Significance of Notch Signaling in Salivary Gland Development and Diseases
Abstract
1. Introduction
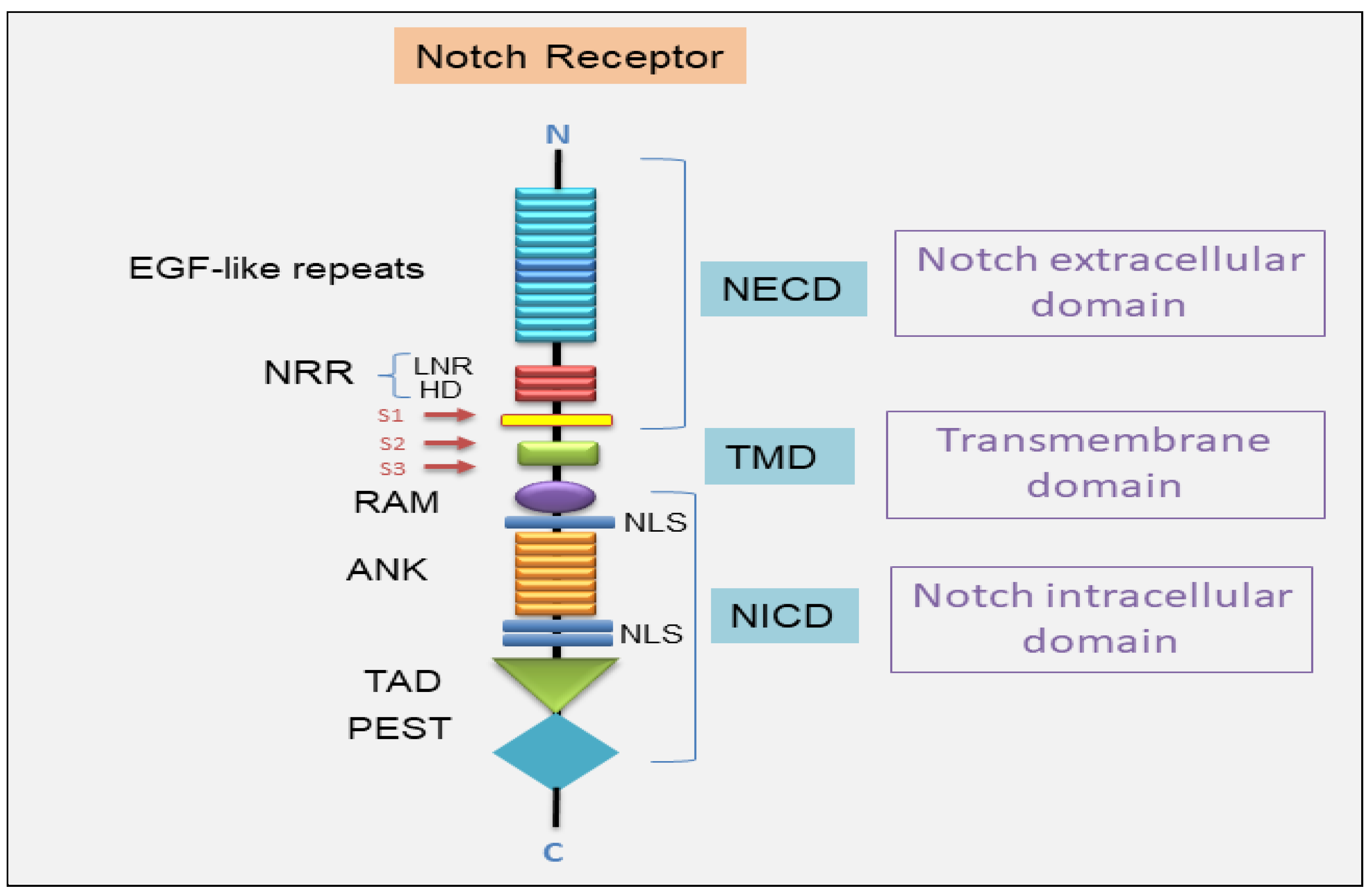
2. Notch Signal Transduction System
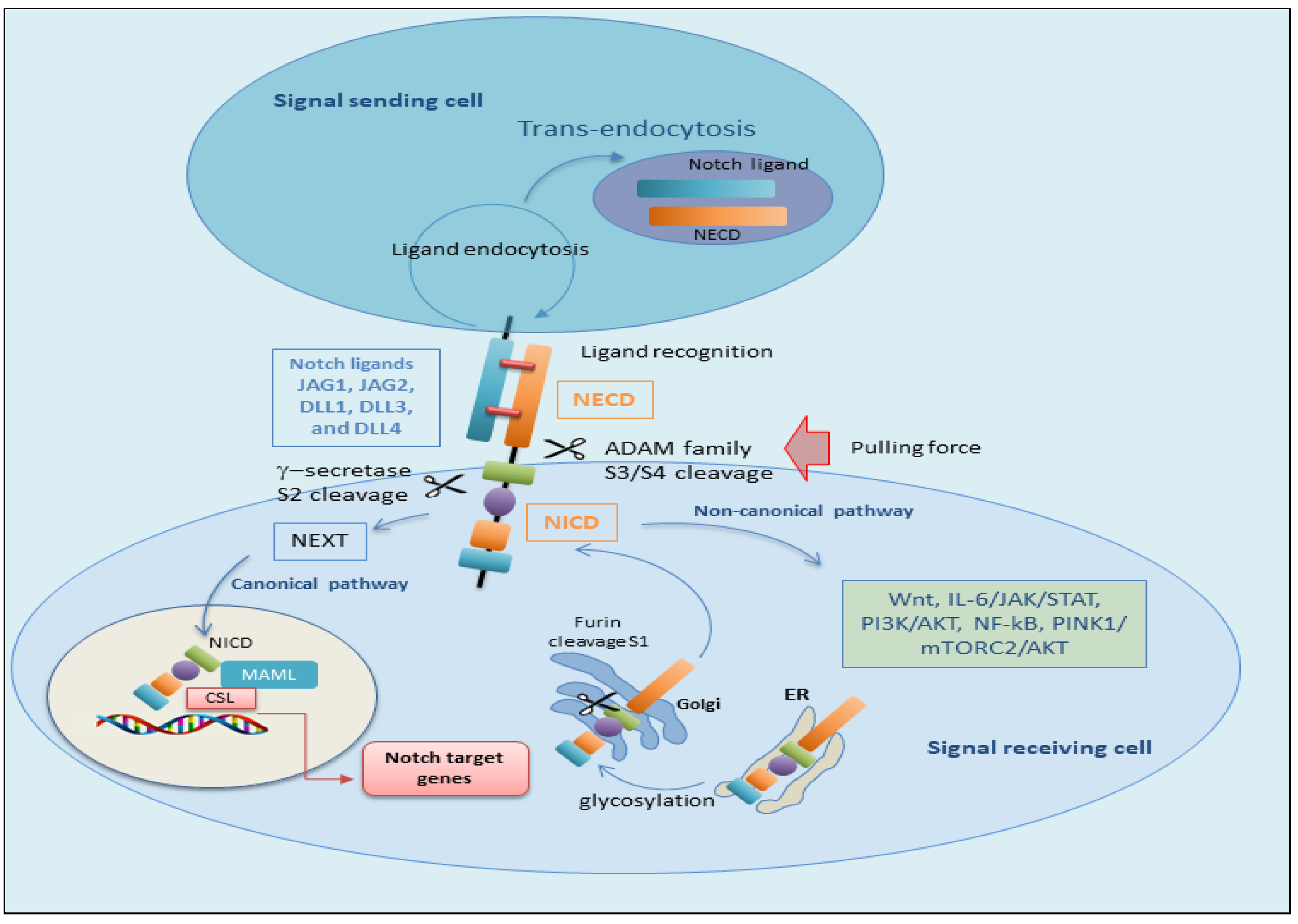
2.1. The Canonical Notch Signaling Pathway
2.2. The Non-Canonical Notch Signaling Pathway
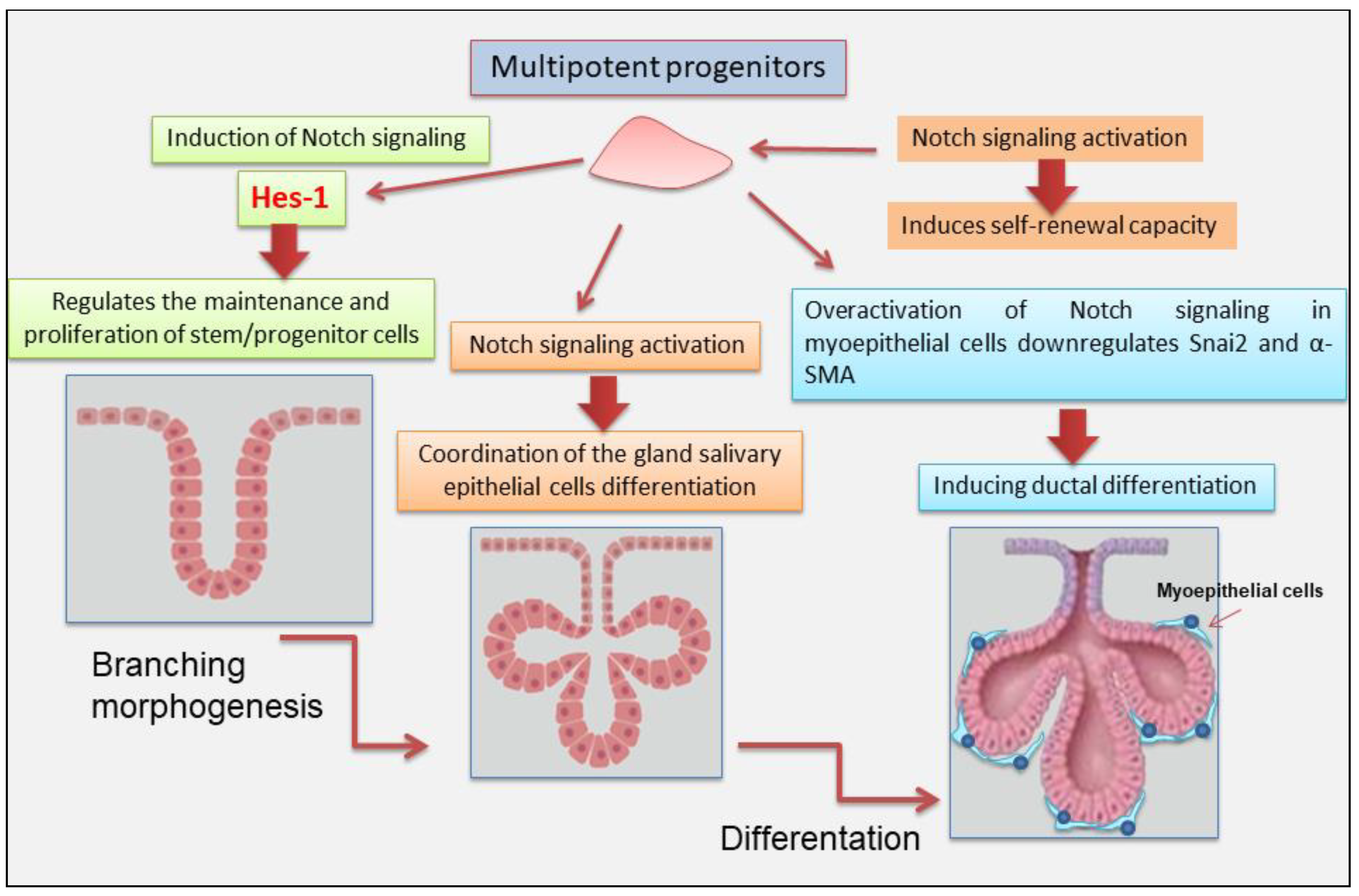
3. Notch Signaling in Salivary Gland Development
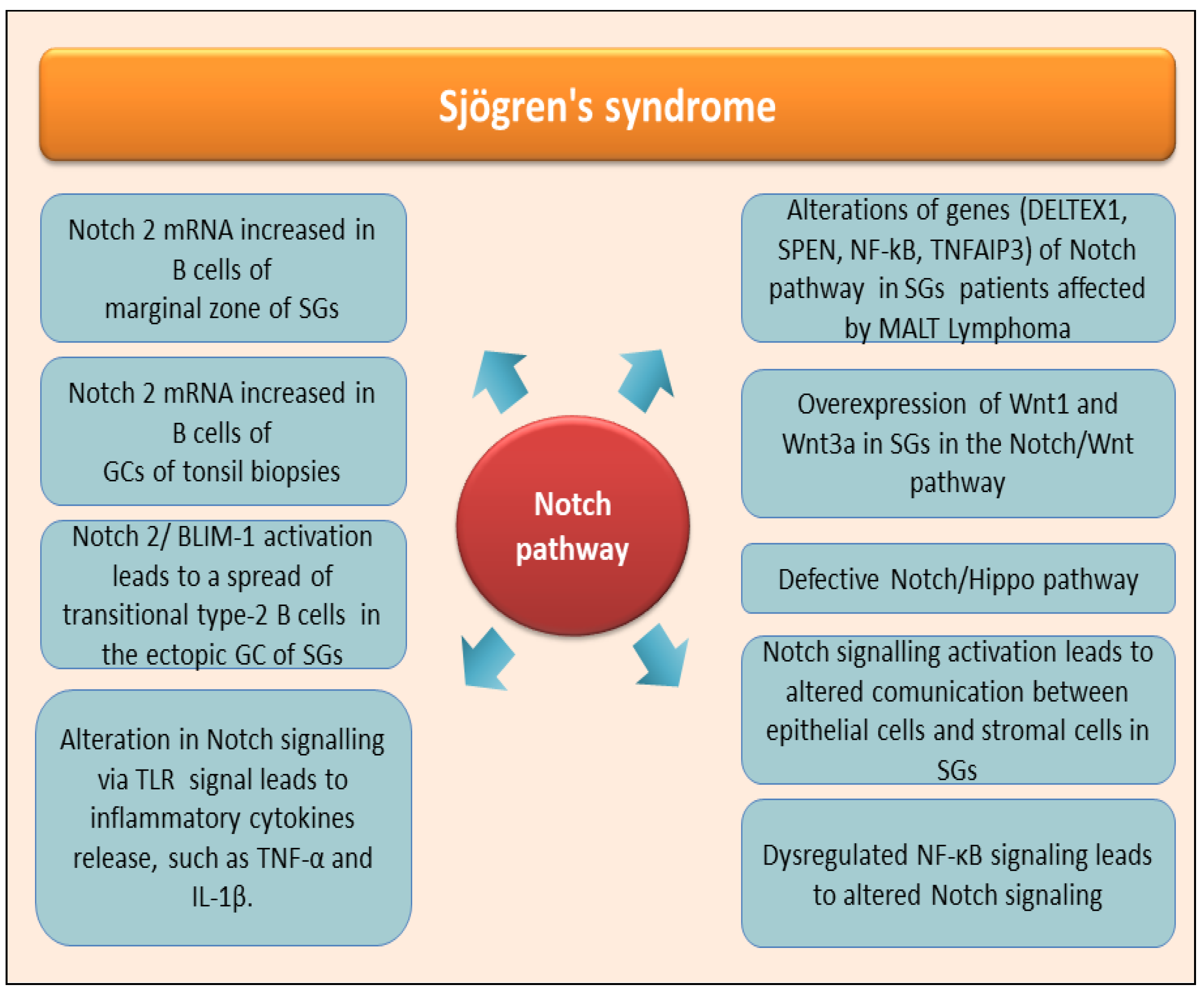
4. The Role of Notch in the Autoimmune Disease Sjögren’s Syndrome
5. Knowledge Regards Notch Pathways Activation in Non-Autoimmune SGs Diseases
5.1. Notch Signaling in Adenoid Cystic Carcinoma of Salivary Glands
5.2. The Intriguing Role of Tumor Stem Cells in NOTCH Signaling in Salivary Glands AdCC
5.3. Notch Signaling in Mucoepidermoid Carcinoma of Salivary Glands
6. Notch-Targeted Therapies in SGs Diseases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parambath, S.; Selvraj, N.R.; Venugopal, P.; Aradhya, R. Notch Signaling: An Emerging Paradigm in the Pathogenesis of Reproductive Disorders and Diverse Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 5423. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Sharma, V.; Mutsuddi, M.; Mukherjee, A. Notch signalling: Multifaceted role in development and disease. FEBS J. 2024, 291, 3030–3059. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, A.G.; El Hachem, M.; Zambruno, G.; Nystrom, A.; Candi, E.; Castiglia, D. Notching up knowledge on molecular mechanisms of skin fibrosis: Focus on the multifaceted Notch signalling pathway. J. Biomed. Sci. 2021, 28, 36. [Google Scholar] [CrossRef]
- Bray, S. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target Ther. 2022, 7, 95. [Google Scholar]
- Cinat, D.; Maturi, R.; Gunawan, J.P.; Jellema-de Bruin, A.L.; Kracht, L.; Martinez, P.S.; Wu, Y.; Soto-Gamez, A.; van Goethem, M.J.; Holtman, I.R.; et al. Notch Signaling Drives Pro-Regenerative and Migratory Traits in Glandular 2 Stem/Progenitor cells. bioRixv 2025. [Google Scholar] [CrossRef]
- Bray, S.J.; Bigas, A. Modes of Notch signalling in development and disease. Nat. Rev. Mol. Cell Biol. 2025; in press. [Google Scholar] [CrossRef]
- Purow, B.W.; Haque, R.M.; Noel, M.W.; Su, Q.; Burdick, M.J.; Lee, J.; Sundaresan, T.; Pastorino, S.; Park, J.K.; Mikolaenko, I.; et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005, 65, 2353–2363. [Google Scholar] [CrossRef]
- Brou, C.; Logeat, F.; Gupta, N.; Bessia, C.; LeBail, O.; Doedens, J.R.; Cumano, A.; Roux, P.; Black, R.A.; Israël, A. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol. Cell 2000, 5, 207–216. [Google Scholar] [CrossRef]
- Gazdik, T.R.; Crow, J.J.; Lawton, T.; Munroe, C.J.; Theriault, H.; Wood, T.M.; Albig, A.R. Notch intracellular domains form transcriptionally active heterodimeric complexes on sequence-paired sites. Sci. Rep. 2024, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Varnum-Finney, B.; Bernstein, I.D. The notch pathway: Modulation of cell fate decisions in hematopoiesis. Int. J. Hematol. 2002, 75, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, M.; De Mattei, C.; Giniger, E. Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 2008, 313, 556–567. [Google Scholar] [CrossRef]
