Neutrophil Percentage-to-Albumin Ratio as a Prognostic Marker in Pneumonia Patients Aged 80 and Above in Intensive Care
Abstract
:1. Introduction
2. Materials and Methods
- Symptoms of lower respiratory tract infection: Fever (>38 °C), cough, purulent sputum, or a change in the character of respiratory secretions.
- Radiographic findings consistent with pneumonia: Newly developed infiltrates on chest radiography or thoracic computed tomography.
- Laboratory findings suggestive of infection: Leukocytosis, leukopenia, or elevated acute phase reactants.
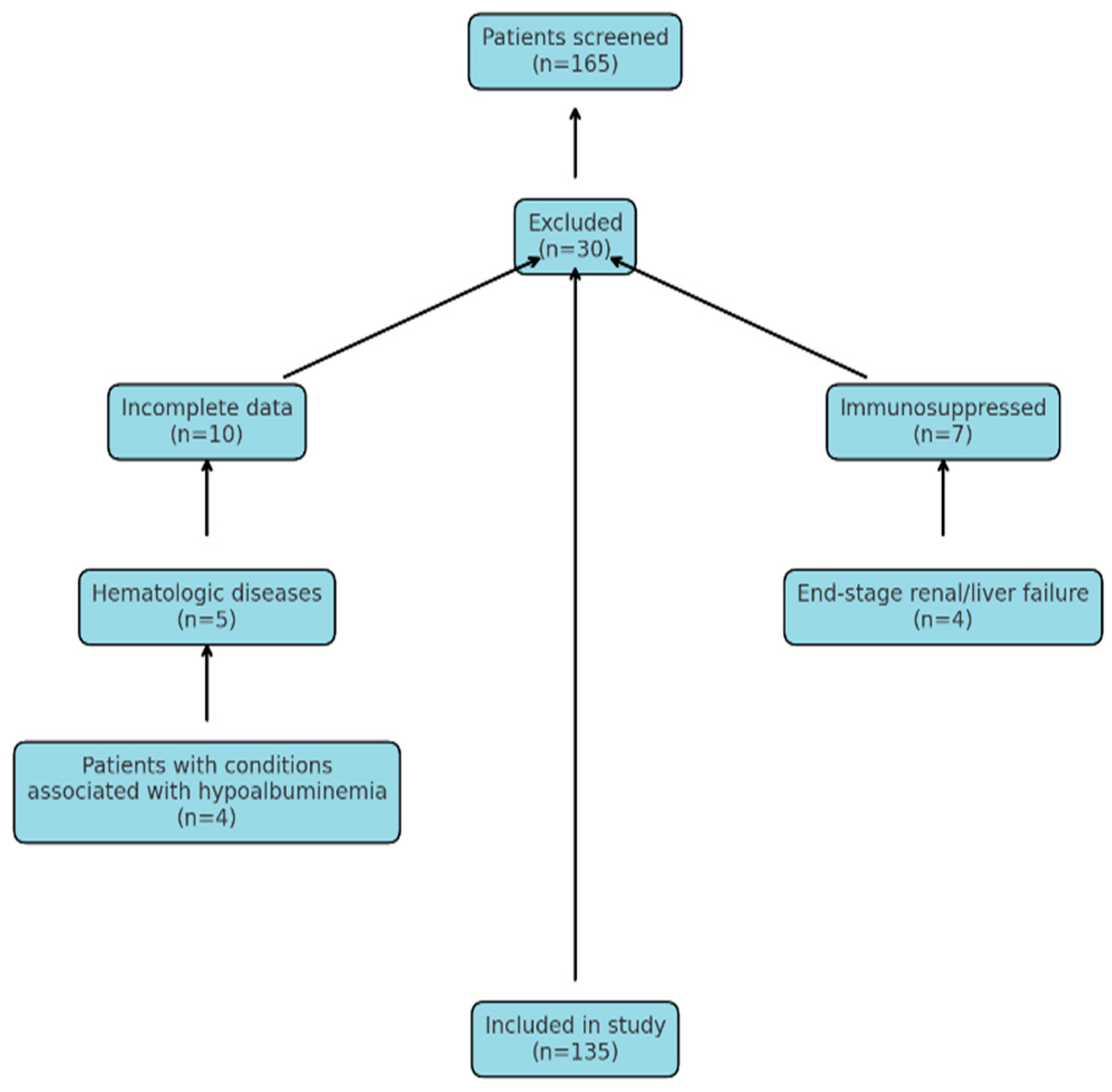
2.1. Exclusion Criteria: Patients Who Were Not Included in the Study Were Identified Based on the Following Exclusion Criteria
2.2. Data Collection and Evaluation
2.3. Assessment of Sepsis and Disease Severity
2.4. Calculation of SpO2/FiO2
2.5. Patient Selection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cillóniz, C.; Dominedò, C.; Pericàs, J.M.; Rodriguez-Hurtado, D.; Torres, A. Community-acquired pneumonia in critically ill very old patients: A growing problem. Eur. Respir. Rev. 2020, 29, 190126. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. Population Division: World Population Prospects 2019. Ten Key Findings; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Lee, S.I.; Huh, J.W.; Hong, S.B.; Koh, Y.; Lim, C.M. Age Distribution and Clinical Results of Critically Ill Patients Above 65-Year-Old in an Aging Society: A Retrospective Cohort Study. Tuberc. Respir. Dis. 2024, 87, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Laporte, L.; Hermetet, C.; Jouan, Y.; Gaborit, C.; Rouve, E.; Shea, K.M.; Si-Tahar, M.; Dequin, P.F.; Grammatico-Guillon, L.; Guillon, A. Ten-year trends in intensive care admissions for respiratory infections in the elderly. Ann. Intensive Care 2018, 8, 84. [Google Scholar] [CrossRef]
- Othman, A.; Sekheri, M.; Filep, J.G. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022, 289, 3932–3953. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef]
