Use of Intraoperative Ultrasonography of the Small Bowel to Reduce Histologically Positive Margins in Crohn’s Disease Surgery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
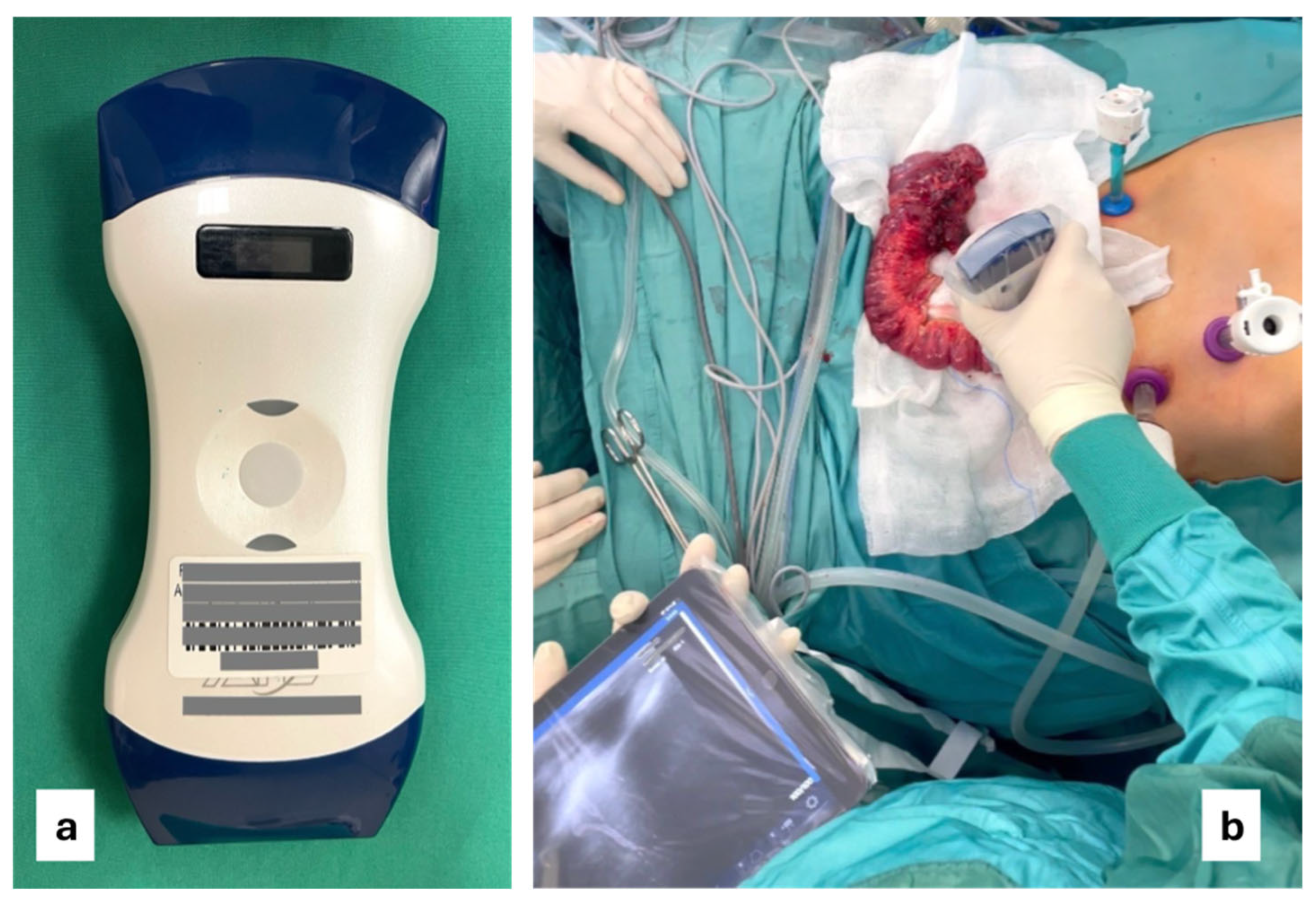
2.2. Intraoperative Ultrasound
2.3. Data Collection
2.4. Endpoint
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Results of the Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| IOUS | Intraoperative ultrasound |
| IBD | Intestinal bowel disease |
| POR | Postoperative recurrence |
| TAB | Table |
| BMI | Body mass index |
| BWP | Bowel wall pattern |
| SD | Standard deviation |
| OR | ODD ratio |
| CI | Confidence interval |
| RR | Relative risk |
| HR | Hazard ratio |
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reese, G.E.; Nanidis, T.; Borysiewicz, C.; Yamamoto, T.; Orchard, T.; Tekkis, P.P. The effect of smoking after surgery for Crohn’s disease: A meta-analysis of observational studies. Int. J. Color. Dis. 2008, 23, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Rogers, A.C.; O’Toole, A.; Burke, J.P. Meta-analysis of Histological Margin Positivity in the Prediction of Recurrence After Crohn’s Resection. Dis. Colon. Rectum. 2019, 62, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Yzet, C.; Riault, C.; Brazier, F.; Grados, L.; Nguyen-Khac, E.; Chatelain, D.; Sabbagh, C.; Buisson, A.; Diouf, M.; Fumery, M. Positive margins and plexitis increase the risk of recurrence after ileocecal resection: A systematic review and meta-analysis. Dig. Liver Dis. 2023, 55, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Mineccia, M.; Bertolino, F.; Giraldi, F.; Rigazio, C.; Rocca, R.; Ferrero, A. Intraoperative ultrasonography in patients undergoing surgery for Crohn’s disease. Prospective evaluation of an innovative approach to optimize staging and treatment planning. Updates Surg. 2019, 71, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Furfaro, F.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Point-of-Care Ultrasound in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Pouillon, L.; Mañosa, M.; Savarino, E.; Allez, M.; Kapizioni, C.; Arebi, N.; Carvello, M.; Myrelid, P.; De Vries, A.C.; et al. Results of the Eighth Scientific Workshop of ECCO: Prevention and Treatment of Postoperative Recurrence in Patients with Crohn’s Disease Undergoing an Ileocolonic Resection with Ileocolonic Anastomosis. J. Crohns Colitis 2023, 17, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Riault, C.; Diouf, M.; Chatelain, D.; Yzet, C.; Turpin, J.; Brazier, F.; Dupas, J.L.; Sabbagh, C.; Nguyen-Khac, E.; Fumery, M. Positive histologic margins is a risk factor of recurrence after ileocaecal resection in Crohn’s disease. