Is Breastfeeding an Effective Approach to Reduce Metabolic Risk After GDM in Mothers and Infants?
Abstract
1. Introduction
2. BF and Glycolipid Profile in Mothers with History of GDM
2.1. Early Effects of BF on Metabolic Health of Mothers with History of GDM
| Study | Study Design | Population | Groups | Postpartum Evaluation | Lactation Classification | Main Findings |
|---|---|---|---|---|---|---|
| Gunderson et al., 2012 [36] | Prospective | GDM n = 522 | Exclusive BF (n = 211) Mostly BF (n = 99) Mixed or inconsistent (n = 77) Exclusive or mostly FF (n = 135) | 6–9 wks | Exclusive BF: no formula; Mostly BF: ≤6 oz formula/24 h; Mixed or inconsistent feeding of breast milk and formula: 7–17 oz/24 h or change in feeding status to increase formula Exclusive or mostly FF: >17 oz formula/24 h | Exclusive BF and mostly BF vs. exclusive or mostly FF: lower FPG, FI, and 2 h insulin; lower prevalence of diabetes or prediabetes |
| Shub et al., 2019 [40] | Prospective | GDM n = 159 NGT n = 84 | Exclusive BF (n = 106) Not exclusive BF (n = 53) | 6–10 wks | BF status categorized as exclusively BF or not exclusively BF, including women who were feeding both formula and BF | GDM group Exclusive BF vs. not exclusive BF: lower FPG, no difference in lipids |
| O’Reilly et al., 2011 [33] | Prospective | GDM n = 300 NGT n = 220 | BF (n = 319) FF (n = 20) | 12 wks | All the following criteria required for lactation definition: ongoing feeding (at least 4 times per day; meeting maternal expectations; duration > 8 wks; infant reaching developmental milestones, in particular, gaining weight; infant receiving scheduled immunizations | BF vs. FF: lower prevalence of persistent OGTT alterations |
| Yasuhi et al., 2019 [41] | Prospective | GDM n = 222 | High-intensity BF (n = 166) Non-high intensity BF (n = 56) | 6–12 wks | High-intensity BF: “BF alone”, “mostly BF with a minimal additional formula”, and “≥80% of the volume by BF; <20% by formula” Non-high intensity BF: “60–70% by BF; 30–40% by formula”, “≤50% by BF; >50% by formula”, and “by formula alone” | High-intensity BF vs. non-high-intensity BF: lower FPG and FI at OGTT; In obese patients: higher HOMA-IR in non-high intensity BF vs. high-intensity BF |
| Corrado et al., 2019 [42] | Retrospective | GDM n = 97 | BF (n = 81) No BF (n = 16) | 3–4 mo | NA | BF vs. no BF: lower FPG, 2 h glucose, TG, HOMA-IR; lower prevalence of IFG and IGT |
| Gunderson et al., 2014 [37] | Prospective | GDM n = 1007 | Exclusive BF (n = 437) Mostly BF (n = 183) Mixed or inconsistent breast milk and formula (n = 128) Mostly or exclusively FF (n = 259) | 6–9 wks | Exclusive BF: 0 ounces of formula; mostly BF: ≤6 ounces of formula/24 h; mixed (breast milk and formula >6 to ≤17 ounces/24 h) or inconsistent feeding method; mostly FF > 17 ounces/24 h; exclusive FF | Exclusive BF and mostly BF groups vs. mostly or exclusive FF groups: lower fasting glucose, FI, 2 h glucose 2 h insulin; higher insulin sensitivity index; lower insulin resistance and insulin secretion indices; less likely to be glucose intolerant ↑ lactation intensity: ↓ TG, ↑ HDL-c, ↓ leptin |
| Vanlaer et al., 2024 [38] | Prospective | GDM n = 1008 | Exclusive BF (n = 567) Mixed BF/FF (n = 102) Exclusive FF (n = 339) | 12 wks | Exclusive BF (<45 mL FF/day); mixed BF/FF; exclusive FF (≥150 mL FF/day) | Exclusive and mixed BF vs. no BF: ↓ rate of T2D, prediabetes. The effect was no longer significant for the mixed BF group when adjusting for pre-pregnancy BMI, race, education, and income. |
| Hebeisen et al., 2024 [39] | Prospective | GDM n = 171 | BF < 6 mo (n = 69) BF ≥ 6 mo (n = 102) | 1 yr | BF < 6 mo; BF ≥ 6 mo | Inverse association of BF duration with weight, weight retention, visceral adipose tissue, insulin resistance indices, and C-reactive protein after adjustment for pre-pregnancy BMI, education, and therapy during pregnancy |
| Zhang et al., 2021 [44] | Prospective | GDM n = 350 | Intensive BF (n = 216) Intensive FF (n = 134) | 6–9 wks | BF intensity and duration ratio: the number of breast milk feeds/24 divided by the total number of all liquid feeds/24 h during the past 7 days to yield a score from 0 to 1. 1 = exclusive BF, 0 = exclusive FF, fractional scores = levels of lactation intensity | Intensive BF: ↓ TG and DAG; ↑ phospholipids and sphingolipids |