- Liu, C.F.; Chien, L.W. Predictive Role of Neutrophil-Percentage-to-Albumin Ratio (NPAR) in Nonalcoholic Fatty Liver Disease and Advanced Liver Fibrosis in Nondiabetic US Adults: Evidence from NHANES 2017–2018. Nutrients 2023, 15, 1892. [Google Scholar] [CrossRef]
- Lan, C.C.; Su, W.L.; Yang, M.C.; Chen, S.Y.; Wu, Y.K. Predictive role of neutrophil-percentage-to-albumin, neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratios for mortality in patients with COPD: Evidence from NHANES 2011–2018. Respirology 2023, 28, 1136–1146. [Google Scholar] [CrossRef]
- Lv, X.N.; Shen, Y.Q.; Li, Z.Q.; Deng, L.; Wang, Z.J.; Cheng, J.; Hu, X.; Pu, M.J.; Yang, W.S.; Xie, P.; et al. Neutrophil percentage to albumin ratio is associated with stroke-associated pneumonia and poor outcome in patients with spontaneous intracerebral hemorrhage. Front. Immunol. 2023, 14, 1173718. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, M.; Wang, W.; Luo, R.; Zhang, Z.; Liu, H. The Relationship Between the Neutrophil Percentage-to-Albumin Ratio and Rates of 28-Day Mortality in Atrial Fibrillation Patients 80 Years of Age or Older. J. Inflamm. Res. 2023, 16, 1629–1638. [Google Scholar] [CrossRef]
- Xu, M.; Huan, J.; Zhu, L.; Xu, J.; Song, K. The neutrophil percentage-to-albumin ratio is an independent risk factor for poor prognosis in peritoneal dialysis patients. Ren. Fail. 2024, 46, 2294149. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Z.; Yang, W.; Yu, J.; Li, J.; Guo, X.; Chen, J.; Mao, H.; Li, Z. Neutrophil Percentage-to-Albumin Ratio and Risk of Mortality in Patients on Peritoneal Dialysis. J. Inflamm. Res. 2023, 16, 6271–6281. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Cooper-smith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis 3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Montull, B.; Menéndez, R.; Torres, A.; Reyes, S.; Méndez, R.; Zalacaín, R.; Capelastegui, A.; Rajas, O.; Borderías, L.; Martin-Villasclaras, J.; et al. Predictors of Severe Sepsis among Patients Hospitalized for Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0145929. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Lin, C.J.; Chang, Y.C.; Tsou, M.T.; Chan, H.L.; Chen, Y.J.; Hwang, L.C. Factors associated with hospitalization for community-acquired pneumonia in home health care patients in Taiwan. Aging Clin. Exp. Res. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Ma, X. Neutrophil extracellular traps and coagulation dysfunction in sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 752–755. [Google Scholar]
- Wang, X.; Zhang, Y.; Wang, Y.; Liu, J.; Xu, X.; Liu, J.; Chen, M.; Shi, L. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with chronic heart failure. BMC Cardiovasc. Disord. 2023, 23, 568. [Google Scholar] [CrossRef]
- Hu, C.; He, Y.; Li, J.; Zhang, C.; Hu, Q.; Li, W.; Hao, C. Association between neutrophil percentage-to-albumin ratio and 28-day mortality in Chinese patients with sepsis. J. Int. Med. Res. 2023, 51, 3000605231178512. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.; Nasrollahi, E.; Bahramvand, Y.; Mohammadkarimi, V.; Dalfardi, B.; Anushiravani, A. The use of APACHE II Scoring System for predicting clinical outcome of patients admitted to the intensive care unit: A report from a resource-limited center. Shraz E-Med. J. 2021, 22, e102858. [Google Scholar] [CrossRef]
- Mumtaz, H.; Ejaz, M.K.; Tayyab, M.; Vohra, L.I.; Sapkota, S.; Hasan, M.; Saqib, M. APACHE scoring as an indicator of mortality rate in ICU patients: A cohort study. Ann. Med. Surg. 2023, 85, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Shafigh, N.; Hasheminik, M.; Shafigh, E.; Alipour, H.; Sayyadi, S.; Kazeminia, N.; Khoundabi, B.; Salarian, S. Prediction of mortality in ICU patients: A comparison between the SOFA score and other indicators. Nurs. Crit. Care 2023, 29, 1619–1622. [Google Scholar] [CrossRef]

| Survivors (N = 82, 60.8%) | Non-Survivors (N = 53, 39.2%) | p-Value | |
|---|---|---|---|
| Age, years (Mean ± SD) | 86.37 ± 4.90 | 87.64 ± 4.97 | 0.463 |
| Female Sex | 38 (57.6%) | 28 (42.4%) | 0.163 |
| Male Sex | 44 (63.8%) | 25 (36.2%) | |
| SOFA Score | 5.59 ± 2.57 | 8.79 ± 2.56 | <0.001 |
| APACHE-II Score | 19.35 ± 5.71 | 28.21 ± 3.73 | <0.001 |
