Updates in Intestinal Failure Management
Abstract
1. Introduction
2. Diagnosis and Clinical Manifestations
3. Nutritional Management
3.1. Enteral Nutrition
3.2. Parenteral Nutrition
3.3. Comparing Enteral and Parenteral Nutrition
4. Medical Management
5. Surgical Management
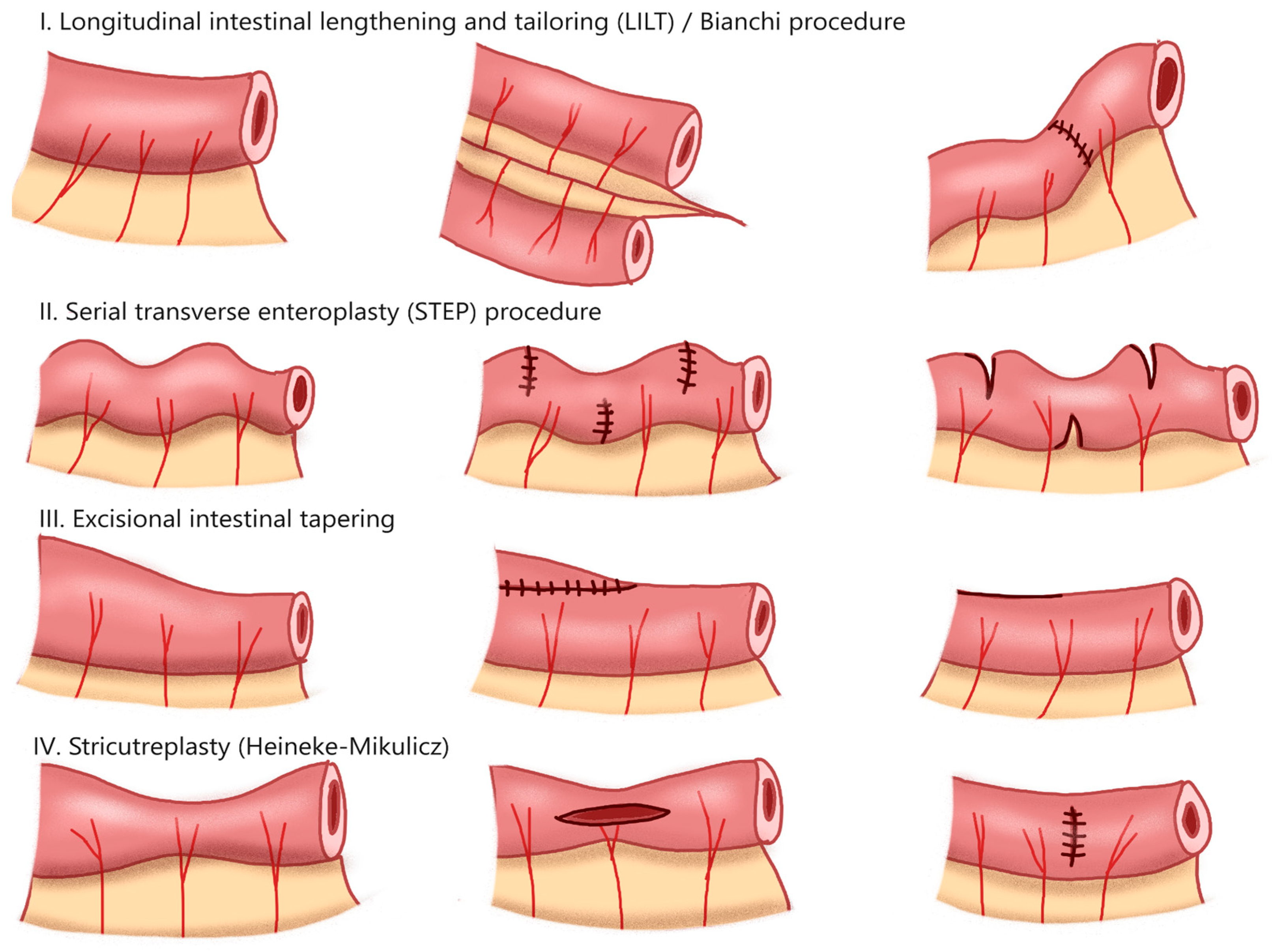
5.1. Autologous Intestinal Reconstruction
5.2. Intestinal Transplantation
5.3. Experimental Therapies
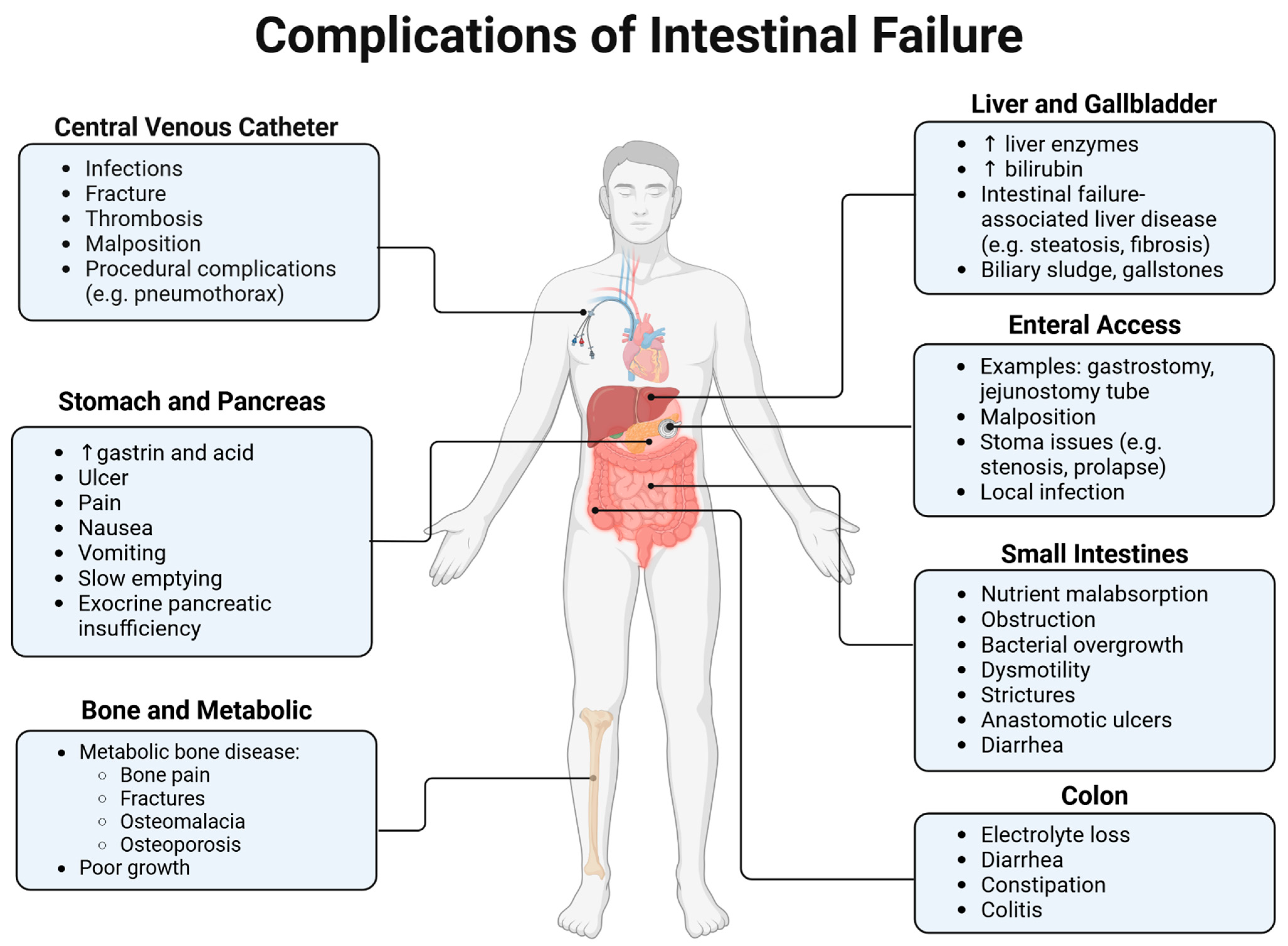
6. Complications
6.1. IFALD
6.2. Metabolic Bone Disease
6.3. Kidney Disease
6.4. Intestinal Inflammation
7. Clinical Case Scenarios
7.1. Case A: Intestinal Atresia
7.2. Case B: Necrotizing Enterocolitis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASPEN | American Society of Parenteral and Enteral Nutrition |
| CLABSI | Central line-associated bloodstream infections |
| CRP | C-reactive protein |
| CVC | Central venous catheter |
| DXA | Dual-energy X-ray absorptiometry |
| EDTA | Ethylenediaminetetraacetic acid |
| FTT | Failure to thrive |
| GFR | Glomerular filtration rate |
| GLP-2 | Glucagon-like peptide 2 |
| IBD | Inflammatory bowel disease |
| IDSA | Infectious Diseases Society of America |
| IF | Intestinal failure |
| IFALD | Intestinal failure-associated liver disease |
| INSPIRE | Ingestible self-propelling device for intestinal reanimation |
| LILT | Longitudinal intestinal lengthening and tapering |
| MBD | Metabolic bone disease |
| NEC | Necrotizing enterocolitis |
| OPTN | Organ Procurement and Transplantation Network |
| PICC | Peripherally-inserted central catheter |
| PN | Parenteral nutrition |
| PNALD | Parenteral nutrition-associated liver disease |
| SBBO | Small bowel bacterial overgrowth |
| SBS | Short bowel syndrome |
| SRTR | Scientific Registry of Transplant Recipients |
| STEP | Serial transverse enteroplasty |
References
- Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.E. Intestinal failure: A new era in clinical management. J. Gastroenterol. Hepatol. 2009, 24 (Suppl. S3), S86–S92. [Google Scholar] [CrossRef] [PubMed]
- Dudrick, S.J. History of parenteral nutrition. J. Am. Coll. Nutr. 2009, 28, 243–251. [Google Scholar] [CrossRef]
- Jaksic, T. Current short bowel syndrome management: An era of improved outcomes and continued challenges. J. Pediatr. Surg. 2023, 58, 789–798. [Google Scholar] [CrossRef]
- Salazar, J.A.; Carey, A.N.; Duggan, C.P. Nutritional and medical approaches to intestinal failure. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 201–209. [Google Scholar] [CrossRef]
- Pironi, L. Definition, classification, and causes of short bowel syndrome. Nutr. Clin. Pract. 2023, 38 (Suppl. S1), S9–S16. [Google Scholar] [CrossRef]
- Bering, J.; DiBaise, J.K. Short Bowel Syndrome in Adults. Am. J. Gastroenterol. 2022, 117, 876–883. [Google Scholar] [CrossRef]
- Modi, B.P.; Galloway, D.P.; Gura, K.; Nucci, A.; Plogsted, S.; Tucker, A.; Wales, P.W. ASPEN definitions in pediatric intestinal failure. JPEN J. Parenter. Enteral Nutr. 2022, 46, 42–59. [Google Scholar] [CrossRef]