| Suthasmalee et al., 2024 [43] | Prospective | GDM n = 130 | BF maintained at 6 mo (exclusive BF n = 49 or partial BF n = 24) BF < 6 mo n = 57 | 6 mo | BF maintained at 6 mo (exclusive BF or partial BF) BF < 6 mo | Maintaining BF at 6 mo vs. BF < 6 mo ↓ rate of prediabetes. The protective effect of BF against prediabetes was significant only in the EBF group, not in the partial BF group. |
2.2. BF and GDM Recurrence
2.3. Long-Standing Effects of BF on Metabolic Health of Mothers with History of GDM
| Study | Study Design | Population | Groups by BF Habits | Follow-Up | Lactation Classification | Main Findings |
|---|---|---|---|---|---|---|
| Gunderson et al., 2015 [49] | Prospective | N = 1010 | Exclusive BF (n = 205) Mostly BF (n = 387) Mostly FF, Mixed/ inconsistent (n = 214) Exclusive FF (n = 153) | 2 yrs | Exclusive BF (no FF), mostly BF (>0 to 6 oz of formula per 24 h), mostly FF (>17 oz per 24 h), and mixed (7 to 17 oz of formula per 24 h) or inconsistent lactation pattern, and exclusive FF (formula only; no BF or BF < 3 weeks) | Inverse association for lactation intensity at baseline and duration with incident T2D |
| O’Shea et al., 2023 [50] | Retrospective | N = 74 | BF (n = 50) No BF (n = 24) | 4 yrs | BF status categorized as any BF yes or no | BF and BF duration associated with lower likelihood of abnormal glucose tolerance, adjusting for age, ethnicity, relatives with T2D, weight gain, and occupation |
| Hewage et al., 2021 [51] | Retrospective | N = 116 | No BF or <1 (n = 21) >1 to <6 (n = 50) >6 mo (n = 45) | 4–7 yrs | No BF or <1, >1 to <6, and >6 mo | BF duration > 6 mo 50% reduced incidence of OGTT alteration compared with women who did not breastfeed or with lactation duration < 1 mo |
| Feleke et al., 2020 [52] | Prospective | N = 1649 | NS | 10 yrs | BF status categorized as any BF yes or no | Frequency of BF inversely associated with T2D incidence, adjusting for age, parity, history of GDM, regular physical activity, family history of T2D, and history of stillbirth or abortion |
| Gunderson et al., 2018 [53] | Prospective | N = 155 | NS | 30 yrs | BF duration: none; <6 weeks; 6–11 weeks; 3–6 mo; or 6 mo or more | Graded inverse association between longer lactation duration and diabetes incidence, adjusting for ethnicity, pre-pregnancy BMI, waist circumference, FPG, HOMA-IR, age, parity, and history of GDM and T2D |
| Ziegler et al., 2012 [54] | Prospective | N = 304 (N = 264 with BF information) | No BF (n = 63) BF < 3 mo (n = 109) BF > 3 mo (n = 92) | 20 yrs | BF duration: no BF; BF ≤ 3 mo; BF > 3 mo | Duration of lactation inversely associated with postpartum diabetes risk. BF > 3 mo had 45% lower risk of diabetes compared with no BF or BF < 3 mo |
| Wander et al., 2022 [55] | Prospective | N = 577 (N = 532 with BF information) | No BF (n = 57) <6 mo (n = 101) 6–12 mo (n = 171) 12–24 mo (n = 143) ≥24 mo (n = 60) | 9–16 yrs | No BF; <6; 6–12; 12–24; ≥24 mo | Longer lactation duration not predictive of reduced risk of T2D, prediabetes, and obesity after adjustment for pre-pregnancy BMI, parity, and lifestyle factors |
| Stuebe et al., 2005 [56] | Retrospective | N = 266 | No BF (n = 265) >0 to 3 mo (n = 197) >3 to 6 mo (n = 114) >6 to 11 mo (n = 185) >11 to 23 mo (n = 224) >23 mo (n = 147) | 10 yrs | No BF >0 to 3 mo >3 to 6 mo >6 to 11 mo >11 to 23 mo >23 mo | Duration of lactation inversely associated with T2D incidence but no significant effect when stratifying by GDM history |
| Ley et al., 2020 [57] | Prospective | N = 4372 | No BF (n = 766) >0 to 6 mo (n = 770) >6 to 12 mo (n = 871) >12 to 24 mo (n = 1082) >24 mo (n = 833) | 25 yrs | No BF >0 to 6 mo >6 to 12 mo >12 to 24 mo >24 mo | Longer lifetime lactation duration and longer exclusive lifetime duration associated with lower risk of T2D after adjustment for age, ethnicity, family history of diabetes, parity, age at first birth, and lifestyle factors |
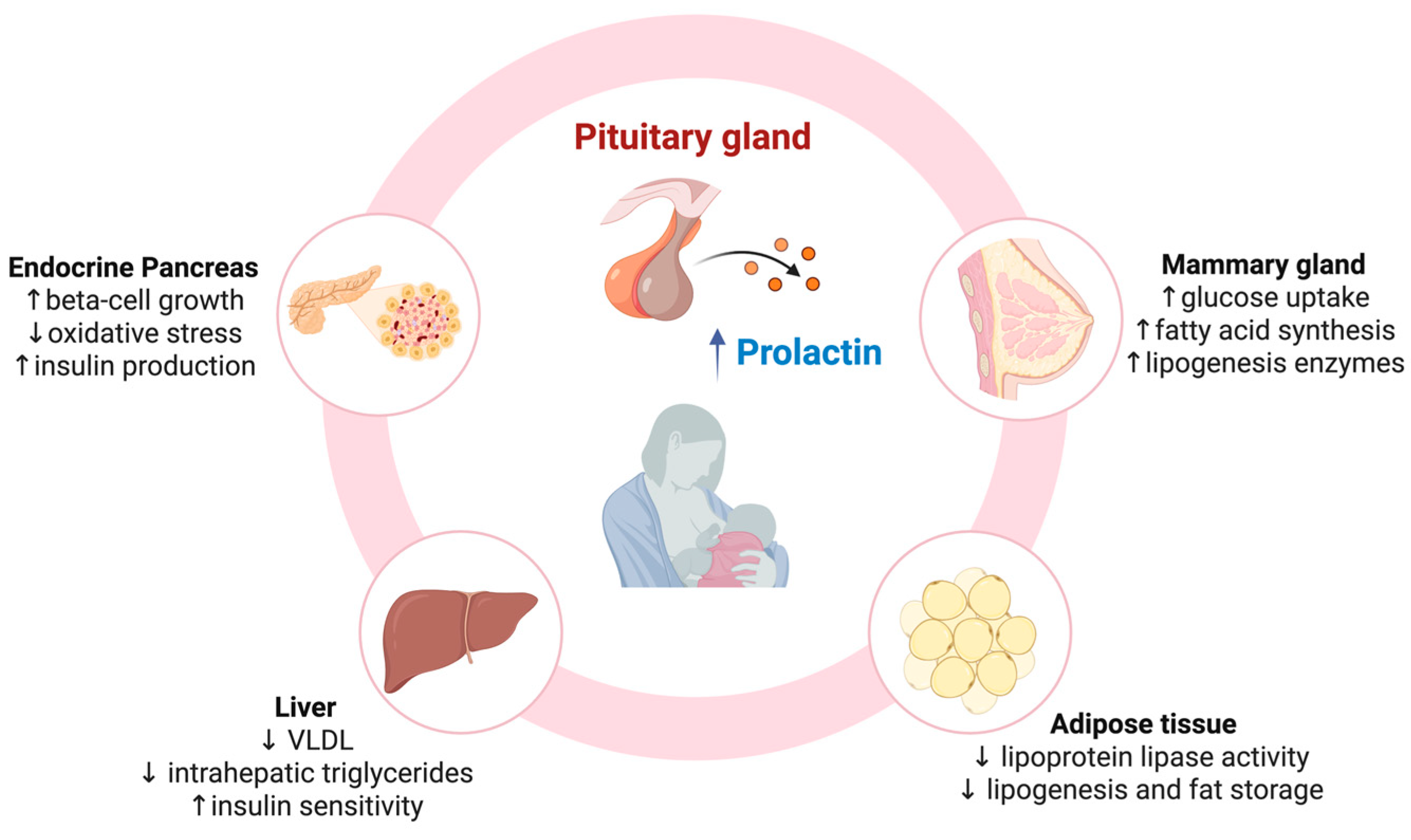
3. BF and Progression to T2D in Mothers: The Mechanisms Behind the Possible Beneficial Effects
3.1. Role of the Mammary Gland in Glucose Homeostasis During Lactation
