Salivary Gland Tumors in Pregnancy—Treatment Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval
2.3. Study Population and Inclusion/Exclusion Criteria
2.4. Definitions of Variables
3. Results
3.1. SGTs in Female Patients
3.2. SGTs in Pregnant Women in the Case Study
4. Discussion
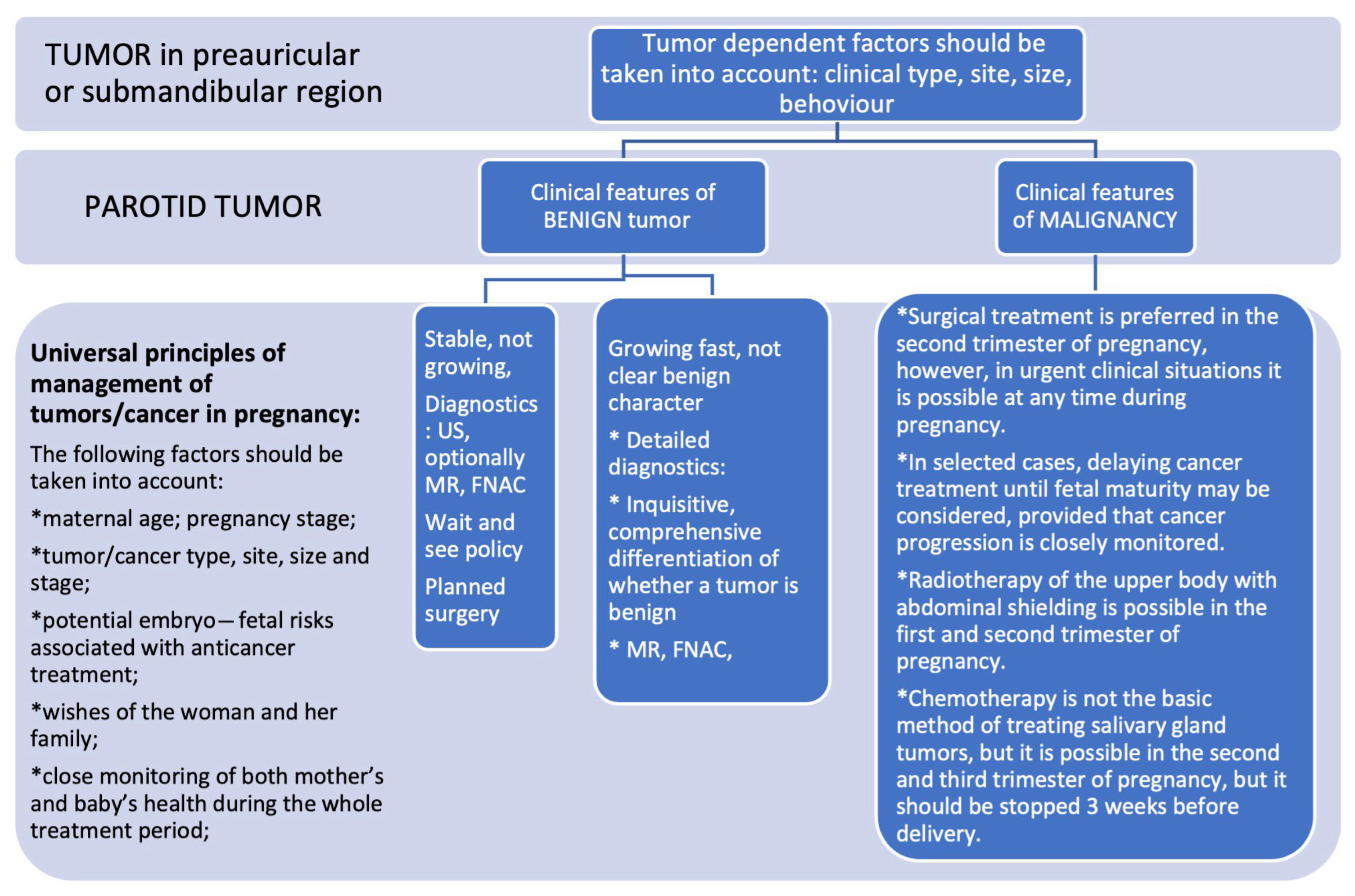
4.1. Malignant Tumors
4.2. Benign Tumors
4.3. Diagnostics and Decision-Making
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cellich, P.P.; Nayyar, R.; Wong, E. Acinic cell carcinoma of the parotid gland in pregnancy: An approach to cancer in pregnancy. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, K.; Skorek, A.; Stankiewicz, C. Diagnostyka i terapia nowotworów złośliwych głowy i szyi u ciężarnych [Managment of head and neck cancers during pregnancy]. Otolaryngol. Pol. 2011, 65, 326–332. [Google Scholar] [CrossRef]
- Puzzi-Fernandes, C.; Surita, F.G.; Schettini, C.S.; Parpinelli, M.A.; Guida, J.P.; Costa, M.L. Awareness towards an increasing concern during pregnancy: Maternal and perinatal outcomes of women with cancer. Am. J. Obstet. Gynecol. MFM. 2020, 2, 100168. [Google Scholar] [CrossRef] [PubMed]
- de Haan, J.; Verheecke, M.; Van Calsteren, K.; Van Calster, B.; Shmakov, R.G.; Gziri, M.M.; Halaska, M.J.; Fruscio, R.; Lok, C.A.R.; A Boere, I.; et al. International Network on Cancer and Infertility Pregnancy (INCIP). Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018, 19, 337–346. [Google Scholar] [CrossRef]
- Pavlidis, N.A. Coexistence of pregnancy and malignancy. Oncologist 2002, 7, 279–287. [Google Scholar]
- de Haan, J.; Lok, C.A.R.; Schutte, J.S.; van Zuylen, L.; de Groot, C.J.M. Cancer related maternal mortality and delay in diagnosis and treatment: A case series on 26 cases. BMC Pregnancy Childbirth 2018, 18, 10. [Google Scholar] [CrossRef]
- Bergamini, C.; Cavalieri, S.; Resteghini, C.; Alfieri, S.; Nuzzolese, I.; Colombo, E.; Ottini, A.; Calareso, G.; Vingiani, A.; Iacovelli, N.A.; et al. Multidisciplinary management of pregnancy-associated and early postpartum head and neck cancer patients. Front. Oncol. 2023, 13, 1298439. [Google Scholar] [CrossRef]