- Sanalkumar, R.; Dhanesh, S.B.; James, J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol. Life Sci. 2010, 67, 2957–2968. [Google Scholar] [CrossRef]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell. 2017, 41, 2. [Google Scholar] [CrossRef]
- Henrique, D.; Schweisguth, F. Mechanisms of Notch signaling: A simple logic deployed in time and space. Development 2019, 146, dev172148. [Google Scholar] [CrossRef]
- Sprinzak, D.; Blacklow, S.C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021, 50, 157–189. [Google Scholar] [CrossRef]
- Seib, E.; Klein, T. The role of ligand endocytosis in notch signalling. Biol. Cell. 2021, 113, 401–418. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef]
- Varshney, S.; Stanley, P. Multiple roles for O-glycans in Notch signalling. FEBS Lett. 2018, 592, 3819–3834. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Luther, K.B.; Haltiwanger, R.S. Diseases related to Notch glycosylation. Mol. Asp. Med. 2021, 79, 100938. [Google Scholar] [CrossRef] [PubMed]
- Logeat, F.; Bessia, C.; Brou, C.; LeBail, O.; Jarriault, S.; Seidah, N.G.; Israël, A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. USA 1998, 95, 8108–8112. [Google Scholar] [CrossRef] [PubMed]
- Lieber, T.; Kidd, S.; Young, M.W. Kuzbanian-mediated cleavage of Drosophila Notch. Genes Dev. 2002, 16, 209–221. [Google Scholar] [CrossRef]
- Zolkiewska, A. ADAM proteases: Ligand processing and modulation of the Notch pathway. Cell Mol. Life Sci. 2008, 65, 2056–2068. [Google Scholar] [CrossRef]
- Deatherage, C.L.; Lu, Z.; Kim, J.H.; Sanders, C.R. Notch transmembrane domain: Secondary structure and topology. Biochemistry 2015, 54, 3565–3568. [Google Scholar] [CrossRef]
- Majumder, S.; Crabtree, J.S.; Golde, T.E.; Minter, L.M.; Osborne, B.A.; Miele, L. Targeting notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021, 20, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Park, H.S.; Lee, Y.C. OPTHiS identifies the molecular basis of the direct interaction between CSL and SMRT corepressor. Mol. Cells 2018, 41, 842–852. [Google Scholar]
- Kwon, C.; Cheng, P.; King, I.N.; Andersen, P.; Shenje, L.; Nigam, V.; Srivastava, D. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat. Cell Biol. 2011, 13, 1244–1251. [Google Scholar] [CrossRef]
- Ayaz, F.; Osborne, B.A. Non-canonical notch signaling in cancer and immunity. Front. Oncol. 2014, 4, 345. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, J.; Osborne, B.A. Noncanonical Notch Signaling. In Targeting Notch in Cancer; Miele, L., Artavanis-Tsakonas, S., Eds.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Jiang, N.; Hu, Y.; Wang, M.; Zhao, Z.; Li, M. The Notch Signaling Pathway Contributes to Angiogenesis and Tumor Immunity in Breast Cancer. Breast Cancer Targets Ther. 2022, 14, 291–309. [Google Scholar] [CrossRef]
- Andersen, P.; Uosaki, H.; Shenje, L.T.; Kwon, C. Non-canonical Notch signaling: Emerging role and mechanism. Trends Cell Biol. 2012, 22, 257–265. [Google Scholar] [CrossRef]