3.2. Prolactin-Mediated Crosstalk Between Mammary Gland and Adipose Tissue in Metabolic Homeostasis
3.3. White Adipose Tissue Adaptation During Lactation
3.4. Role of the Liver in Metabolic Homeostasis During Lactation
3.5. Endocrine Pancreas Response to Lactation
4. Impact of BF on Metabolic Profile and Risk of T2D in Offspring of Mothers with Previous GDM
5. Mechanisms Behind the Possible Beneficial Effects of BF Against Obesity and Metabolic Diseases in Offspring
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Nachum, Z.; Green, M.S. Risk factors of gestational diabetes mellitus recurrence: A meta-analysis. Endocrine 2016, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Berger, D.K.; Chamany, S. Recurrence of gestational diabetes mellitus: A systematic review. Diabetes Care 2007, 30, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, J.N. Recurrent gestational diabetes: Risk factors, diagnosis, management, and implications. Semin. Perinatol. 2007, 31, 176–184. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Rayanagoudar, G.; Hashi, A.A.; Zamora, J.; Khan, K.S.; Hitman, G.A.; Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia 2016, 59, 1403–1411. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Xiao, S.; Qiu, L.; Yu, Y.; Zhang, Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: Systematic review and meta-analysis. BMJ 2022, 378, e070244. [Google Scholar] [CrossRef]
- Christensen, M.H.; Bistrup, C.; Rubin, K.H.; Nohr, E.A.; Vinter, C.A.; Andersen, M.S.; Moller, S.; Jensen, D.M. Kidney Disease in Women With Previous Gestational Diabetes Mellitus: A Nationwide Register-Based Cohort Study. Diabetes Care 2024, 47, 401–408. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, E.; Qiao, Y.; Katzmarzyk, P.T.; Chaput, J.P.; Fogelholm, M.; Johnson, W.D.; Kuriyan, R.; Kurpad, A.; Lambert, E.V.; et al. Maternal gestational diabetes and childhood obesity at age 9-11: Results of a multinational study. Diabetologia 2016, 59, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Holder, T.; Giannini, C.; Santoro, N.; Pierpont, B.; Shaw, M.; Duran, E.; Caprio, S.; Weiss, R. A low disposition index in adolescent offspring of mothers with gestational diabetes: A risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014, 57, 2413–2420. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Lamichhane, A.P.; D’Agostino, R.B., Jr.; Liese, A.D.; Vehik, K.S.; Narayan, K.M.; Zeitler, P.; Hamman, R.F. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: The SEARCH Case-Control Study. Diabetes Care 2008, 31, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Cui, D.; Li, C.; Ma, R.C.W.; Li, J.; Yang, X. Effects of lifestyle intervention on long-term risk of diabetes in women with prior gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2021, 22, e13122. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Patnode, C.D.; Henrikson, N.B.; Webber, E.M.; Blasi, P.R.; Senger, C.A.; Guirguis-Blake, J.M. Breastfeeding and Health Outcomes for Infants and Children: A Systematic Review. Pediatrics 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.M.; Lynch, K.F.; Uusitalo, U.; Yang, J.; Lonnrot, M.; Virtanen, S.M.; Hyoty, H.; Norris, J.M.; for the TEDDY Study Group. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Gao, Y.C.; Hu, X.; Tan, Y.T.; Deng, J.J.; Pan, H.F.; Tao, S.S. Association between breastfeeding and the risk of autoimmune diseases: A systematic review and meta-analysis. Autoimmun. Rev. 2025, 24, 103801. [Google Scholar] [CrossRef]
- Blotsky, A.L.; Rahme, E.; Dahhou, M.; Nakhla, M.; Dasgupta, K. Gestational diabetes associated with incident diabetes in childhood and youth: A retrospective cohort study. CMAJ 2019, 191, E410–E417. [Google Scholar] [CrossRef]
- Dinleyici, E.C. Breastfeeding and Health Benefits for the Mother-Infant Dyad: A Perspective on Human Milk Microbiota. Ann. Nutr. Metab. 2025, 1–13, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Breastfeeding Scorecard 2023. Rates of Breastfeeding Increase Around the World Through Inproved Protection and Support. Available online: https://www.who.int/publications/i/item/WHO-HEP-NFS-23.17 (accessed on 24 December 2024).