- Boucek, J.; de Haan, J.; Halaska, M.J.; Plzak, J.; Van Calsteren, K.; de Groot, C.J.M.; Steffensen, K.D.; Fruscio, R.; Massolt, E.T.; Klaritsch, P.; et al. International Network on Cancer, Infertility, and Pregnancy. Maternal and obstetrical outcome in 35 cases of well-differentiated thyroid carcinoma during pregnancy. Laryngoscope 2018, 128, 1493–1500. [Google Scholar] [CrossRef]
- Maggen, C.; Dierickx, D.; Lugtenburg, P.; Laenen, A.; Cardonick, E.; Smakov, R.G.; Bellido, M.; Cabrera-Garcia, A.; Gziri, M.M.; Halaska, M.J.; et al. International Network on Cancer, Infertility and Pregnancy. Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: A multicentre, retrospective, cohort study. Lancet Haematol. 2019, 6, e551–e561. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; von Minckwitz, G.; Gwyn, K.; Ellis, P.; Blohmer, J.U.; Schlegelberger, B.; Keller, M.; Harder, S.; Theriault, R.L.; Crivellari, D.; et al. Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer 2006, 106, 237–246. [Google Scholar] [CrossRef]
- Sato, K.; Shimamoto, H.; Mochizuki, Y.; Hirai, H.; Tomioka, H.; Shimizu, R.; Marukawa, E.; Fukayama, H.; Yoshimura, R.; Ishida, H.; et al. Treatment of oral cancers during pregnancy: A case-based discussion. J. Otolaryngol. Head Neck Surg. 2019, 48, 9. [Google Scholar] [CrossRef] [PubMed]
- Pugi, J.; Levin, M.; Gupta, M. Supraglottic p16+ squamous cell carcinoma during pregnancy: A case report and review of the literature. J. Otolaryngol. Head Neck Surg. 2019, 48, 47. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.L.; Chan, J.Y.; Ng, R.W.; Wei, W.I. Management of head and neck tumours during pregnancy: Case report and literature review. Asian J. Surg. 2008, 31, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Uwa, N.; Sagawa, K.; Mohri, T.; Kida, K.; Saeki, N.; Sakagami, M. A case of tongue carcinoma resection and reconstruction with microsurgical free flap during pregnancy. Nippon. Jibiinkoka Gakkai Kaiho 2015, 118, 46–52. [Google Scholar] [CrossRef]
- Tagliabue, M.; Elrefaey, S.H.; Peccatori, F.; Favia, G.; Navach, V.; Pignataro, L.; Capaccio, P.; Venturino, M.; Tredici, S.; Alterio, D.; et al. Tongue cancer during pregnancy: Surgery and more, a multidisciplinary challenge. Crit. Rev. Oncol./Hematol. 2016, 98, 1–11. [Google Scholar] [CrossRef]
- Terenzi, V.; Cassoni, A.; Della Monaca, M.; Priore, P.; De Felice, F.; Musio, D.; Battisti, A.; Fadda, M.T.; Tombolini, V.; Valentini, V. Oral cancer during pregnancy. Oral Oncol. 2016, 59, e1–e3. [Google Scholar] [CrossRef]