- Hurlbut, G.D.; Kankel, M.W.; Lake, R.J.; Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 2007, 19, 166–175. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhao, S.; Zhao, X.-Y.; Min, P.-X.; Ma, Y.-D.; Wang, Y.-Y.; Chen, Y.; Tang, S.-J.; Zhang, Y.-J.; et al. Non-canonical notch signaling regulates actin remodeling in cell migration by activating PI3K/AKT/Cdc42 pathway. Front. Pharm. 2019, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Sun, Y.J.; Weger, S.; Raouf, A. Notch-induced expression of FZD7 requires noncanonical NOTCH3 signaling in human breast epithelial cells. Stem Cells Dev. 2016, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Mutvei, A.P.; Chivukula, I.V.; Andersson, E.R.; Ramsköld, D.; Sandberg, R.; Lee, K.L.; Kronqvist, P.; Mamaeva, V.; Ostling, P.; et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene 2013, 32, 4892–4902. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 2017, 8, 709. [Google Scholar] [CrossRef]
- Lee, K.-S.; Wu, Z.; Song, Y.; Mitra, S.S.; Feroze, A.H.; Cheshier, S.H.; Lu, B. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 2013, 27, 2642–2647. [Google Scholar] [CrossRef]
- Perumalsamy, L.R.; Nagala, M.; Banerjee, P.; Sarin, A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR—Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009, 16, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Negulescu, A.; Bulusu, S.; Gibert, B.; Delcros, J.G.; Ducarouge, B.; Rama, N.; Gadot, N.; Treilleux, I.; Saintigny, P.; et al. Non-canonical NOTCH3 signalling limits tumour angiogenesis. Nat. Commun. 2017, 8, 16074. [Google Scholar] [CrossRef]
- Ma, J.; Tang, X.; Wong, P.; Jacobs, B.; Borden, E.C.; Bedogni, B. Noncanonical activation of Notch1 protein by membrane type 1 matrix metalloproteinase (MT1-MMP) controls melanoma cell proliferation. J. Biol. Chem. 2014, 289, 8442–8449. [Google Scholar] [CrossRef]
- Lammel, U.; Meadows, L.; Saumweber, H. Analysis of Drosophila salivary gland, epidermis and CNS development suggests an additional function of brinker in anterior-posterior cell fate specification. Mech. Dev. 2000, 92, 179–191. [Google Scholar] [CrossRef]
- Morgan, T.H. The theory of the gene. Am. Nat. 1917, 51, 513–544. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Bin Jang, S.; Lim, N.; Yeo, H.C.; Kwak, Y.; Lee, S.-H.; Shin, H.J.; Cho, S.-G. Advances in lacrimal gland organoid development: Techniques and therapeutic applications. Biomed. Pharmacother. 2025, 183, 117870. [Google Scholar]
- Šale, S.; Lafkas, D.; Artavanis-Tsakonas, S. Notch2 genetic fate mapping reveals two previously unrecognized mammary epithelial lineages. Nat. Cell Biol. 2013, 15, 451–460. [Google Scholar] [CrossRef]
- Dang, H.; Lin, A.L.; Zhang, B.; Zhang, H.-M.; Katz, M.S.; Yeh, C.-K. Role for Notch signaling in salivary acinar cell growth and differentiation. Dev. Dyn. 2009, 238, 724–731. [Google Scholar] [CrossRef]
- Chatzeli, L.; Bordeu, I.; Han, S.; Bisetto, S.; Waheed, Z.; Koo, B.-K.; Alcolea, M.P.; Simons, B.D. A cellular hierarchy of Notch and Kras signaling controls cell fate specification in the developing mouse salivary gland. Dev. Cell 2023, 58, 94–109. [Google Scholar] [CrossRef]
- Yasuhara, R.; Kang, S.; Irié, T.; Mabuchi, Y.; Kujiraoka, S.; Yukimori, A.; Ishida, S.; Tanaka, J.; Mishima, K. Role of Snai2 and Notch signaling in salivary gland myoepithelial cell fate. Lab. Investig. 2022, 102, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Qian, J.; Liang, W.; Xiang, H.; Ding, P.; Jin, M.; Lin, Z.; Chen, Y.; Wang, Z.; Huang, X.; et al. Establishment of human minor salivary gland organoids in laminin-GelMA hydrogel from healthy individuals and Sjögren’s disease patients. Chem. Eng. J. 2025, 503, 158257. [Google Scholar] [CrossRef]