- Tappenden, K.A. Intestinal adaptation following resection. JPEN J. Parenter. Enteral Nutr. 2014, 38, 23S–31S. [Google Scholar] [CrossRef]
- Gatti, S.; Quattrini, S.; Palpacelli, A.; Catassi, G.N.; Lionetti, M.E.; Catassi, C. Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review. Nutrients 2022, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Fisher, J.G.; Bairdain, S.; Sparks, E.A.; Zurakowski, D.; Modi, B.P.; Duggan, C.; Jaksic, T. Metabolic bone disease in pediatric intestinal failure patients: Prevalence and risk factors. J. Pediatr. Surg. 2015, 50, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Engelstad, H.J.; Barron, L.; Moen, J.; Wylie, T.N.; Wylie, K.; Rubin, D.C.; Davidson, N.; Cade, W.T.; Warner, B.B.; Warner, B.W. Remnant Small Bowel Length in Pediatric Short Bowel Syndrome and the Correlation with Intestinal Dysbiosis and Linear Growth. J. Am. Coll. Surg. 2018, 227, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.V.; Nes, E.K.; Fénnich, H.; Staffa, S.J.; Etskovitz, H.B.; Duggan, C.P.; Jaksic, T.; Carey, A.N.; Modi, B.P. Risk Factors and Treatment Strategies for Anastomotic Ulcers in Pediatric Intestinal Failure. J. Pediatr. Surg. 2024, 162118. [Google Scholar] [CrossRef]
- Sundaram, A.; Koutkia, P.; Apovian, C.M. Nutritional management of short bowel syndrome in adults. J. Clin. Gastroenterol. 2002, 34, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Olieman, J.F.; Penning, C.; Ijsselstijn, H.; Escher, J.C.; Joosten, K.F.; Hulst, J.M.; Tibboel, D. Enteral nutrition in children with short-bowel syndrome: Current evidence and recommendations for the clinician. J. Am. Diet. Assoc. 2010, 110, 420–426. [Google Scholar] [CrossRef]
- Andorsky, D.J.; Lund, D.P.; Lillehei, C.W.; Jaksic, T.; Dicanzio, J.; Richardson, D.S.; Collier, S.B.; Lo, C.; Duggan, C. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J. Pediatr. 2001, 139, 27–33. [Google Scholar] [CrossRef]
- Duggan, C.; Stark, A.R.; Auestad, N.; Collier, S.; Fulhan, J.; Gura, K.; Utter, S.; Teixeira-Pinto, A.; Donovan, K.; Lund, D. Glutamine supplementation in infants with gastrointestinal disease: A randomized, placebo-controlled pilot trial. Nutrition 2004, 20, 752–756. [Google Scholar] [CrossRef]
- Bines, J.; Francis, D.; Hill, D. Reducing parenteral requirement in children with short bowel syndrome: Impact of an amino acid-based complete infant formula. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 123–128. [Google Scholar] [CrossRef]
- Eisenberg, M.; Monuteaux, M.C.; Fell, G.; Goldberg, V.; Puder, M.; Hudgins, J. Central Line-Associated Bloodstream Infection among Children with Intestinal Failure Presenting to the Emergency Department with Fever. J. Pediatr. 2018, 196, 237–243.e231. [Google Scholar] [CrossRef]