- Murphy, J.; Berman, D.R.; Edwards, S.P.; Prisciandaro, J.; Eisbruch, A.; Ward, B.B. Squamous cell carcinoma of the tongue during pregnancy: A case report and review of the literature. J. Oral Maxillofac. Surg. 2016, 74, 2557–2566. [Google Scholar] [CrossRef]
- Atabo, A.; Bradley, P.J. Management principles of head and neck cancers during pregnancy: A review and case series. Oral Oncol. 2008, 44, 236–241. [Google Scholar] [CrossRef]
- Al-Zaher, N.N.; Obeid, A.A. Acinic cell carcinoma in pregnancy: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 91. [Google Scholar] [CrossRef]
- Prabhu, R.V.; Dinkar, A.; Spadigam, A.; Prabhu, V. Low-grade papillary adenocarcinoma of minor salivary glands in pregnancy. Ind. J. Cancer 2015, 52, 644–645. [Google Scholar] [CrossRef]
- Bergamini, C.; Cavalieri, S.; Sanguineti, G.; Farneti, A.; Licitra, L. Treatment of HER2+ metastatic salivary ductal carcinoma in a pregnant woman: A case report. Oxf. Med. Case Rep. 2019, 2019, omz102. [Google Scholar] [CrossRef]
- Koh, I.S.; Cho, H.J.; Kim, J.W. Rapidly growing giant pilomatricoma in the right parotid region of a pregnant woman. Arch. Craniofac. Surg. 2020, 21, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Palluch, F.; Lehmann, M.; Volz, J.; Upile, T.; Sudhoff, H. The rapid growth of a pleomorphic adenoma of the parotid gland in the third trimester of pregnancy. J. Med. Case Rep. 2011, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; Bartkowiak, E.; Pietruszewska, W.; Stodulski, D.; Markowski, J.; Burduk, P.; Olejniczak, I.; Piernicka-Dybich, A.; Wierzchowska, M.; Amernik, K.; et al. Rationale for Increasing Oncological Vigilance in Relation to Clinical Findings in Accessory Parotid Gland-Observations Based on 2192 Cases of the Polish Salivary Network Database. Cancers 2024, 16, 463. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Slootweg, P.J.; El-Naggar, A.K. World Health Organization 4th edition of head and neck tumor classification: Insight into the consequential modifications. Virchows Arch. 2018, 472, 311–313. [Google Scholar] [CrossRef]
- Seçin, I.; Uijen, M.J.M.; Driessen, C.M.L.; van Herpen, C.M.L.; Scheepers, P.T.J. Case Report: Two Cases of Salivary Duct Carcinoma in Workers With a History of Chromate Exposure. Front. Med. 2021, 8, 730403. [Google Scholar] [CrossRef]