- Gorodetskiy, V.R.; Probatova, N.A.; Vasilyev, V.I. Characteristics of diffuse large B-cell lymphoma in patients with primary Sjögren’s syndrome. Int. J. Rheum. Dis. 2020, 23, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, L.; Azgomi, M.S.; La Manna, M.P.; Guggino, G.; Botta, C.; Dieli, F.; Caccamo, N. B-Cell Receptor Signaling Is Thought to Be a Bridge between Primary Sjogren Syndrome and Diffuse Large B-Cell Lymphoma. Int. J. Mol. Sci. 2023, 24, 8385. [Google Scholar] [CrossRef]
- Le Pottier, L.; Devauchelle, V.; Fautrel, A.; Daridon, C.; Saraux, A.; Youinou, P.; Pers, J.O. Ectopic germinal centers are rare in Sjogren’s syndrome salivary glands and do not exclude autoreactive B cells. J. Immunol. 2009, 182, 3540–3547. [Google Scholar] [CrossRef]
- Guerrier, T.; Le Pottier, L.; Devauchelle, V.; Pers, J.-O.; Jamin, C.; Youinou, P. Role of Toll-like receptors in primary Sjögren’s syndrome with a special emphasis on B-cell maturation within exocrine tissues. J. Autoimmun. 2012, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Han, J.; Yang, M.; Zhu, J.; Jin, T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020, 18, 131. [Google Scholar] [CrossRef]
- Titsinides, S.; Nikitakis, N.; Piperi, E.; Sklavounou, A. MALT Lymphoma of Minor Salivary Glands in a Sjögren’s Syndrome Patient: A Case Report and Review of Literature. J. Oral Maxillofac. Res. 2017, 8, e5. [Google Scholar] [CrossRef]
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Famà, R.; et al. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551. [Google Scholar] [CrossRef]
- Bertoni, F.; Zucca, E. Delving deeper into MALT lymphoma biology. J. Clin. Investig. 2006, 116, 22–26. [Google Scholar] [CrossRef]
- Chanudet, E.; Huang, Y.; Zeng, N.; Streubel, B.; Chott, A.; Raderer, M.; Du, M.-Q. TNFAIP3 abnormalities in MALT lymphoma with autoimmunity. Br. J. Haematol. 2011, 154, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.; Escudero-Ibarz, L.; Wang, M.; Clipson, A.; Ruiz, E.O.; Dunn-Walters, D.; Xue, X.; Zeng, N.; Robson, A.; Chuang, S.S.; et al. Significant association between TNFAIP3 inactivation and biased immunoglobulin heavy chain variable region 4–34 usage in mucosa-associated lymphoid tissue lymphoma. J. Pathol. 2017, 243, 3–8. [Google Scholar] [CrossRef]
- Kuksin, C.A.; Minter, L.M. The Link between Autoimmunity and Lymphoma: Does NOTCH Signaling Play a Contributing Role? Front. Oncol. 2015, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Feng, Y.; Yang, Z.; Ding, Y.; Cheng, D.; Shi, Z.; Li, R.; Xue, L. Primary Sjögren’s syndrome: New perspectives on salivary gland epithelial cells. Eur. J. Med. Res. 2024, 29, 371. [Google Scholar] [CrossRef]
- Kang, B.K.; Zhu, Z.; Wang, J.; Zhou, J.; Yu, S.; Zhou, X.; Zhao, Z.; Xie, A.; Lu, L.; Yang, J. Maintenance of adult stem cells from human minor salivary glands via the Wnt signaling pathway. Stem Cell Res. Ther. 2023, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, L.; Zhao, L.; Su, Y. The interaction of Notch and Wnt signaling pathways in vertebrate regeneration. Cell Regen. 2021, 10, 11. [Google Scholar] [CrossRef]