- Nguyen, P.T.H.; Pham, N.M.; Chu, K.T.; Van Duong, D.; Van Do, D. Gestational Diabetes and Breastfeeding Outcomes: A Systematic Review. Asia Pac. J. Public Health 2019, 31, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Wang, Y.; You, H.X.; Luo, B.R. Exploring the breastfeeding knowledge level and its influencing factors of pregnant women with gestational diabetes mellitus. BMC Pregnancy Childbirth 2020, 20, 723. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Dennison, R.A.; Chen, E.S.; Green, M.E.; Legard, C.; Kotecha, D.; Farmer, G.; Sharp, S.J.; Ward, R.J.; Usher-Smith, J.A.; Griffin, S.J. The absolute and relative risk of type 2 diabetes after gestational diabetes: A systematic review and meta-analysis of 129 studies. Diabetes Res. Clin. Pract. 2021, 171, 108625. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- de Gennaro, G.; Bianchi, C.; Aragona, M.; Battini, L.; Baronti, W.; Brocchi, A.; Del Prato, S.; Bertolotto, A. Postpartum screening for type 2 diabetes mellitus in women with gestational diabetes: Is it really performed? Diabetes Res. Clin. Pract. 2020, 166, 108309. [Google Scholar] [CrossRef]
- Brown, S.D.; Hedderson, M.M.; Zhu, Y.; Tsai, A.L.; Feng, J.; Quesenberry, C.P.; Ferrara, A. Uptake of guideline-recommended postpartum diabetes screening among diverse women with gestational diabetes: Associations with patient factors in an integrated health system in USA. BMJ Open Diabetes Res. Care 2022, 10, e002726. [Google Scholar] [CrossRef]
- Ogonowski, J.; Miazgowski, T. The prevalence of 6 weeks postpartum abnormal glucose tolerance in Caucasian women with gestational diabetes. Diabetes Res. Clin. Pract. 2009, 84, 239–244. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Avalos, G.; Dennedy, M.C.; O’Sullivan, E.P.; Dunne, F. Atlantic DIP: High prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur. J. Endocrinol. 2011, 165, 953–959. [Google Scholar] [CrossRef]
- Bianchi, C.; de Gennaro, G.; Brocchi, A.; Minaldi, E.; Del Prato, S.; Bertolotto, A. Risk factors associated with postpartum impaired glucose regulation in women with previous gestational diabetes. J. Diabetes Complicat. 2021, 35, 107854. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodriguez, M.; Melero, V.; Barabash, A.; Valerio, J.; Del Valle, L.; O’Connor, R.M.; de Miguel, P.; Diaz, J.A.; Familiar, C.; Moraga, I.; et al. Modifiable Risk Factors and Trends in Changes in Glucose Regulation during the First Three Years Postdelivery: The St Carlos Gestational Diabetes Mellitus Prevention Cohort. Nutrients 2023, 15, 4995. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Hedderson, M.M.; Chiang, V.; Crites, Y.; Walton, D.; Azevedo, R.A.; Fox, G.; Elmasian, C.; Young, S.; Salvador, N.; et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: The SWIFT cohort. Diabetes Care 2012, 35, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Kim, C.; Quesenberry, C.P., Jr.; Marcovina, S.; Walton, D.; Azevedo, R.A.; Fox, G.; Elmasian, C.; Young, S.; Salvador, N.; et al. Lactation intensity and fasting plasma lipids, lipoproteins, non-esterified free fatty acids, leptin and adiponectin in postpartum women with recent gestational diabetes mellitus: The SWIFT cohort. Metabolism 2014, 63, 941–950. [Google Scholar] [CrossRef]
- Vanlaer, Y.; Minschart, C.; Vrolijk, H.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Devlieger, R.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; et al. Impact of breastfeeding on risk of glucose intolerance in early postpartum after gestational diabetes. Front. Endocrinol. 2024, 15, 1374682. [Google Scholar] [CrossRef]
- Hebeisen, I.; Gonzalez Rodriguez, E.; Arhab, A.; Gross, J.; Schenk, S.; Gilbert, L.; Benhalima, K.; Horsch, A.; Quansah, D.Y.; Puder, J.J. Prospective associations between breast feeding, metabolic health, inflammation and bone density in women with prior gestational diabetes mellitus. BMJ Open Diabetes Res. Care 2024, 12, e004117. [Google Scholar] [CrossRef]
- Shub, A.; Miranda, M.; Georgiou, H.M.; McCarthy, E.A.; Lappas, M. The effect of breastfeeding on postpartum glucose tolerance and lipid profiles in women with gestational diabetes mellitus. Int. Breastfeed. J. 2019, 14, 46. [Google Scholar] [CrossRef]
- Yasuhi, I.; Yamashita, H.; Maeda, K.; Nomiyama, M.; Mizunoe, T.; Tada, K.; Yorozu, M.; Ogawa, M.; Kodama, T.; Yamaguchi, K.; et al. High-intensity breastfeeding improves insulin sensitivity during early post-partum period in obese women with gestational diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3127. [Google Scholar] [CrossRef]