- Rollon-Mayordomo, A.; Avellaneda-Camarena, A.; Gutierrez-Domingo, A.; Martinez-Carapeto, E.; Infante-Cossio, P. Synchronous occurrence of IgG4-related sialadenitis and ductal carcinoma of the parotid gland: A case report. Gland. Surg. 2021, 10, 2069–2075. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Morrow, M.; Ljung, B.M. Menstrual and reproductive factors for salivary gland cancer risk in women. Epidemiology 1999, 10, 528–530. [Google Scholar] [CrossRef]
- Glas, A.S.; Hollema, H.; Nap, R.E.; Plukker, J.T. Expression of estrogen receptor, progesterone receptor, and insulin-like growth factor receptor-1 and of MIB-1 in patients with recurrent pleomorphic adenoma of the parotid gland. Cancer 2002, 94, 2211–2216. [Google Scholar] [CrossRef]
- Lu, Y.; Lagergren, J.; Eloranta, S.; Lambe, M. Childbearing and salivary gland cancer: A population-based nested case–control study. Epidemiology 2009, 20, 780–782. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Schrock, A.B.; Erlich, R.L.; Miller, V.A.; Knost, J.; Le-Lindqwister, N.; Jujjavarapu, S.; Ali, S.M.; Liu, J.J. Significant and durable clinical benefit from trastuzumab in 2 patients with HER2-amplified salivary gland cancer and a review of the literature. Head Neck. 2017, 39, E40–E44. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.G.; Wang, K.; Torman, D.; Binks, B.J.; Rubin, M.L.; Andersen, C.R.; Lewis, W.E.; Rivera, M.J.; Kaya, D.; El-Naggar, A.K.; et al. Treatment patterns and outcomes of palliative systemic therapy in patients with salivary duct carcinoma and adenocarcinoma, not otherwise specified. Cancer 2022, 128, 509–518. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, P.; Wan, X.; Xu, L.; Gong, Y.; Zhang, W. Androgen Deprivation Therapy for Patients with Androgen-Receptor-Positive Metastatic Salivary Duct Carcinoma: A Case Report and Review of the Literature. Onco. Targets Ther. 2021, 14, 3481–3486. [Google Scholar] [CrossRef]
- Laine, M.; Ojanotko-Harri, A. Progesterone metabolism by major salivary glands of rat. Parotid Gland. J. Steroid Biochem. Mol. Biol. 1990, 37, 605–611. [Google Scholar] [CrossRef]