- Varelas, X.; Wrana, J.L. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol. 2012, 22, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Enger, T.B.; Samad-Zadeh, A.; Bouchie, M.P.; Skarstein, K.; Galtung, H.K.; Mera, T.; Walker, J.; Menko, A.S.; Varelas, X.; Faustman, D.L.; et al. The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren’s syndrome. Lab. Investig. 2013, 93, 1203–1218. [Google Scholar] [CrossRef]
- Peng, B.; Ling, J.; Lee, A.J.; Wang, Z.; Chang, Z.; Jin, W.; Kang, Y.; Zhang, R.; Shim, D.; Wang, H.; et al. Defective feedback regulation of NF-kappaB underlies Sjögren’s syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc. Natl. Acad. Sci. USA 2010, 107, 15193–15198. [Google Scholar] [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. Understanding the Complexity of Sjögren’s Syndrome: Remarkable Progress in Elucidating NF-κB Mechanisms. J. Clin. Med. 2020, 9, 2821. [Google Scholar] [CrossRef]
- Huang, J.; Tang, J.; Zhang, C.; Liu, T.; Deng, Z.; Liu, L. Single-cell transcriptomic analysis uncovers heterogeneity in the labial gland microenvironment of primary Sjögren’s syndrome. J. Transl. Autoimmun. 2024, 9, 100248. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Dolcino, M.; Del Papa, N.; Minniti, A.; Pignataro, F.; Maglione, W.; Lunardi, C.; Puccetti, A. Gene expression profiles in primary Sjögren’s Syndrome with and without systemic manifestations. ACR Open Rheumatol. 2019, 1, 603–613. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N., Jr.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef]
- Zupancic, M.; Näsman, A.; Berglund, A.; Dalianis, T.; Friesland, S. Adenoid Cystic Carcinoma (AdCC): A Clinical Survey of a Large Patient Cohort. Cancers 2023, 15, 1499. [Google Scholar] [CrossRef]
- Tasoulas, J.; Schrank, T.P.; Bharambe, H.; Mehta, J.; Johnson, S.; Divaris, K.; Hackman, T.G.; Sheth, S.; Kirtane, K.; Hernandez-Prera, J.C.; et al. Molecular characterization of the salivary adenoid cystic carcinoma immune landscape by anatomic subsites. Sci. Rep. 2024, 14, 15821. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Murase, T.; Ueda, K.; Nagao, T.; Kusafuka, K.; Nakaguro, M.; Urano, M.; Taguchi, K.I.; Yamamoto, H.; Kano, S.; et al. Pathological evaluation of tumor grade for salivary adenoid cystic carcinoma: A proposal of an objective grading system. Cancer Sci. 2021, 112, 1184–1195. [Google Scholar] [CrossRef]
- Bell, D.; Roberts, D.; Kies, M.; Rao, P.; Weber, R.S.; El-Naggar, A.K. Cell type-dependent biomarker expression in adenoid cystic carcinoma: Biologic and therapeutic implications. Cancer 2010, 116, 5749–5756. [Google Scholar] [CrossRef]
- Barsky, S.H.; Karlin, N.J. Myoepithelial cells: Autocrine and paracrine suppressors of breast cancer progression. J. Mammary Gland Neoplasm Biol. 2005, 10, 249–260. [Google Scholar] [CrossRef]
- Hong, S.D.; Katuwal, N.B.; Kang, M.S.; Ghosh, M.; Park, S.M.; Kim, T.H.; Baek, Y.S.; Lee, S.R.; Moon, Y.W. Trastuzumab-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) Enhances Natural Killer Cell Cytotoxicity in HER2-Overexpressing Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 11733. [Google Scholar] [CrossRef]
- Bell, D.; Hanna, E.Y.; Miele, L.; Roberts, D.; Weber, R.S.; El-Naggar, A.K. Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann. Diagn. Pathol. 2014, 18, 10–13. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.-L.; Ma, S.-R.; Wang, W.-M.; Huang, C.-F.; Yu, G.-T.; Wu, T.-F.; Bu, L.-L.; Wang, Y.-F.; Zhao, Y.-F.; Zhang, W.-F.; et al. Notch signaling induces epithelial-mesenchymal transition to promote invasion and metastasis in adenoid cystic carcinoma. Am. J. Transl. Res. 2015, 7, 162–174. [Google Scholar]