- Corrado, F.; Giunta, L.; Granese, R.; Corrado, S.; Micali, M.; Santamaria, A.; D’Anna, R.; Di Benedetto, A. Metabolic effects of breastfeeding in women with previous gestational diabetes diagnosed according to the IADPSG criteria. J. Matern. Fetal Neonatal Med. 2019, 32, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Suthasmalee, S.; Phaloprakarn, C. Lactation duration and development of type 2 diabetes and metabolic syndrome in postpartum women with recent gestational diabetes mellitus. Int. Breastfeed. J. 2024, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lai, M.; Piro, A.L.; Alexeeff, S.E.; Allalou, A.; Rost, H.L.; Dai, F.F.; Wheeler, M.B.; Gunderson, E.P. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: The SWIFT study. BMC Med. 2021, 19, 241. [Google Scholar] [CrossRef]
- Funai, K.; Lodhi, I.J.; Spears, L.D.; Yin, L.; Song, H.; Klein, S.; Semenkovich, C.F. Skeletal Muscle Phospholipid Metabolism Regulates Insulin Sensitivity and Contractile Function. Diabetes 2016, 65, 358–370. [Google Scholar] [CrossRef]
- Zuarez-Easton, S.; Berkovich, I.; Birenbaum-Carmeli, D.; Tal, A.; Zoabi, R.; Salim, R. Effect of lactation on the recurrence rate of gestational diabetes mellitus: A retrospective cohort study. Arch. Gynecol. Obstet. 2020, 301, 973–979. [Google Scholar] [CrossRef]
- Melov, S.J.; White, L.; Simmons, M.; Kirby, A.; Stulz, V.; Padmanabhan, S.; Alahakoon, T.I.; Pasupathy, D.; Cheung, N.W. The BLIiNG study—Breastfeeding length and intensity in gestational diabetes and metabolic effects in a subsequent pregnancy: A cohort study. Midwifery 2022, 107, 103262. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.J.; Elhindi, J.; White, L.; McNab, J.; Lee, V.W.; Donnolley, K.; Alahakoon, T.I.; Padmanabhan, S.; Cheung, N.W.; Pasupathy, D. Previous High-Intensity Breastfeeding Lowers the Risk of an Abnormal Fasting Glucose in a Subsequent Pregnancy Oral Glucose Tolerance Test. Nutrients 2023, 16, 28. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Hurston, S.R.; Ning, X.; Lo, J.C.; Crites, Y.; Walton, D.; Dewey, K.G.; Azevedo, R.A.; Young, S.; Fox, G.; et al. Lactation and Progression to Type 2 Diabetes Mellitus After Gestational Diabetes Mellitus: A Prospective Cohort Study. Ann. Intern. Med. 2015, 163, 889–898. [Google Scholar] [CrossRef]
- O’Shea, E.; Awang, M.H.; Kgosidialwa, O.; Tuthill, A. Abnormal glucose tolerance in women with prior gestational diabetes mellitus: A 4-year follow-up study. Ir. J. Med. Sci. 2023, 192, 641–648. [Google Scholar] [CrossRef]
- Hewage, S.S.; Koh, X.Y.H.; Soh, S.E.; Pang, W.W.; Fok, D.; Cai, S.; Muller-Riemenschneider, F.; Yap, F.; Tan, K.H.; Chua, M.C.; et al. Breastfeeding Duration and Development of Dysglycemia in Women Who Had Gestational Diabetes Mellitus: Evidence from the GUSTO Cohort Study. Nutrients 2021, 13, 408. [Google Scholar] [CrossRef]
- Feleke, B.E.; Feleke, T.E.; Kassahun, M.B.; Adane, W.G.; Achenefe, D.; Genetu, A.; Nigussie, A.A.; Engedaw, H.A. Progression of pregnancy induced diabetes mellitus to type two diabetes mellitus, an ambidirectional cohort study. Prim. Care Diabetes 2021, 15, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Lewis, C.E.; Lin, Y.; Sorel, M.; Gross, M.; Sidney, S.; Jacobs, D.R., Jr.; Shikany, J.M.; Quesenberry, C.P., Jr. Lactation Duration and Progression to Diabetes in Women Across the Childbearing Years: The 30-Year CARDIA Study. JAMA Intern. Med. 2018, 178, 328–337. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Wallner, M.; Kaiser, I.; Rossbauer, M.; Harsunen, M.H.; Lachmann, L.; Maier, J.; Winkler, C.; Hummel, S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes 2012, 61, 3167–3171. [Google Scholar] [CrossRef]
- Wander, P.L.; Hinkle, S.N.; Enquobahrie, D.A.; Wu, J.; Ley, S.H.; Grunnet, L.G.; Chavarro, J.E.; Li, M.; Bjerregaard, A.A.; Liu, A.; et al. Cumulative Lactation and Clinical Metabolic Outcomes at Mid-Life among Women with a History of Gestational Diabetes. Nutrients 2022, 14, 650. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Rich-Edwards, J.W.; Willett, W.C.; Manson, J.E.; Michels, K.B. Duration of lactation and incidence of type 2 diabetes. JAMA 2005, 294, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Chavarro, J.E.; Li, M.; Bao, W.; Hinkle, S.N.; Wander, P.L.; Rich-Edwards, J.; Olsen, S.; Vaag, A.; Damm, P.; et al. Lactation Duration and Long-term Risk for Incident Type 2 Diabetes in Women With a History of Gestational Diabetes Mellitus. Diabetes Care 2020, 43, 793–798. [Google Scholar] [CrossRef]