- Nasser, S.M.; Faquin, W.C.; Dayal, Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am. J. Clin. Pathol. 2003, 119, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Lynch, C.D.; Schneider, P.; Thung, S.; Costantine, M.M.; O’malley, D.; Landon, M.B.; Pawlik, T.M.; Venkatesh, K.K. Postoperative complications after nonobstetric surgery among pregnant patients in the National Surgical Quality Improvement Program, 2005–2012. Am. J. Surg. 2022, 223, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.N.; Ng, B.R.J.; Arafat, Y.; Mendis, B.A.S.; Dharmawardhane, A.; Lucky, T. Evaluation of safety and foeto-maternal outcome following nonobstetric surgery in pregnancy: A retrospective single-site Australian study. ANZ J. Surg. 2021, 91, 627–632. [Google Scholar] [CrossRef]
- Haataja, A.; Kokki, H.; Uimari, O.; Kokki, M. Nonobstetric surgery during pregnancy and the effects on maternal and fetal outcomes: A systematic review. Scand. J. Surg. 2023, 112, 187–205. [Google Scholar] [CrossRef]
- Ravindra, G.L.; Madamangalam, A.S.; Seetharamaiah, S. Anaesthesia for nonobstetric surgery in obstetric patients. Ind. J. Anaesth. 2018, 62, 710–716. [Google Scholar] [CrossRef]
- Wilson, M.S.J.; Powell-Bowns, M.; Robertson, A.G.; Luhmann, A.; Richards, C.H. Scottish Surgical Research Group. National multicentre audit of pregnancy status in general surgery admissions in Scotland. Postgrad. Med. J. 2017, 93, 480–483. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Preoperative Tests: The Use of Routine Preoperative Tests for Elective Surgery: NICE; National Institute for Health and Care Excellence: London, UK, 2003. [Google Scholar]
- Brodsky, J.B.; Cohen, E.N.; Brown, B.W., Jr.; Wu, M.L.; Whitcher, C. Surgery during pregnancy and fetal outcome. Am. J. Obstet. Gynecol. 1980, 138, 1165–1167. [Google Scholar] [PubMed]
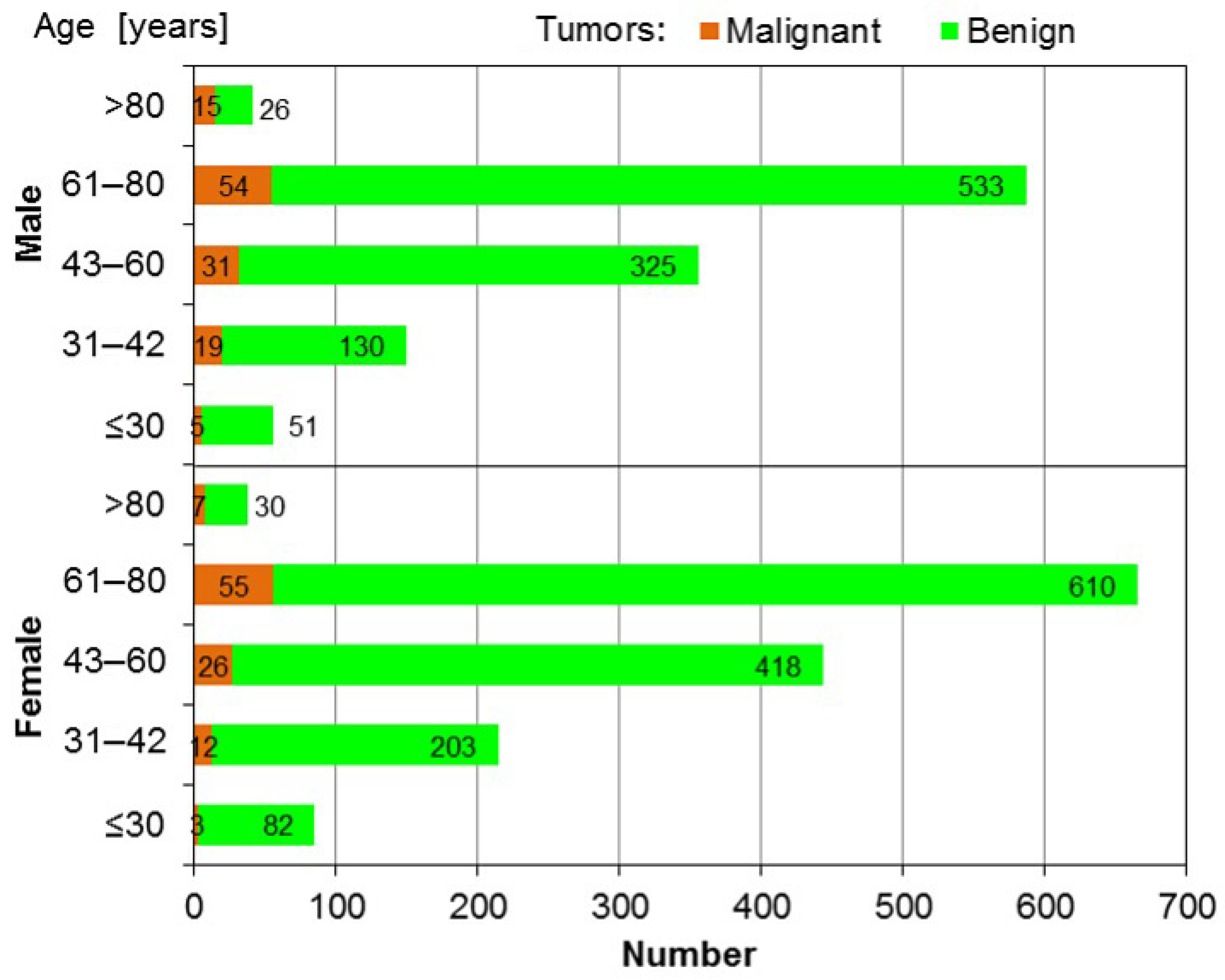
| Malignant Tumors | Benign Tumors | |||
|---|---|---|---|---|
| Age | n | % | n | % |
| 16–30 | 3 | 0.113% | 82 | 3.091% |
| 31–42 | 12 | 0.452% | 203 | 7.652% |
| 16–42 | 15 | 0.565% | 285 | 10.743% |
| No | Age | Gravida | Familyl History of Cancer | Gestational Age at Diagnosis | Tumor Growth During Pregnancy | Delivery | Histology (HP) | Tumor Size (cm) | Timing of Surgery | Final Diagnosis | DFS/Outcome | Special Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | 1 | No | Pre-pregnancy (surgery delayed) | Stable | C-section (twains) | FNAC Milano III | 4.0 | 6 months postpartum | PA | 5 years/Vital | Infertility treatment |