- Chang, M.I.; Carlson, S.J.; Nandivada, P.; O’Loughlin, A.A.; Potemkin, A.K.; Cowan, E.; Mitchell, P.D.; Gura, K.M.; Puder, M. Challenging the 48-Hour Rule-Out for Central Line-Associated Bloodstream Infections in the Pediatric Intestinal Failure Population: A Retrospective Pilot Study. JPEN J. Parenter. Enteral Nutr. 2016, 40, 567–573. [Google Scholar] [CrossRef]
- Fell, G.L.; Cho, B.S.; Anez-Bustillos, L.; Dao, D.T.; Baker, M.A.; Nandivada, P.; O’Loughlin, A.A.; Hurley, A.P.; Mitchell, P.D.; Rangel, S.; et al. Optimizing Duration of Empiric Management of Suspected Central Line-Associated Bloodstream Infections in Pediatric Patients with Intestinal Failure. J. Pediatr. 2020, 227, 69–76.e63. [Google Scholar] [CrossRef] [PubMed]
- Fligor, S.C.; Hirsch, T.I.; Tsikis, S.T.; Joiner, M.M.; Mitchell, P.D.; Carbeau, S.; McClelland, J.; Carey, A.; Gura, K.M.; Puder, M. Ethanol lock therapy increases mechanical catheter complications in a pediatric intestinal failure population: A retrospective cohort study. JPEN J. Parenter. Enteral Nutr. 2023, 47, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, T.I.; Fligor, S.C.; Tsikis, S.T.; Mitchell, P.D.; DeVietro, A.; Carbeau, S.; Wang, S.Z.; McClelland, J.; Carey, A.N.; Gura, K.M.; et al. Administration of 4% tetrasodium EDTA lock solution and central venous catheter complications in high-risk pediatric patients with intestinal failure: A retrospective cohort study. JPEN J. Parenter. Enteral Nutr. 2024, 48, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Lobo, R.; Riveiro, J.; Vega-Piñero, B.; Botella-Carretero, J.I. Infectious Complications in Home Parenteral Nutrition: A Systematic Review and Meta-Analysis Comparing Peripherally-Inserted Central Catheters with Other Central Catheters. Nutrients 2019, 11, 2083. [Google Scholar] [CrossRef]
- Buetti, N.; Marschall, J.; Drees, M.; Fakih, M.G.; Hadaway, L.; Maragakis, L.L.; Monsees, E.; Novosad, S.; O’Grady, N.P.; Rupp, M.E.; et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 553–569. [Google Scholar] [CrossRef]
- Chang, M.I.; Puder, M.; Gura, K.M. The use of fish oil lipid emulsion in the treatment of intestinal failure associated liver disease (IFALD). Nutrients 2012, 4, 1828–1850. [Google Scholar] [CrossRef]
- Puder, M.; Valim, C.; Meisel, J.A.; Le, H.D.; de Meijer, V.E.; Robinson, E.M.; Zhou, J.; Duggan, C.; Gura, K.M. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 2009, 250, 395–402. [Google Scholar] [CrossRef]
- Ho, B.E.; Chan, S.C.; Faino, A.V.; Mortensen, M.; Williamson, N.; Javid, P.J.; Horslen, S.P.; Wendel, D. Evaluation of SMOFlipid in Pediatric Intestinal-Failure Patients and Its Effects on Essential Fatty Acid Levels. JPEN J. Parenter. Enteral Nutr. 2021, 45, 546–552. [Google Scholar] [CrossRef]
- Le, H.D.; Fallon, E.M.; de Meijer, V.E.; Malkan, A.D.; Puder, M.; Gura, K.M. Innovative parenteral and enteral nutrition therapy for intestinal failure. Semin. Pediatr. Surg. 2010, 19, 27–34. [Google Scholar] [CrossRef]