| Need for Hemodialysis | 9 (10.9%) | 24 (89.1%) | <0.001 |
| Need for Vasopressor Support | 11 (12.1%) | 32 (87.9%) | <0.001 |
| Need for Invasive Mechanical Ventilation | 12 (14.6%) | 48 (85.4%) | <0.001 |
| Presence of Comorbidities | 63 (76.8%) | 48 (90.6%) | 0.042 |
| COPD * | 22 (26.8%) | 24 (45.3%) | 0.028 |
| Malignancy | 3 (3.7%) | 7 (13.2%) | 0.039 |
| Neutrophil Percentage (%) | 85 (78–90) | 89 (85–92) | 0.004 |
| Albumin (g/L) | 32 (27–35) | 29 (24–34) | 0.006 |
| NPAR ** | 2.91 (2.44–21.60) | 4.22 (2.98–30.92) | <0.001 |
| Procalcitonin (ng/mL) | 0.19 (0.06–0.67) | 0.46 (0.21–2.63) | 0.003 |
| Lactate (mmol/L) | 2.30 (1.40–3.00) | 2.90 (2.40–3.40) | <0.001 |
| Blood Urea Nitrogen (mg/dL) | 51 (34–75) | 82 (54–108) | <0.001 |
| Creatinine (mg/dL) | 1.13 (0.89–1.76) | 1.52 (1.18–2.10) | 0.010 |
| Potassium (mmol/L) | 4.00 (3.70–5.00) | 4.49 (4.00–4.88) | 0.102 |
| CRP (mg/L) | 32 (8–148) | 62 (10–136) | 0.456 |
| Platelets (×103/µL) | 223 (181–293) | 169 (144–202) | <0.001 |
| Variable | All Patients 135 (100%) N (%) Mean ± SD | NPAR ≤ 0.286 48 (35.6%) N (%) Mean ± SD * | NPAR > 0.286 87 (64.4%) N (%) Mean ± SD | p-Value |
|---|---|---|---|---|
| Age (years) | 86.87 ± 4.95 | 86.65 ± 5.23 | 86.99 ± 4.82 | 0.638 |
| Male sex | 66 (48.9%) | 22 (33.3%) | 44 (66.7%) | 0.599 |
| SOFA score | 6.84 ± 3.00 | 5.85 ± 2.94 | 7.39 ± 2.90 | 0.002 |
| APACHE II score | 22.83 ± 6.63 | 20.90 ± 7.53 | 23.90 ± 5.85 | 0.007 |
| Renal replacement therapy | 33 (24.4%) | 10 (20.8%) | 23 (26.4%) | 0.470 |
| Vasopressor requirement | 43 (31.9%) | 10 (20.8%) | 33 (37.9%) | 0.042 |
| Invasive mechanical ventilation | 60 (44.4%) | 13 (27.1%) | 47 (54.0%) | 0.003 |
| Requirement for high-flow nasal oxygen | 82 (60.7%) | 26 (54.1%) | 56 (64.3%) | 0.102 |
| Requirement for noninvasive mechanical ventilation | 68 (50.3%) | 17 (35.4%) | 51 (58.6%) | 0.090 |
| Presence of comorbidities | 111 (82.2%) | 47 (97.9%) | 64 (73.6%) | <0.001 |
| Cardiovascular disease | 50 (37.0%) | 25 (52.1%) | 25 (28.7%) | 0.007 |
| Hypertension | 67 (49.6%) | 34 (70.8%) | 33 (37.9%) | <0.001 |
| Severity of the disease | ||||