- Ma, S.; Hu, S.; Liang, H.; Xiao, Y.; Tan, H. Metabolic effects of breastfeed in women with prior gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3108. [Google Scholar] [CrossRef] [PubMed]
- Anhe, G.F.; Bordin, S. The adaptation of maternal energy metabolism to lactation and its underlying mechanisms. Mol. Cell Endocrinol. 2022, 553, 111697. [Google Scholar] [CrossRef]
- Burnol, A.F.; Leturque, A.; Ferre, P.; Kande, J.; Girard, J. Increased insulin sensitivity and responsiveness during lactation in rats. Am. J. Physiol. 1986, 251, E537–E541. [Google Scholar] [CrossRef]
- Jones, R.G.; Ilic, V.; Williamson, D.H. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem. J. 1984, 220, 455–460. [Google Scholar] [CrossRef]
- Burnol, A.F.; Loizeau, M.; Girard, J. Insulin receptor activity and insulin sensitivity in mammary gland of lactating rats. Am. J. Physiol. 1990, 259, E828–E834. [Google Scholar] [CrossRef]
- Butte, N.F.; Hopkinson, J.M.; Mehta, N.; Moon, J.K.; Smith, E.O. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am. J. Clin. Nutr. 1999, 69, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Crites, Y.; Chiang, V.; Walton, D.; Azevedo, R.A.; Fox, G.; Elmasian, C.; Young, S.; Salvador, N.; Lum, M.; et al. Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstet. Gynecol. 2012, 120, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Kawakubo, M.; Kjos, S.L.; Buchanan, T.A. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006, 29, 613–617. [Google Scholar] [CrossRef]
- Monroy, G.; Fernandez, C.; Caballe, T.; Altimira, L.; Corcoy, R. Breastfeeding effect on glucose tolerance assessment in women with previous gestational diabetes mellitus: A randomized controlled trial. Diabet. Med. 2022, 39, e14954. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; O’Hara, H.; Foderingham, N.; Dupont, W.D.; Shu, X.O.; Peterson, N.; Fair, A.M.; Disher, A.C. Type 2 diabetes and mammographic breast density among underserved women. Cancer Causes Control 2015, 26, 303–309. [Google Scholar] [CrossRef]
- Ken-Dror, G.; Fluck, D.; Lean, M.E.J.; Casanueva, F.F.; Han, T.S. The relationship between low prolactin and type 2 diabetes. Rev. Endocr. Metab. Disord. 2024, 25, 1087–1095. [Google Scholar] [CrossRef]
- Retnakaran, R.; Ye, C.; Kramer, C.K.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B. Maternal Serum Prolactin and Prediction of Postpartum beta-Cell Function and Risk of Prediabetes/Diabetes. Diabetes Care 2016, 39, 1250–1258. [Google Scholar] [CrossRef]
- Zhang, Z.; Piro, A.L.; Allalou, A.; Alexeeff, S.E.; Dai, F.F.; Gunderson, E.P.; Wheeler, M.B. Prolactin and Maternal Metabolism in Women With a Recent GDM Pregnancy and Links to Future T2D: The SWIFT Study. J. Clin. Endocrinol. Metab. 2022, 107, 2652–2665. [Google Scholar] [CrossRef]
- Vasavada, R.C.; Gonzalez-Pertusa, J.A.; Fujinaka, Y.; Fiaschi-Taesch, N.; Cozar-Castellano, I.; Garcia-Ocana, A. Growth factors and beta cell replication. Int. J. Biochem. Cell Biol. 2006, 38, 931–950. [Google Scholar] [CrossRef]
- Freemark, M.; Avril, I.; Fleenor, D.; Driscoll, P.; Petro, A.; Opara, E.; Kendall, W.; Oden, J.; Bridges, S.; Binart, N.; et al. Targeted deletion of the PRL receptor: Effects on islet development, insulin production, and glucose tolerance. Endocrinology 2002, 143, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Hugo, E.R.; Brandebourg, T.D.; LaPensee, C.R. Focus on prolactin as a metabolic hormone. Trends Endocrinol. Metab. 2006, 17, 110–116. [Google Scholar] [CrossRef]
- Bensadoun, A. Lipoprotein lipase. Annu. Rev. Nutr. 1991, 11, 217–237. [Google Scholar] [CrossRef]
- McNestry, C.; Crowley, R.K.; O’Reilly, S.L.; Kasemiire, A.; Callanan, S.; Delahunt, A.; Twomey, P.J.; McAuliffe, F.M. Breastfeeding duration is associated with favorable body composition and lower glycoprotein acetyls in later life. Int. J. Gynaecol. Obstet. 2024, 166, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Roman, M.A.; Syed-Abdul, M.M.; Casey, B.M.; Alger, J.R.; Liu, Y.L.; Parks, E.J. Lactation alters the relationship between liver lipid synthesis and hepatic fat stores in the postpartum period. J. Lipid Res. 2022, 63, 100288. [Google Scholar] [CrossRef]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Otero, N.; Tessem, J.S.; Banerjee, R.R. Pancreatic islet adaptation in pregnancy and postpartum. Trends Endocrinol. Metab. 2024, 35, 834–847. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, H.; Kim, H.; Park, J.; Choi, W.; Choi, W.; Hong, H.J.; Ro, H.J.; Jun, S.; Choi, S.H.; et al. Lactation improves pancreatic beta cell mass and function through serotonin production. Sci. Transl. Med. 2020, 12, eaay0455. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Rodekamp, E.; Kohlhoff, R. Rapid neonatal weight gain increases risk of childhood overweight in offspring of diabetic mothers. J. Perinat. Med. 2012, 40, 557–563. [Google Scholar] [CrossRef]