- Seres, D.S.; Valcarcel, M.; Guillaume, A. Advantages of enteral nutrition over parenteral nutrition. Therap. Adv. Gastroenterol. 2013, 6, 157–167. [Google Scholar] [CrossRef]
- Lewis, S.R.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Database Syst. Rev. 2018, 6, CD012276. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Puoti, M.G.; Köglmeier, J. Nutritional Management of Intestinal Failure due to Short Bowel Syndrome in Children. Nutrients 2022, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Hansen, N.I.; Higgins, R.D.; Ziegler, T.R.; Stoll, B.J.; Network, E.K.S.N.N.R. Very low birth weight preterm infants with surgical short bowel syndrome: Incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008, 122, e573–e582. [Google Scholar] [CrossRef]
- Raghu, V.K.; Leraas, H.J.; Samoylova, M.; Park, C.; Rothenberger, S.D.; Sudan, D.; Avitzur, Y. Predictors of 1-year enteral autonomy in children with intestinal failure: A descriptive retrospective cohort study. JPEN J. Parenter. Enteral Nutr. 2023, 47, 1047–1055. [Google Scholar] [CrossRef]
- Lauro, A.; Lacaille, F. Short bowel syndrome in children and adults: From rehabilitation to transplantation. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 55–70. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Shahraz, S.; Hopkins, T.; Worsfold, A.; Genestin, E. Impact of intestinal failure and parenteral support on adult patients with short-bowel syndrome: A multinational, noninterventional, cross-sectional survey. JPEN J. Parenter. Enteral Nutr. 2022, 46, 1650–1659. [Google Scholar] [CrossRef]
- Nordsten, C.B.; Molsted, S.; Bangsgaard, L.; Fuglsang, K.A.; Brandt, C.F.; Niemann, M.J.; Jeppesen, P.B. High Parenteral Support Volume Is Associated With Reduced Quality of Life Determined by the Short-Bowel Syndrome Quality of Life Scale in Nonmalignant Intestinal Failure Patients. JPEN J. Parenter. Enteral Nutr. 2021, 45, 926–932. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, M.; Chen, H.; Sheng, Y.; Wang, Z.; Wu, B. A Systematic Review of Quality of Life in Patients with Short Bowel Syndrome and Their Caregivers. Patient Prefer. Adherence 2024, 18, 1217–1230. [Google Scholar] [CrossRef]
- Tappenden, K.A. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology 2006, 130, S93–S99. [Google Scholar] [CrossRef]
- Tappenden, K.A. Anatomical and physiological considerations in short bowel syndrome: Emphasis on intestinal adaptation and the role of enterohormones. Nutr. Clin. Pract. 2023, 38 (Suppl. S1), S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.; Cermak, S.A.; Merritt, R.J. Oral Feeding Difficulties in Children With Short Bowel Syndrome: A Narrative Review. Nutr. Clin. Pract. 2018, 33, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fourati, S.; de Dreuille, B.; Bettolo, J.; Hutinet, C.; Le Gall, M.; Bado, A.; Joly, F.; Le Beyec, J. Hyperphagia is prominent in adult patients with short bowel syndrome: A role for the colon? Clin. Nutr. 2023, 42, 2109–2115. [Google Scholar] [CrossRef]
