 3.1 † | 31.5 ± 3.1 |
| LV-ESV (mL) | 36.5 ± 9.0 | 38.2 ± 9.4 | 37.1 ± 7.5 | 33.2 ± 7.5 | 38.6 ± 9.6 †† | 37.6 ± 6.6 |
| IVS (mm) | 9.0 ± 1.5 | 9.3 ± 1.2 | 9.4 ± 1.4 | 8.9 ± 1.5 | 9.3 ± 1.3 | 9.2 ± 1.3 |
| LV-PW (mm) | 9.0 ± 1.1 | 9.3 ± 1.5 | 10.1 ± 1.7 # | 9.4 ± 1.6 | 9.4 ± 1.6 | 9.6 ± 1.1 |
| LV-EF (%) | 65.3 ± 2.8 | 64.4 ± 3.9 | 64.6 ± 3.1 | 66.9 ± 4.0 | 64.0 ± 3.6 ††† | 64.6 ± 2.8 |
| MAPSE (mm) | 12.3 ± 3.9 | 14.6 ± 2.7 * | 14.8 ± 3.7 | 13.8 ± 2.1 | 14.2 ± 3.4 | 15.6 ± 1.9 ††††††† |
| Three-dimensional speckle-tracking echocardiography | ||||||
| apical LV rotation (°) | 10.7 ± 3.6 | 10.1 ± 3.3 | 9.4 ± 4.5 | 4.8 ± 1.2 | 9.8 ± 1.9 †††† | 16.0 ± 1.7 ††††††††/‡ |
| basal LV rotation (°) | -1.5 ± 0.3 | -3.6 ± 1.0 ** | -8.0 ± 0.9 ****/## | -5.0 ± 2.2 | -3.9 ± 2.0 ††††† | -4.3 ± 2.4 |
| LV twist (°) | 12.2 ± 3.7 | 13.8 ± 3.4 | 17.4 ± 4.9 *****/### | 9.9 ± 2.9 | 13.7 ± 2.6 †††††† | 20.3 ± 3.3 †††††††††/‡‡ |
| LV twist time (ms) | 270.1 ± 97.2 | 352.1 ± 137.2 *** | 350.2 ± 91.7 ****** | 297.2 ± 173.9 | 346.6 ± 122.1 | 356.1 ± 111.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Bordács, B.; Ambrus, N.; Lengyel, C. Longitudinal Systolic Excursion of the Mitral Annular Plane and Left Ventricular Rotational Mechanics Are Associated in Healthy Adults—Three-Dimensional Speckle-Tracking Echocardiography-Derived Insights from the MAGYAR-Healthy Study. J. Clin. Med. 2025, 14, 3201. https://doi.org/10.3390/jcm14093201
Nemes A, Bordács B, Ambrus N, Lengyel C. Longitudinal Systolic Excursion of the Mitral Annular Plane and Left Ventricular Rotational Mechanics Are Associated in Healthy Adults—Three-Dimensional Speckle-Tracking Echocardiography-Derived Insights from the MAGYAR-Healthy Study. Journal of Clinical Medicine. 2025; 14(9):3201. https://doi.org/10.3390/jcm14093201
Chicago/Turabian StyleNemes, Attila, Barbara Bordács, Nóra Ambrus, and Csaba Lengyel. 2025. "Longitudinal Systolic Excursion of the Mitral Annular Plane and Left Ventricular Rotational Mechanics Are Associated in Healthy Adults—Three-Dimensional Speckle-Tracking Echocardiography-Derived Insights from the MAGYAR-Healthy Study" Journal of Clinical Medicine 14, no. 9: 3201. https://doi.org/10.3390/jcm14093201
APA StyleNemes, A., Bordács, B., Ambrus, N., & Lengyel, C. (2025). Longitudinal Systolic Excursion of the Mitral Annular Plane and Left Ventricular Rotational Mechanics Are Associated in Healthy Adults—Three-Dimensional Speckle-Tracking Echocardiography-Derived Insights from the MAGYAR-Healthy Study. Journal of Clinical Medicine, 14(9), 3201. https://doi.org/10.3390/jcm14093201