- Luo, J.; Wang, P.; Wang, R.; Wang, J.; Liu, M.; Xiong, S.; Li, Y.; Cheng, B. The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget 2015, 7, 9525. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target Ther. 2024, 9, 170. [Google Scholar]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Aval, S.F.; Lotfi, H.; Sheervalilou, R.; Zarghami, N. Tuning of major signaling networks (TGF-β, Wnt, Notch and Hedgehog) by miRNAs in human stem cells commitment to different lineages: Possible clinical application. Biomed. Pharmacother. 2017, 91, 849–860. [Google Scholar] [CrossRef]
- Manni, W.; Min, W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. Med. Comm. 2022, 3, e176. [Google Scholar] [CrossRef]
- Olsauskas-Kuprys, R.; Zlobin, A.; Osipo, C. Gamma secretase inhibitors of Notch signaling. OncoTargets Ther. 2013, 6, 943–955. [Google Scholar]
- McCaw, T.R.; Inga, E.; Chen, H.; Jaskula-Sztul, R.; Dudeja, V.; Bibb, J.A.; Ren, B.; Rose, J.B. Gamma Secretase Inhibitors in Cancer: A Current Perspective on Clinical Performance. Oncologist 2021, 26, e608–e621. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Xu, C.; Gao, M.; Li, N.; Geng, Q. The critical role of γ-secretase and its inhibitors in cancer and cancer therapeutics. Int. J. Biol. Sci. 2023, 19, 5089–5103. [Google Scholar] [CrossRef]
- Fujita, S.; Ikeda, T. Cancer stem-like cells in adenoid cystic carcinoma of salivary glands: Relationship with morphogenesis of histological variants. J. Oral Pathol. Med. 2012, 41, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Saffar, H.; Taheri, P.; Yazdani, F.; Etebarian, A. Prognostic significance of cancer stem cell markers in patients with salivary gland carcinomas. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 284–290. [Google Scholar] [CrossRef]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T. Multifactorial contribution of notch signaling in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2019, 20, 1520. [Google Scholar] [CrossRef] [PubMed]
- Feeney, L.; Hapuarachi, B.; Adderley, H.; Rack, S.; Morgan, D.; Walker, R.; Rauch, R.; Herz, E.; Kaye, J.; Harrington, K.; et al. Clinical disease course and survival outcomes following disease recurrence in adenoid cystic carcinoma with and without NOTCH signaling pathway activation. Oral Oncol. 2022, 133, 106028. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, Z.; Qiao, Q.; Zhou, D.; Sun, M. Anti-NOTCH1 therapy with OMP-52 M51 inhibits salivary adenoid cystic carcinoma by depressing epithelial-mesenchymal transition (EMT) process and inducing ferroptosis. Toxicol. Appl. Pharmacol. 2024, 484, 116825. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, R. Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells. J. Clin. Med. 2019, 8, 642. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, J.; Waheed, A.; Karki, N.R.; Goodbee, M.; Yasinzai, A.Q.K.; Tareen, B.; Wali, A.; Khan, K.A.; Zarak, M.S.; et al. Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management. Cancers 2022, 15, 250. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, S.; Li, J.-L.; Ni, W.; Guo, R.; Lu, J.; Kaye, F.J.; Wu, L. CRTC1-MAML2 fusion-induced lncRNA LINC00473 expression maintains the growth and survival of human mucoepidermoid carcinoma cells. Oncogene 2018, 37, 1885–1895. [Google Scholar] [CrossRef]