| 2 | 28 | 2 | Yes | 12 weeks | Enlarged (12–16 w), then stable | Natural at 38 wks | FNAC Milano III | 3.0 | 1 months postpartum | PA | Not-reported/Vital | Civil lawsuit withdraw |
| 3. | 34 | 3 | No | 28 weeks; | Slightly enlarged | Natural at 38 wks | FNAC Milano III | 3.0 | 2 months postpartum | PA | 2 years/Vital | Factor VII mutation |
| 4. | 34 | 1 | No | 20 weeks | Gradual growth | C-section at 38 wks | FNAC Benign cells | 2.0 | 3 months postpartum | PA | 3 years/Vital | myopia |
| 5. | 33 | 1 | Mother breast cancer | 34 weeks | Rapid growth (doubling) | Induced at 38 wks | FNAC Cancer cells | 6.0 | 4 months postpartum | SDC ex PA (T4N3bM0) | 15 months, Alive with metastases | HER2+, AR-, Ki67 60–80% |
| 6 | 32 | 2 | Ovarian cancer (pt)+leukemia (child) | 32 weeks | Not specified | C-section at 38 wks | FNAC–malignant cells | 3.0 | postpartum | Mucoepidermoid carcinoma (low grade) | 6 years/Vital | Later ovarian cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, M.; Radomska, K.; Pietruszewska, W.; Stodulski, D.; Mikaszewski, B.; Markowski, J.; Burduk, P.; Woźniak, A.; Lubiński, J.; Rzepakowska, A. Salivary Gland Tumors in Pregnancy—Treatment Strategies. J. Clin. Med. 2025, 14, 3136. https://doi.org/10.3390/jcm14093136
Wierzbicka M, Radomska K, Pietruszewska W, Stodulski D, Mikaszewski B, Markowski J, Burduk P, Woźniak A, Lubiński J, Rzepakowska A. Salivary Gland Tumors in Pregnancy—Treatment Strategies. Journal of Clinical Medicine. 2025; 14(9):3136. https://doi.org/10.3390/jcm14093136
Chicago/Turabian StyleWierzbicka, Małgorzata, Katarzyna Radomska, Wioletta Pietruszewska, Dominik Stodulski, Bogusław Mikaszewski, Jarosław Markowski, Paweł Burduk, Aldona Woźniak, Jakub Lubiński, and Anna Rzepakowska. 2025. "Salivary Gland Tumors in Pregnancy—Treatment Strategies" Journal of Clinical Medicine 14, no. 9: 3136. https://doi.org/10.3390/jcm14093136
APA StyleWierzbicka, M., Radomska, K., Pietruszewska, W., Stodulski, D., Mikaszewski, B., Markowski, J., Burduk, P., Woźniak, A., Lubiński, J., & Rzepakowska, A. (2025). Salivary Gland Tumors in Pregnancy—Treatment Strategies. Journal of Clinical Medicine, 14(9), 3136. https://doi.org/10.3390/jcm14093136