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101569. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Cazals-Hatem, D.; Auzolle, C.; Gardair, C.; Ngollo, M.; Bottois, H.; Nancey, S.; Pariente, B.; Buisson, A.; Treton, X.; et al. Association Between Microscopic Lesions at Ileal Resection Margin and Recurrence After Surgery in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 141–149.e2. [Google Scholar] [CrossRef] [PubMed]
- de Buck van Overstraeten, A.; Eshuis, E.J.; Vermeire, S.; Van Assche, G.; Ferrante, M.; D’Haens, G.R.; Ponsioen, C.Y.; Belmans, A.; Buskens, C.J.; Wolthuis, A.M.; et al. Short- and medium-term outcomes following primary ileocaecal resection for Crohn’s disease in two specialist centres. Br. J. Surg. 2017, 104, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Poredska, K.; Kunovsky, L.; Marek, F.; Kala, Z.; Prochazka, V.; Dolina, J.; Zboril, V.; Kovalcikova, P.; Pavlik, T.; Jabandziev, P.; et al. The Influence of Microscopic Inflammation at Resection Margins on Early Postoperative Endoscopic Recurrence After Ileocaecal Resection for Crohn’s Disease. J. Crohns Colitis 2020, 14, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Malhi, G.; Abdali, D.; Pogue, E.; Marshall, J.K.; de Buck van Overstraeten, A.; Riddell, R.; Narula, N. Active Margins, Plexitis, and Granulomas Increase Postoperative Crohn’s Recurrence: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Joustra, V.; Duijvestein, M.; Mookhoek, A.; Bemelman, W.; Buskens, C.; Koželj, M.; Novak, G.; Hindryckx, P.; Mostafavi, N.; D’Haens, G. Natural History and Risk Stratification of Recurrent Crohn’s Disease After Ileocolonic Resection: A Multicenter Retrospective Cohort Study. Inflamm. Bowel Dis. 2022, 28, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamilton, S.R.; Reese, J.; Pennington, L.; Boitnott, J.K.; Bayless, T.M.; Cameron, J.L. The role of resection margin frozen section in the surgical management of Crohn’s disease. Surg. Gynecol. Obstet. 1985, 160, 57–62. [Google Scholar] [PubMed]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Morris, S.; Quinn, L.; Tomini, F.; Miles, A.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; et al. Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: The METRIC diagnostic accuracy study. Health Technol. Assess. 