- Fenger-Gron, J.; Fenger-Gron, M.; Blunck, C.H.; Schonemann-Rigel, H.; Wielandt, H.B. Low breastfeeding rates and body mass index in Danish children of women with gestational diabetes mellitus. Int. Breastfeed. J. 2015, 10, 26. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Greenspan, L.C.; Faith, M.S.; Hurston, S.R.; Quesenberry, C.P., Jr.; Investigators, S.O.S. Breastfeeding and growth during infancy among offspring of mothers with gestational diabetes mellitus: A prospective cohort study. Pediatr. Obes. 2018, 13, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Longmore, D.K.; Titmuss, A.; Barr, E.; Barzi, F.; Simmonds, A.; Lee, I.L.; Hawthorne, E.; Derkenne, R.; Connors, C.; Boyle, J.; et al. Breastfeeding and infant growth in offspring of mothers with hyperglycaemia in pregnancy: The pregnancy and neonatal diabetes outcomes in remote Australia study. Pediatr. Obes. 2022, 17, e12891. [Google Scholar] [CrossRef]
- Aris, I.M.; Soh, S.E.; Tint, M.T.; Saw, S.M.; Rajadurai, V.S.; Godfrey, K.M.; Gluckman, P.D.; Yap, F.; Chong, Y.S.; Lee, Y.S. Associations of infant milk feed type on early postnatal growth of offspring exposed and unexposed to gestational diabetes in utero. Eur. J. Nutr. 2017, 56, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Ogden, L.G.; Mayer-Davis, E.J.; Hamman, R.F.; Norris, J.M.; Bischoff, K.J.; McDuffie, R.; Dabelea, D. The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero: The EPOCH Study. Int. J. Obes. 2012, 36, 529–534. [Google Scholar] [CrossRef]
- Sauder, K.A.; Bekelman, T.A.; Harrall, K.K.; Glueck, D.H.; Dabelea, D. Gestational diabetes exposure and adiposity outcomes in childhood and adolescence: An analysis of effect modification by breastfeeding, diet quality, and physical activity in the EPOCH study. Pediatr. Obes. 2019, 14, e12562. [Google Scholar] [CrossRef]
- Cantoral, A.; Tellez-Rojo, M.M.; Ettinger, A.S.; Hu, H.; Hernandez-Avila, M.; Peterson, K. Early introduction and cumulative consumption of sugar-sweetened beverages during the pre-school period and risk of obesity at 8–14 years of age. Pediatr. Obes. 2016, 11, 68–74. [Google Scholar] [CrossRef]
- Vandyousefi, S.; Whaley, S.E.; Widen, E.M.; Asigbee, F.M.; Landry, M.J.; Ghaddar, R.; Davis, J.N. Association of breastfeeding and early exposure to sugar-sweetened beverages with obesity prevalence in offspring born to mothers with and without gestational diabetes mellitus. Pediatr. Obes. 2019, 14, e12569. [Google Scholar] [CrossRef]
- Vandyousefi, S.; Goran, M.I.; Gunderson, E.P.; Khazaee, E.; Landry, M.J.; Ghaddar, R.; Asigbee, F.M.; Davis, J.N. Association of breastfeeding and gestational diabetes mellitus with the prevalence of prediabetes and the metabolic syndrome in offspring of Hispanic mothers. Pediatr. Obes. 2019, 14, e12515. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Gunderson, E.P.; Gyllenhammer, L.E.; Goran, M.I. Impact of gestational diabetes mellitus on pubertal changes in adiposity and metabolic profiles in Latino offspring. J. Pediatr. 2013, 162, 741–745. [Google Scholar] [CrossRef]
- Grummer-Strawn, L.M.; Mei, Z.; Centers for Disease, C.; Prevention Pediatric Nutrition Surveillance, S. Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics 2004, 113, e81–e86. [Google Scholar] [CrossRef]
- Weng, S.F.; Redsell, S.A.; Swift, J.A.; Yang, M.; Glazebrook, C.P. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunesova, M.; Hejgaard, T.; Garcia Solano, M.; Fijalkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Rollins, N.; Dias, M.S.; Garcez, V.; Perez-Escamilla, R. Systematic review and meta-analysis of breastfeeding and later overweight or obesity expands on previous study for World Health Organization. Acta Paediatr. 2023, 112, 34–41. [Google Scholar] [CrossRef]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef]
- Cioana, M.; Deng, J.; Nadarajah, A.; Hou, M.; Qiu, Y.; Chen, S.S.J.; Rivas, A.; Banfield, L.; Toor, P.P.; Zhou, F.; et al. The Prevalence of Obesity Among Children With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2247186. [Google Scholar] [CrossRef]