| No sepsis | 41 (30.4%) | 20 (41.7%) | 21 (24.1%) | 0.035 |
| Sepsis | 94 (69.6%) | 28 (58.3%) | 66 (75.9%) | |
| SpO2/FiO2 | ||||
| SpO2/FiO2 > 315 | 21 (15.6%) | 6 (12.5%) | 15 (17.2%) | 0.087 |
| 235 < SpO2/FiO2 ≤ 315 | 43 (31.9%) | 21 (43.8%) | 22 (25.3%) | |
| 148 < SpO2/FiO2 ≤ 235 | 42 (31.8%) | 17 (35.4%) | 25 (28.7%) | |
| SpO2/FiO2 ≤ 148 | 29 (21.5%) | 4 (8.3%) | 25 (28.7%) | |
| Mortality | 53 (39.3%) | 9 (18.8%) | 44 (50.6%) | <0.001 |
| Laboratory Parameters | All Patients 135 (100%) Median (IQR) | NPAR ≤ 0.286 48 (35.6%) Median (IQR) | NPAR > 0.286 87 (64.4%) Median (IQR) | p-Value |
|---|---|---|---|---|
| Neutrophil percentage (%) | 86.8 (79.6–91.8) | 84.90 (75.52–90.35) | 88.20 (81.40–92.20) | 0.008 |
| Albumin (g/L) | 30 (36–35) | 34 (32–37) | 28 (25–31) | <0.001 |
| NPAR * | 0.32 (0.25–2.58) | 0.24 (0.22–0.26) | 2.27 (0.33–2.89) | <0.001 |
| Procalcitonin (ng/mL) | 0.30 (0.12–0.92) | 0.18 (0.05–0.59) | 0.36 (0.15–1.59) | 0.020 |
| Lactate | 2.60 (1.62–3.10) | 2.35 (1.45–2.75) | 2.90 (1.90–3.30) | 0.003 |
| Blood urea nitrogen (mg/dL) | 60 (36–90) | 58 (33–88) | 65 (48–97) | 0.167 |
| Creatinine (mg/dL) | 1.29 (1.0–1.95) | 1.20 (0.90–1.90) | 1.34 (1.06–1.97) | 0.206 |
| Potassium (mmol/L) | 4.30 (3.89–4.90) | 4.02 (3.70–4.80) | 4.60 (4.10–5.08) | <0.001 |
| C-reactive protein (CRP) (mg/L) | 51 (9.4–141) | 23 (4.9–141) | 76 (39–142) | 0.011 |
| Neutrophil (×103/µL) | 10.20 (7.57–14.30) | 9.58 (7.74–13.04) | 10.80 (7.50–15.50) | 0.176 |
| Platelet count (×103/µL) | 197 (163–247) | 200 (160–251) | 193 (164–242) | 0.811 |
| AUC | 95% Confidence Interval | Cut-Off Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ * | LR− ** | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| NPAR *** | 0.692 | 0.599–0.784 | 0.286 | 83 | 47.6 | 50.6 | 81.2 | 1.58 | 0.36 | <0.001 |
| Variable | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.045 (0.989–1.104) | 0.114 | ||
| Presence of comorbidities | 2.290 (0.911–5.760) | 0.078 | ||
| Cardiovascular disease | 0.830 (0.469–1.471) | 0.524 | ||
| Hypertension | 0.682 (0.394–1.180) | 0.172 | ||