2019, 23, 1–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Celentano, V.; Beable, R.; Ball, C.; Flashman, K.G.; Reeve, R.; Holmes, A.; Fogg, C.; Harper, M.; Higginson, A. The Portsmouth protocol for intra-operative ultrasound of the small bowel in Crohn’s disease. Color. Dis. 2020, 22, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Celentano, V.; Beable, R.; Ball, C.; Flashman, K.G.; Reeve, R.; Fogg, C.; Harper, M.; Higginson, A. Feasibility of intraoperative ultrasound of the small bowel during Crohn’s disease surgery. Tech. Coloproctol. 2020, 24, 965–969, Erratum in Tech. Coloproctol. 2023, 27, 343. [Google Scholar] [CrossRef] [PubMed]
- Minordi, L.M.; Larosa, L.; Brizi, M.G.; Armuzzi, A.; Manfredi, R. Length of the healthy and pathological small intestine in patients with Crohn’s disease: Calculations using computed tomography and magnetic resonance enterography. Diagn. Interv. Radiol. 2023, 29, 24–28. [Google Scholar] [CrossRef] [PubMed]




| KERRYPNX | Historical Cohort (n = 205) | IOUS (n = 27) | p Value |
|---|---|---|---|
| Gender | 0.55 | ||
| F | 109 (53.2) | 16 (59.3) | |
| M | 96 (46.8) | 11 (40.7) | |
| Age (years) | |||
| Mean (SD) | 42.5 (15.7) | 38.3 (16.5) | 0.19 |
| BMI | |||
| Mean (SD) | 21.90 (4.14) | 21.24 (2.03) | 0.42 |
| Smoking | |||
| No | 145 (70.7) | 22 (81.5) | |
| Yes | 60 (29.3) | 5 (18.5) | 0.24 |
| Stricturing disease | |||
| No | 76 (37.1) | 15 (55.6) | |
| Yes | 129 (62.9) | 12 (44.4) | 0.064 |
| Penetrating disease | |||
| No | 102 (49.8) | 13 (48.1) | |
| Yes | 103 (50.2) | 14 (51.9) | 0.87 |
| Localization | |||
| Ileal | 62 (30.2) | 8 (29.6) | |
| Ileocolonic | 143 (69.8) | 19 (70.4) | 0.95 |
| Perianal disease | |||
| No | 166 (81.0) | 26 (96.3) | |
| Yes | 39 (19.0) | 1 (3.7) | 0.048 |
| Laparoscopic | |||
| No | 95 (46.3) | 9 (33.3) | |
| Yes | 110 (53.7) | 18 (66.7) | 0.20 |
| Surgery for recurrence | |||
| No | 145 (70.7) | 20 (74.1) | |
| Yes | 60 (29.3) | 7 (25.9) | 0.72 |
| Historical Cohort (n = 27) | IOUS (n = 27) | p Value | |
|---|---|---|---|
| Gender | 0.78 | ||
| F | 15 (55.6) | 16 (59.3) | |
| M | 12 (44.4) | 11 (40.7) | |
| Age (years) | |||
| Mean (SD) | 40.4 (15.2) | 38.3 (16.5) | 0.61 |
| BMI | |||
| Mean (SD) | 20.59 (5.14) | 21.24 (2.03) | 0.54 |
| Smoking | |||
| No | 20 (74.1) | 22 (81.5) | |
| Yes | 7 (25.9) | 5 (18.5) | 0.51 |
| Stricturing disease | |||
| No | 11 (40.7) | 15 (55.6) | |
| Yes | 16 (59.3) | 12 (44.4) | 0.28 |
| Penetrating disease | |||