- Ekelund, U.; Ong, K.; Linne, Y.; Neovius, M.; Brage, S.; Dunger, D.B.; Wareham, N.J.; Rossner, S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: The Stockholm Weight Development Study (SWEDES). Am. J. Clin. Nutr. 2006, 83, 324–330. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Belfort, M.B.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009, 123, 1177–1183. [Google Scholar] [CrossRef]
- Dewey, K.G. Is breastfeeding protective against child obesity? J. Hum. Lact. 2003, 19, 9–18. [Google Scholar] [CrossRef]
- Durmus, B.; Heppe, D.H.; Gishti, O.; Manniesing, R.; Abrahamse-Berkeveld, M.; van der Beek, E.M.; Hofman, A.; Duijts, L.; Gaillard, R.; Jaddoe, V.W. General and abdominal fat outcomes in school-age children associated with infant breastfeeding patterns. Am. J. Clin. Nutr. 2014, 99, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef]
- Arenz, S.; Ruckerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Tang, M. Protein Intake during the First Two Years of Life and Its Association with Growth and Risk of Overweight. Int. J. Environ. Res. Public Health 2018, 15, 1742. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Guo, T.; Platt, R.W.; Vanilovich, I.; Sevkovskaya, Z.; Dzikovich, I.; Michaelsen, K.F.; Dewey, K.; Promotion of Breastfeeding Intervention Trials Study, G. Feeding effects on growth during infancy. J. Pediatr. 2004, 145, 600–605. [Google Scholar] [CrossRef]
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161. [Google Scholar] [CrossRef]
- Ong, K.K.; Petry, C.J.; Emmett, P.M.; Sandhu, M.S.; Kiess, W.; Hales, C.N.; Ness, A.R.; Dunger, D.B.; the ALSPAC Study Team. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 2004, 47, 1064–1070. [Google Scholar] [CrossRef]
- Yu, H.; Dilbaz, S.; Cossmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Kashyap, S.; Heird, W.C.; Thormar, H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch. Dis. Child. 1990, 65, 861–864. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Demmelmair, H.; Grunewald, M.; Auricchio, R.; Castillejo, G.; Korponay-Szabo, I.R.; Polanco, I.; Roca, M.; Vriezinga, S.L.; et al. The impact of human breast milk components on the infant metabolism. PLoS ONE 2018, 13, e0197713. [Google Scholar] [CrossRef]
- George, A.D.; Burugupalli, S.; Paul, S.; Mansell, T.; Burgner, D.; Meikle, P.J. The Role of Human Milk Lipids and Lipid Metabolites in Protecting the Infant against Non-Communicable Disease. Int. J. Mol. Sci. 2022, 23, 7490. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Martinez, C.; Santaella-Pascual, M.; Yague-Guirao, G.; Martinez-Gracia, C. Infant gut microbiota colonization: Influence of prenatal and postnatal factors, focusing on diet. Front. Microbiol. 2023, 14, 1236254. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Oleksiak, A.; Mlodzinska, A.; Bulanda, M.; Salamon, D.; Major, P.; Stanek, M.; Gosiewski, T. Metagenomic Analysis of Duodenal Microbiota Reveals a Potential Biomarker of Dysbiosis in the Course of Obesity and Type 2 Diabetes: A Pilot Study. J. Clin. Med. 2020, 9, 369. [Google Scholar] [CrossRef]
- Ma, J.; Palmer, D.J.; Geddes, D.; Lai, C.T.; Stinson, L. Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth. Nutrients 2022, 14, 5148. [Google Scholar] [CrossRef]
- Stettler, N.; Stallings, V.A.; Troxel, A.B.; Zhao, J.; Schinnar, R.; Nelson, S.E.; Ziegler, E.E.; Strom, B.L. Weight gain in the first week of life and overweight in adulthood: A cohort study of European American subjects fed infant formula. Circulation 2005, 111, 1897–1903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filardi, T.; Bleve, E.; Gorini, S.; Caprio, M.; Morano, S. Is Breastfeeding an Effective Approach to Reduce Metabolic Risk After GDM in Mothers and Infants? J. Clin. Med. 2025, 14, 3065. https://doi.org/10.3390/jcm14093065
Filardi T, Bleve E, Gorini S, Caprio M, Morano S. Is Breastfeeding an Effective Approach to Reduce Metabolic Risk After GDM in Mothers and Infants? Journal of Clinical Medicine. 2025; 14(9):3065. https://doi.org/10.3390/jcm14093065
Chicago/Turabian StyleFilardi, Tiziana, Enrico Bleve, Stefania Gorini, Massimiliano Caprio, and Susanna Morano. 2025. "Is Breastfeeding an Effective Approach to Reduce Metabolic Risk After GDM in Mothers and Infants?" Journal of Clinical Medicine 14, no. 9: 3065. https://doi.org/10.3390/jcm14093065
APA StyleFilardi, T., Bleve, E., Gorini, S., Caprio, M., & Morano, S. (2025). Is Breastfeeding an Effective Approach to Reduce Metabolic Risk After GDM in Mothers and Infants? Journal of Clinical Medicine, 14(9), 3065. https://doi.org/10.3390/jcm14093065