- Hopkins, J.; Merritt, R. Strategies to Promote Success in Oral Feedings in Infants and Children with Intestinal Failure due to Short Bowel Syndrome. Gastroenterol. Clin. North. Am. 2024, 53, 329–341. [Google Scholar] [CrossRef]
- Sabra, H.K.; Remeih, G.S.; Kereet, I.M.; Hamad, M.; Ahmed, Y.A.; Jahangir, K.; Bakr, M.A.; Alagelli, F.A.; Sherif, H.; Elsaid, M. Efficacy and safety of glucagon-like peptide 2 in patients with short bowel syndrome: A systematic review and network meta-analysis. J. Gastrointest. Surg. 2024, 28, 1194–1205. [Google Scholar] [CrossRef]
- Drucker, D.J. The Discovery of GLP-2 and Development of Teduglutide for Short Bowel Syndrome. ACS Pharmacol. Transl. Sci. 2019, 2, 134–142. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’keefe, S.J.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012, 143, 1473–1481.e1473. [Google Scholar] [CrossRef]
- Kocoshis, S.A.; Merritt, R.J.; Hill, S.; Protheroe, S.; Carter, B.A.; Horslen, S.; Hu, S.; Kaufman, S.S.; Mercer, D.F.; Pakarinen, M.P.; et al. Safety and Efficacy of Teduglutide in Pediatric Patients With Intestinal Failure due to Short Bowel Syndrome: A 24-Week, Phase III Study. JPEN J. Parenter. Enteral Nutr. 2020, 44, 621–631. [Google Scholar] [CrossRef]
- Chiba, M.; Masumoto, K.; Kaji, T.; Matsuura, T.; Morii, M.; Fagbemi, A.; Hill, S.; Pakarinen, M.P.; Protheroe, S.; Urs, A.; et al. Efficacy and Safety of Teduglutide in Infants and Children With Short Bowel Syndrome Dependent on Parenteral Support. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 339–346. [Google Scholar] [CrossRef]
- Eliasson, J.; Hvistendahl, M.K.; Freund, N.; Bolognani, F.; Meyer, C.; Jeppesen, P.B. Apraglutide, a novel glucagon-like peptide-2 analog, improves fluid absorption in patients with short bowel syndrome intestinal failure: Findings from a placebo-controlled, randomized phase 2 trial. JPEN J. Parenter. Enteral Nutr. 2022, 46, 896–904. [Google Scholar] [CrossRef]
- Reiner, J.; Thiery, J.; Held, J.; Berlin, P.; Skarbaliene, J.; Vollmar, B.; Jaster, R.; Eriksson, P.O.; Lamprecht, G.; Witte, M. The dual GLP-1 and GLP-2 receptor agonist dapiglutide promotes barrier function in murine short bowel. Ann. N. Y. Acad. Sci. 2022, 1514, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Solar, H.; Ortega, M.L.; Gondolesi, G. Current Status of Chronic Intestinal Failure Management in Adults. Nutrients 2024, 16, 2648. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.; Gura, K.M. Pharmacologic challenges in pediatric intestinal failure: A review. Intest. Fail. 2024, 4, 100039. [Google Scholar] [CrossRef]
- Iyer, K.R. Surgical management of short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 2014, 38, 53S–59S. [Google Scholar] [CrossRef]
- Bianchi, A. Intestinal loop lengthening—A technique for increasing small intestinal length. J. Pediatr. Surg. 1980, 15, 145–151. [Google Scholar] [CrossRef]