| No | 16 (59.3) | 13 (48.1) | |
| Yes | 11 (40.7) | 14 (51.9) | 0.41 |
| Localization | |||
| Ileal | 8 (29.6) | 8 (29.6) | |
| Ileocolonic | 19 (70.4) | 19 (70.4) | 0.99 |
| Perianal disease | |||
| No | 20 (74.1) | 26 (96.3) | |
| Yes | 7 (25.9) | 1 (3.7) | 0.02 |
| Laparoscopic | |||
| No | 14 (51.9) | 9 (33.3) | |
| Yes | 13 (48.1) | 18 (66.7) | 0.17 |
| Surgery for recurrence | |||
| No | 20 (74.1) | 20 (74.1) | |
| Yes | 7 (25.9) | 7 (25.9) | 0.99 |
| Historical Cohort (n = 27) | IOUS (n = 27) | p Value | |
|---|---|---|---|
| Histological margin | 0.021 | ||
| No | 14 (51.9) | 22 (81.5) | |
| Yes | 13 (48.1) | 5 (18.5) | |
| Length of specimen (cm) | |||
| Mean (SD) | 34.1 (23.1) | 24.1 (13.5) | 0.058 |
| Length of surgery (minutes) | |||
| Mean (SD) | 225.0 (77.8) | 254.2 (49.3) | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchetti, F.; Pizzolante, F.; Giambusso, M.; Nesci, C.; Giannarelli, D.; Galiandro, F.; Pugliese, D.; Scaldaferri, F.; Giustiniani, M.C.; Balzano, D.; et al. Use of Intraoperative Ultrasonography of the Small Bowel to Reduce Histologically Positive Margins in Crohn’s Disease Surgery: A Pilot Study. J. Clin. Med. 2025, 14, 3135. https://doi.org/10.3390/jcm14093135
Sacchetti F, Pizzolante F, Giambusso M, Nesci C, Giannarelli D, Galiandro F, Pugliese D, Scaldaferri F, Giustiniani MC, Balzano D, et al. Use of Intraoperative Ultrasonography of the Small Bowel to Reduce Histologically Positive Margins in Crohn’s Disease Surgery: A Pilot Study. Journal of Clinical Medicine. 2025; 14(9):3135. https://doi.org/10.3390/jcm14093135
Chicago/Turabian StyleSacchetti, Franco, Fabrizio Pizzolante, Mauro Giambusso, Carmen Nesci, Diana Giannarelli, Federica Galiandro, Daniela Pugliese, Franco Scaldaferri, Maria C. Giustiniani, Domenico Balzano, and et al. 2025. "Use of Intraoperative Ultrasonography of the Small Bowel to Reduce Histologically Positive Margins in Crohn’s Disease Surgery: A Pilot Study" Journal of Clinical Medicine 14, no. 9: 3135. https://doi.org/10.3390/jcm14093135
APA StyleSacchetti, F., Pizzolante, F., Giambusso, M., Nesci, C., Giannarelli, D., Galiandro, F., Pugliese, D., Scaldaferri, F., Giustiniani, M. C., Balzano, D., Caprino, P., Potenza, A. E., Minordi, L. M., & Sofo, L. (2025). Use of Intraoperative Ultrasonography of the Small Bowel to Reduce Histologically Positive Margins in Crohn’s Disease Surgery: A Pilot Study. Journal of Clinical Medicine, 14(9), 3135. https://doi.org/10.3390/jcm14093135