- Tonon, G.; Modi, S.; Wu, L.; Kubo, A.; Coxon, A.B.; Komiya, T.; O’Neil, K.; Stover, K.; El-Naggar, A.; Griffin, J.D.; et al. t(11;19) (q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003, 33, 208–213. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Gu, Y.; Hu, C.; Li, J.-L.; Lin, S.; Shen, H.; Cao, C.; Gao, R.; Li, J.; et al. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene 2014, 33, 3869–3877. [Google Scholar] [CrossRef]
- Ni, W.; Chen, Z.; Zhou, X.; Yang, R.; Yu, M.; Lu, J.; Kaye, F.J.; Wu, L. Targeting Notch and EGFR signaling in human mucoepidermoid carcinoma. Signal Transduct. Target Ther. 2021, 6, 27. [Google Scholar] [CrossRef]
- Andersson, E.R.; Lendahl, U. Therapeutic modulation of Notch signalling-are we there yet? Nat. Rev. Drug Discov. 2014, 13, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; O’Sullivan Coyne, G.; Do, K.T.; Turkbey, B.; Meltzer, P.S.; Polley, E.; Choyke, P.L.; Meehan, R.; Vilimas, R.; Horneffer, Y.; et al. Clinical Activity of the γ-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis). J. Clin. Oncol. 2017, 35, 1561–1569. [Google Scholar] [CrossRef]
- Dumbrava, E.E.I.; Mills, G.B.; Yap, T.A. Targeting gamma secretase: Has progress moved up a Notch? Ann. Oncol. 2018, 29, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Desai, J.; Iyer, S.P.; Gadgeel, S.M.; Ramalingam, S.S.; Horn, L.; LoRusso, P.; Bajaj, G.; Kollia, G.; Qi, Z.; et al. A phase I study of AL101, a pan-NOTCH inhibitor, in patients (pts) with locally advanced or metastatic solid tumors. J. Clin. Oncol. 2018, 36, 2515. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Metcalf, R.; Rodriguez, C.P.; Muzaffar, J.; Even, C.; Perez, C.A.; Van Herpen, C.M.L.; Oliva, M.; Xia, B.; Bowles, D.W.; et al. Results of ACCURACY: A phase 2 trial of AL101, a selective gamma secretase inhibitor, in subjects with recurrent/metastatic (R/M) adenoid cystic carcinoma (ACC) harboring Notch activating mutations (Notchmut). J. Clin. Oncol. 2022, 40, 6046. [Google Scholar] [CrossRef]
- Massard, C.; Azaro, A.; Soria, J.-C.; Lassen, U.; Le Tourneau, C.; Sarker, D.; Smith, C.; Ohnmacht, U.; Oakley, G.; Patel, B.K.R.; et al. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann. Oncol. 2018, 29, 1911–1917. [Google Scholar] [CrossRef]
- Even, C.; Lassen, U.; Merchan, J.; Le Tourneau, C.; Soria, J.-C.; Ferte, C.; Ricci, F.; Diener, J.T.; Yuen, E.; Smith, C.; et al. Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Investig. New Drugs 2020, 38, 402–409. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Chen, Z.; Ni, W.; Li, J.-L.; Lin, S.; Zhou, X.; Sun, Y.; Li, J.W.; Leon, M.E.; Hurtado, M.D.; et al. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight 2021, 6, e139497. [Google Scholar]
- Sundaram, M.V. The love–hate relationship between Ras and Notch. Genes Dev. 2005, 19, 1825–1839. [Google Scholar] [CrossRef]
- Arasada, R.R.; Amann, J.M.; Rahman, M.A.; Huppert, S.S.; Carbone, D.P. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res. 2014, 74, 5572–5584. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fu, W.; Li, T.; Yuan, Q.; Wang, F.; Lv, G.; Lv, Y.; Fan, X.; Shen, Y.; Lin, F.; et al. Antagonism of EGFR and Notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci. Transl. Med. 2017, 9, eaag0339. [Google Scholar] [CrossRef] [PubMed]