- Kim, H.B.; Fauza, D.; Garza, J.; Oh, J.T.; Nurko, S.; Jaksic, T. Serial transverse enteroplasty (STEP): A novel bowel lengthening procedure. J. Pediatr. Surg. 2003, 38, 425–429. [Google Scholar] [CrossRef]
- Jones, B.A.; Hull, M.A.; Potanos, K.M.; Zurakowski, D.; Fitzgibbons, S.C.; Ching, Y.A.; Duggan, C.; Jaksic, T.; Kim, H.B.; Registry, I.S.D. Report of 111 consecutive patients enrolled in the International Serial Transverse Enteroplasty (STEP) Data Registry: A retrospective observational study. J. Am. Coll. Surg. 2013, 216, 438–446. [Google Scholar] [CrossRef]
- Frongia, G.; Kessler, M.; Weih, S.; Nickkholgh, A.; Mehrabi, A.; Holland-Cunz, S. Comparison of LILT and STEP procedures in children with short bowel syndrome—A systematic review of the literature. J. Pediatr. Surg. 2013, 48, 1794–1805. [Google Scholar] [CrossRef]
- Fujioka, W.K.; Cowles, R.A. Infectious complications following serial transverse enteroplasty in infants and children with short bowel syndrome. J. Pediatr. Surg. 2015, 50, 428–430. [Google Scholar] [CrossRef]
- Lacaille, F.; Boluda, E.R.; Gupte, G.; Hind, J.; Sturm, E.; Hilberath, J.; Herlenius, G.; D’Antiga, L.; Pietrobattista, A.; Hernandez, F.; et al. Indications and successes of intestinal transplantation in children in the 21st century: A retrospective cohort study. Clin. Nutr. ESPEN 2024, 62, 247–252. [Google Scholar] [CrossRef]
- Bryan, N.S.; Russell, S.C.; Ozler, O.; Sugiguchi, F.; Yazigi, N.A.; Khan, K.M.; Ekong, U.D.; Vitola, B.E.; Guerra, J.F.; Kroemer, A.; et al. Evaluation of pediatric patients for intestinal transplantation in the modern era. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Zorzetti, N.; Marino, I.R.; Sorrenti, S.; Navarra, G.G.; D’Andrea, V.; Lauro, A. Small bowel transplant—Novel indications and recent progress. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 677–690. [Google Scholar] [CrossRef]
- Kaufman, S.S.; Atkinson, J.B.; Bianchi, A.; Goulet, O.J.; Grant, D.; Langnas, A.N.; McDiarmid, S.V.; Mittal, N.; Reyes, J.; Tzakis, A.G.; et al. Indications for pediatric intestinal transplantation: A position paper of the American Society of Transplantation. Pediatr. Transplant. 2001, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Horslen, S.P.; Raghu, V.K.; Ahn, Y.S.; Howell, J.; Schumacher, B.; McDermott, M.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2023 Annual Data Report: Intestine. Am. J. Transplant. 2025, 25, S288–S328. [Google Scholar] [CrossRef] [PubMed]
- O’Quin, C.; Clayton, S.D.; Trosclair, L.; Meyer, H.; Dao, N.H.; Minagar, A.; White, L.; Welch, V.; Solitro, G.; Alexander, J.S.; et al. Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome. Pathophysiology 2024, 31, 388–397. [Google Scholar] [CrossRef]
- Rafeeqi, T.A.; Thomas, A.L.; Salimi-Jazi, F.; Diyaolu, M.; Lopez, N.; Dunn, J.C.Y. Mechanical distraction enterogenesis utilizing springs has equal effectiveness in adult and juvenile pigs. Pediatr. Surg. Int. 2024, 41, 18. [Google Scholar] [CrossRef]