| APACHE II score | 1.163 (1.118–1.209) | <0.001 | 1.077 (1.013–1.147) | 0.019 |
| SOFA score | 1.291 (1.193–1.398) | <0.001 | 1.100 (0.964–1.254) | 0.156 |
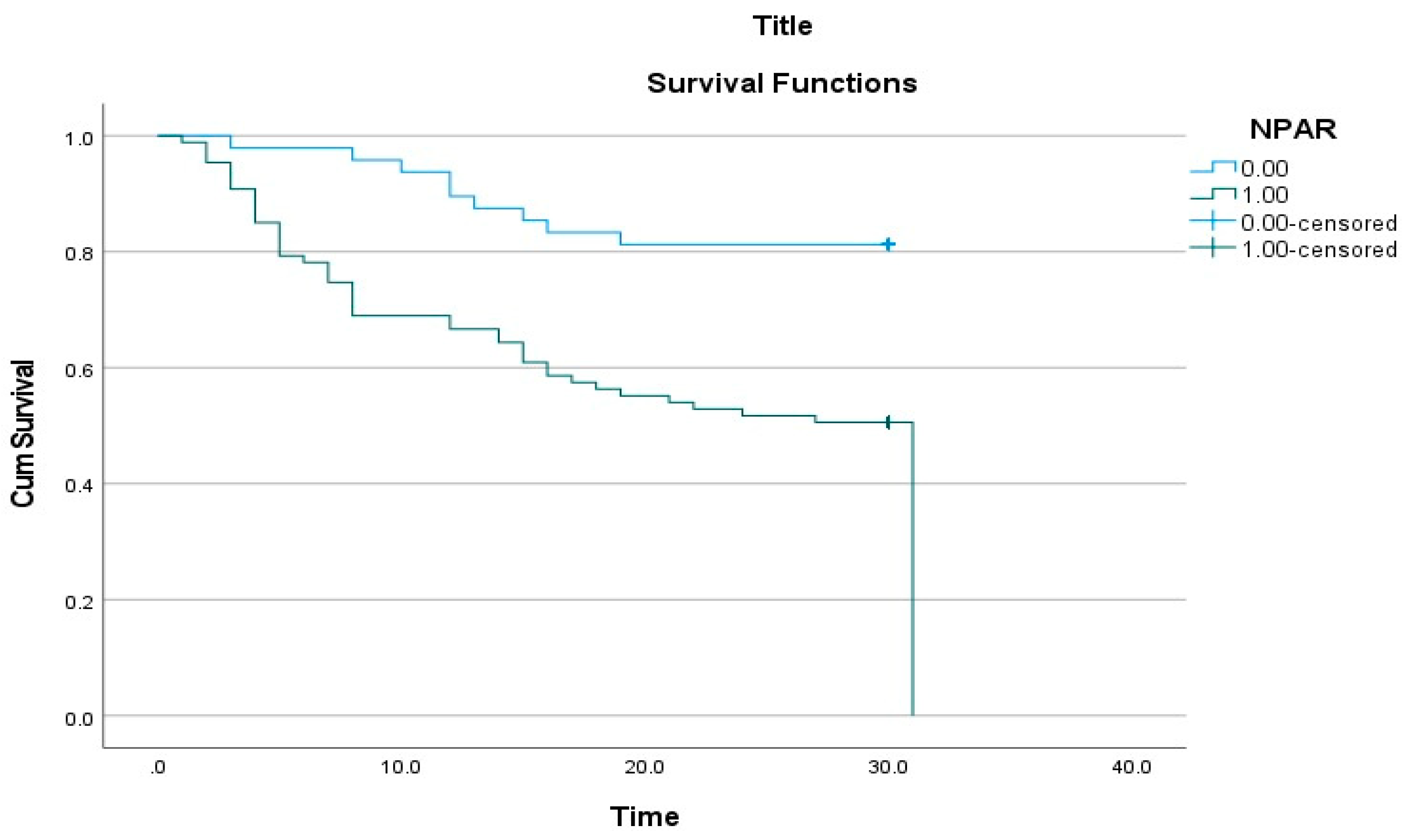
| NPAR * > 0.286 | 3.318 (1.616–6.812) | 0.001 | 2.488 (1.167–5.302) | 0.018 |
| Need for renal replacement therapy | 3.788 (2.185–6.567) | <0.001 | 1.969 (1.046–3.705) | 0.036 |
| Need for vasopressor therapy | 4.166 (2.385–7.279) | <0.001 | 0.616 (0.311–1.220) | 0.165 |
| Need for invasive mechanical ventilation | 20.297 (8.019–51.374) | <0.001 | 9.446 (3.402–26.229) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ari, M.; Haykir Solay, A.; Ozdemir, T.; Yildiz, M.; Mentes, O.; Tuten, O.F.; Tetik Manav, H.; Celik, D.; Doganci, M.; Eraslan Doganay, G.; et al. Neutrophil Percentage-to-Albumin Ratio as a Prognostic Marker in Pneumonia Patients Aged 80 and Above in Intensive Care. J. Clin. Med. 2025, 14, 3033. https://doi.org/10.3390/jcm14093033
Ari M, Haykir Solay A, Ozdemir T, Yildiz M, Mentes O, Tuten OF, Tetik Manav H, Celik D, Doganci M, Eraslan Doganay G, et al. Neutrophil Percentage-to-Albumin Ratio as a Prognostic Marker in Pneumonia Patients Aged 80 and Above in Intensive Care. Journal of Clinical Medicine. 2025; 14(9):3033. https://doi.org/10.3390/jcm14093033
Chicago/Turabian StyleAri, Maside, Aslı Haykir Solay, Tarkan Ozdemir, Murat Yildiz, Oral Mentes, Omer Faruk Tuten, Husra Tetik Manav, Deniz Celik, Melek Doganci, Guler Eraslan Doganay, and et al. 2025. "Neutrophil Percentage-to-Albumin Ratio as a Prognostic Marker in Pneumonia Patients Aged 80 and Above in Intensive Care" Journal of Clinical Medicine 14, no. 9: 3033. https://doi.org/10.3390/jcm14093033
APA StyleAri, M., Haykir Solay, A., Ozdemir, T., Yildiz, M., Mentes, O., Tuten, O. F., Tetik Manav, H., Celik, D., Doganci, M., Eraslan Doganay, G., Ari, E., & Usul, E. (2025). Neutrophil Percentage-to-Albumin Ratio as a Prognostic Marker in Pneumonia Patients Aged 80 and Above in Intensive Care. Journal of Clinical Medicine, 14(9), 3033. https://doi.org/10.3390/jcm14093033