- Mur, E.B.; Bernardo, S.; Papon, L.; Mancini, M.; Fabbrizio, E.; Goussard, M.; Ferrer, I.; Giry, A.; Quantin, X.; Pujol, J.-L.; et al. Notch inhibition overcomes resistance to tyrosine kinase inhibitors in EGFR-driven lung adenocarcinoma. J. Clin. Investig. 2020, 130, 612–624. [Google Scholar]
- Miranda, E.L.; Stathis, A.; Hess, D.; Racca, F.; Quon, D.; Rodon, J.; Gadea, O.S.S.; Garcia, J.M.P.; Nuciforo, P.; Vivancos, A.; et al. Phase 1 study of CB-103, a novel first-in-class inhibitor of the CSL-NICD gene transcription factor complex in human cancers. J. Clin. Oncol. 2021, 39, 3020. [Google Scholar] [CrossRef]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting cancer stemness in the clinic: From hype to hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef]
| Drug | Target | Mode of Action | Effect | Ref |
|---|---|---|---|---|
| AL101 | γ-secretase inhibitor | Notch pathway inhibition during the cleavage process in the intracellular domain | Anti-tumor effect in patients with metastatic solid tumors | [106] |
| Crenigacestat | γ-secretase inhibitor | Inhibition of the release of the Notch intracellular domain by suppression of γ-secretase complex | Anti-tumor effect in patients with advanced or metastatic disease as patients with ADCC | [108] |
| DBZ dibenzazepine | γ-secretase inhibitor | The block of the cleavage of Notch into its active signaling effector, Notch intracellular domain | Anti-tumor efficacy with strong anti-stem cell effect in human mucoepidermoid carcinoma of the salivary gland | [102] |
| Erlotinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti-tumor effect in human mucoepidermoid carcinoma | [102] |
| Gefitinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti-tumor effect in lung adenocarcinoma | [114] |
| Osimertinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti-tumor effect in lung adenocarcinoma | [114] |
| Brontictuzumab | Notch 1 inhibitor | Humanized monoclonal antibody against the Notch1 protein | Repression of the proliferation and EMT inhibiting salivary adenoid cystic carcinoma | [96] |
| CB-103 | Notch 1 inhibitor | Inhibits the CSL–NICD | Downregulation in Notch signaling | [115] |
| Amcasertib | Notch inhibitor | Cancer stemness kinase inhibitor | Impairment of cancer stem cell survival deregulating Notch signaling in AdCC | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisto, M.; Lisi, S. Significance of Notch Signaling in Salivary Gland Development and Diseases. J. Clin. Med. 2025, 14, 3325. https://doi.org/10.3390/jcm14103325
Sisto M, Lisi S. Significance of Notch Signaling in Salivary Gland Development and Diseases. Journal of Clinical Medicine. 2025; 14(10):3325. https://doi.org/10.3390/jcm14103325
Chicago/Turabian StyleSisto, Margherita, and Sabrina Lisi. 2025. "Significance of Notch Signaling in Salivary Gland Development and Diseases" Journal of Clinical Medicine 14, no. 10: 3325. https://doi.org/10.3390/jcm14103325
APA StyleSisto, M., & Lisi, S. (2025). Significance of Notch Signaling in Salivary Gland Development and Diseases. Journal of Clinical Medicine, 14(10), 3325. https://doi.org/10.3390/jcm14103325