- Yu, F.; Cui, X.; Lang, Y.; Huang, F.; Wang, L.; Miao, X.; Ai, F.; Xie, C.; Xin, H.; Yang, C.; et al. Real-time manipulation of intestinal peristalsis by enteric-encapsulated magnetic nanoparticles & wearable 3D-printed devices. NPG Asia Mater. 2019, 11, 33. [Google Scholar]
- Smith, M.E.; May, A.; Schwehr, T.; Erin, O.; Tragesser, C.; Scheese, D.; Mair, L.O.; Diaz-Mercado, Y.; Hackam, D.K.; Krieger, A. MagnetoStalsis: Generating Peristalsis in an Artificial Bowel for Treatment of Short Bowel Syndrome. J. Med. Robot. Res. 2024, 9, 2440006. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Dosso, J.; Huang, H.W.; Selsing, G.; Alshareef, A.; Kuosmanen, J.; Ishida, K.; Jenkins, J.; Madani, W.A.M.; Hayward, A.; et al. An ingestible self-propelling device for intestinal reanimation. Sci. Robot. 2024, 9, eadh8170. [Google Scholar] [CrossRef]
- Fligor, S.C.; Tsikis, S.T.; Hirsch, T.I.; Jain, A.; Sun, L.; Rockowitz, S.; Gura, K.M.; Puder, M. Inflammation drives pathogenesis of early intestinal failure-associated liver disease. Sci. Rep. 2024, 14, 4240. [Google Scholar] [CrossRef]
- Fligor, S.C.; Hirsch, T.I.; Tsikis, S.T.; Pan, A.; Quigley, M.; Gura, K.M.; Puder, M. Intestinal failure-associated liver disease model: A reduced phytosterol intravenous lipid emulsion prevents liver injury. Pediatr. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.R.; de Silva, N.T.; Tomlinson, G.A.; Pencharz, P.B.; Feldman, B.M.; Moore, A.M.; Ling, S.C.; Wales, P.W. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: A multiple-variable analysis. JPEN J. Parenter. Enteral Nutr. 2011, 35, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Secor, J.D.; Yu, L.; Tsikis, S.; Fligor, S.; Puder, M.; Gura, K.M. Current strategies for managing intestinal failure-associated liver disease. Expert. Opin. Drug Saf. 2021, 20, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Yeh, P.J.; Lai, H.H.; Chen, M.C.; Ming, Y.C.; Lai, J.Y.; Lai, M.W. The Ratio of Remaining to Expected Small Bowel Length Predicts Enteral Autonomy in Pediatric Patients with Short Bowel Syndrome. Biomed. J. 2024, 100791. [Google Scholar] [CrossRef]
- Guz Mark, A.; Levi, S.; Davidovits, M.; Marderfeld, L.; Shamir, R. Children with Intestinal Failure Maintain Their Renal Function on Long-Term Parenteral Nutrition. Nutrients 2021, 13, 3647. [Google Scholar] [CrossRef]
- Roszkowska, P.; Klimczak, E.; Ostrycharz, E.; Rączka, A.; Wojciechowska-Koszko, I.; Dybus, A.; Cheng, Y.H.; Yu, Y.H.; Mazgaj, S.; Hukowska-Szematowicz, B. Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases-Current State of Knowledge. Biomedicines 2024, 12, 1030. [Google Scholar] [CrossRef]
- Culbreath, K.; Knell, J.; Keefe, G.; Han, S.M.; Hong, C.R.; Riley, H.B.; Liu, E.; McAdam, A.J.; Modi, B.P.; Jaksic, T.; et al. Antibiotic Therapy for Culture-Proven Bacterial Overgrowth in Children With Intestinal Failure Results in Improved Symptoms and Growth. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 345–350. [Google Scholar] [CrossRef]
- Gutierrez, I.M.; Kang, K.H.; Calvert, C.E.; Johnson, V.M.; Zurakowski, D.; Kamin, D.; Jaksic, T.; Duggan, C. Risk factors for small bowel bacterial overgrowth and diagnostic yield of duodenal aspirates in children with intestinal failure: A retrospective review. J. Pediatr. Surg. 2012, 47, 1150–1154. [Google Scholar] [CrossRef]
- Culbreath, K.; Keefe, G.; Nes, E.; Staffa, S.J.; Carey, A.N.; Jaksic, T.; Goldsmith, J.D.; Modi, B.P.; Ouahed, J.D.; Jimenez, L. Factors Associated With Chronic Intestinal Inflammation Resembling Inflammatory Bowel Disease in Pediatric Intestinal Failure: A Matched Case-Control Study. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 468–474. [Google Scholar] [CrossRef]
- Stanger, J.D.; Oliveira, C.; Blackmore, C.; Avitzur, Y.; Wales, P.W. The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: A systematic review and meta-analysis. J. Pediatr. Surg. 2013, 48, 983–992. [Google Scholar] [CrossRef]
| Medication Class | Function(s)/Mechanism(s) | Example of Generic | Brand Name |
|---|---|---|---|
| Anti-diarrheal | Antimotility | Loperamide | Imodium |
| Antimotility/anticolinergic | Diphenoxylate/atropine | Lomotil | |
| Bile acid binder | Colesevelam | Welchol | |
| Prokinetic | Dopamine-2 receptor antagonist | Metoclopramide | Reglan |
| Motilin receptor agonist | Erythromycin | Erythrocin | |
| Serotonin (5-HT4) receptor agonist | Cisapride | Propulsid | |
| Antacid | Histamine (H2) receptor antagonist | Famotidine | Pepcid |
| Proton pump inhibitor | Pantoprazole | Protonix | |
| Somatostatin analog | Octreotide | Sandostatin | |
| Neutralize excess acid | Calcium carbonate | Tums | |
| Mucosal protectant | Sucralfate | Carafate | |
| Antibiotics and probiotics | Antibiotics: reduce bacterial overgrowth | Metronidazole | Flagyl |
| Probiotics: encourage commensal bacteria | Lactobacillus sp. | Culturelle | |
| GLP-2 1 analog | Promote mucosal growth and nutrient absorption | Teduglutide | Gattex |
| Long-acting GLP-2 analog | Apraglutide | N/A 2 | |
| Enzyme replacement | Pancreatic enzyme replacement | Pancrelipase | Creon |
| Other | Parenteral nutrition | (Patient-specific) | N/A |
| Intravenous lipid emulsion | Mixed oils | SMOFlipid 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.Z.; O’Daniel, E.L. Updates in Intestinal Failure Management. J. Clin. Med. 2025, 14, 3031. https://doi.org/10.3390/jcm14093031
Wang SZ, O’Daniel EL. Updates in Intestinal Failure Management. Journal of Clinical Medicine. 2025; 14(9):3031. https://doi.org/10.3390/jcm14093031
Chicago/Turabian StyleWang, Sarah Z., and Elizabeth L. O’Daniel. 2025. "Updates in Intestinal Failure Management" Journal of Clinical Medicine 14, no. 9: 3031. https://doi.org/10.3390/jcm14093031
APA StyleWang, S. Z., & O’Daniel, E. L. (2025). Updates in Intestinal Failure Management. Journal of Clinical Medicine, 14(9), 3031. https://doi.org/10.3390/jcm14093031






