Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics
Abstract
1. Introduction
2. Methods
2.1. The Standard Protocol and Registration
2.2. The Search Strategy, Selection Criteria, and Process
2.3. Quality Control and Bias Assessment
2.4. Outcomes
2.5. Statistical Analysis
3. Results
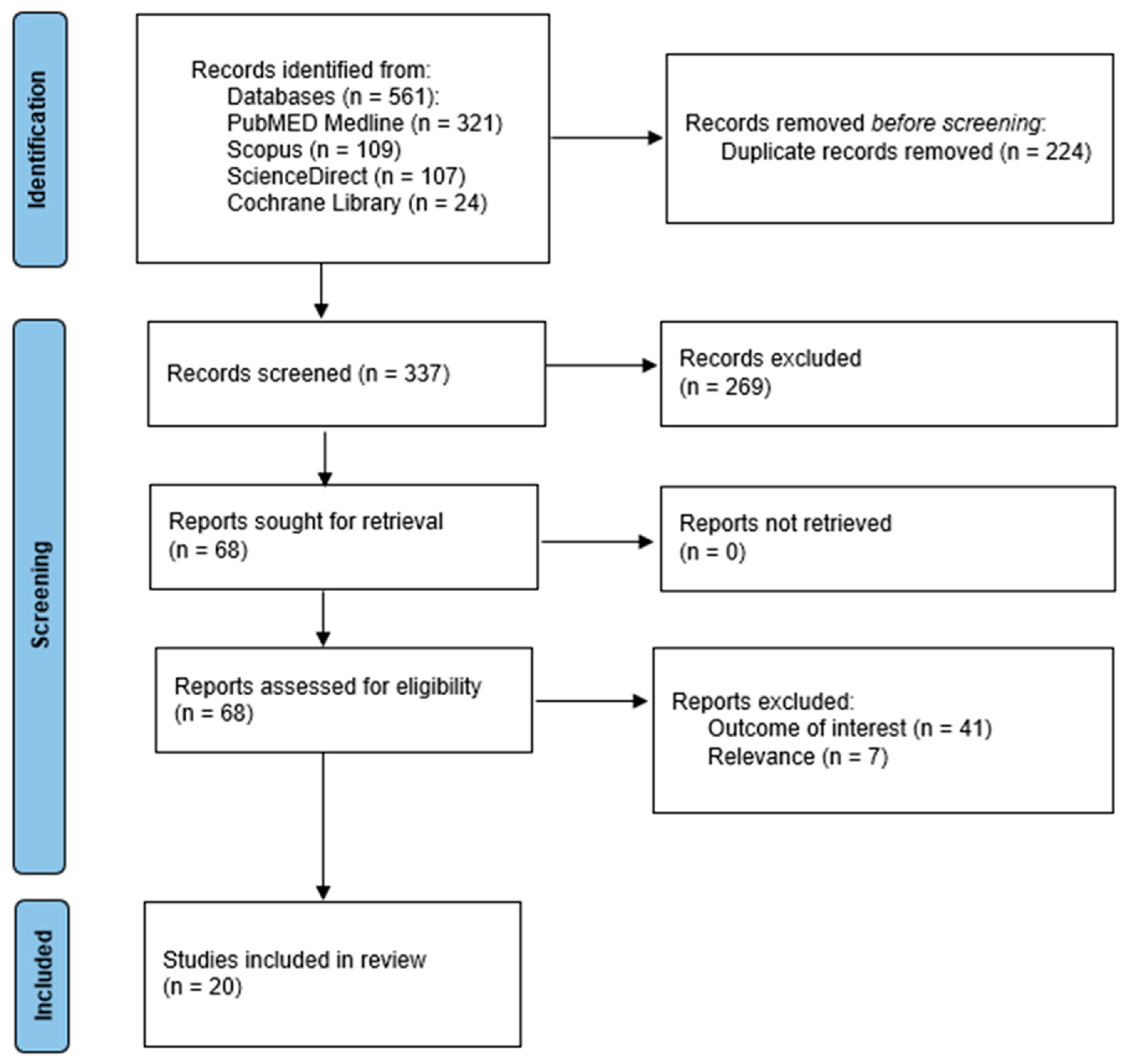
3.1. The Literature Search and Included Studies
3.2. Quality Control
3.3. The Qualitative Results of the Systematic Review on Cognitive Performance and Molecular Biomarkers in Newly Diagnosed pwMS
3.3.1. Biomarkers of Axonal Pathology and NI
3.3.2. Biomarkers Modulating Inflammatory Responses and NI
3.3.3. Biomarkers of Neuronal Survival and Metabolism and NI
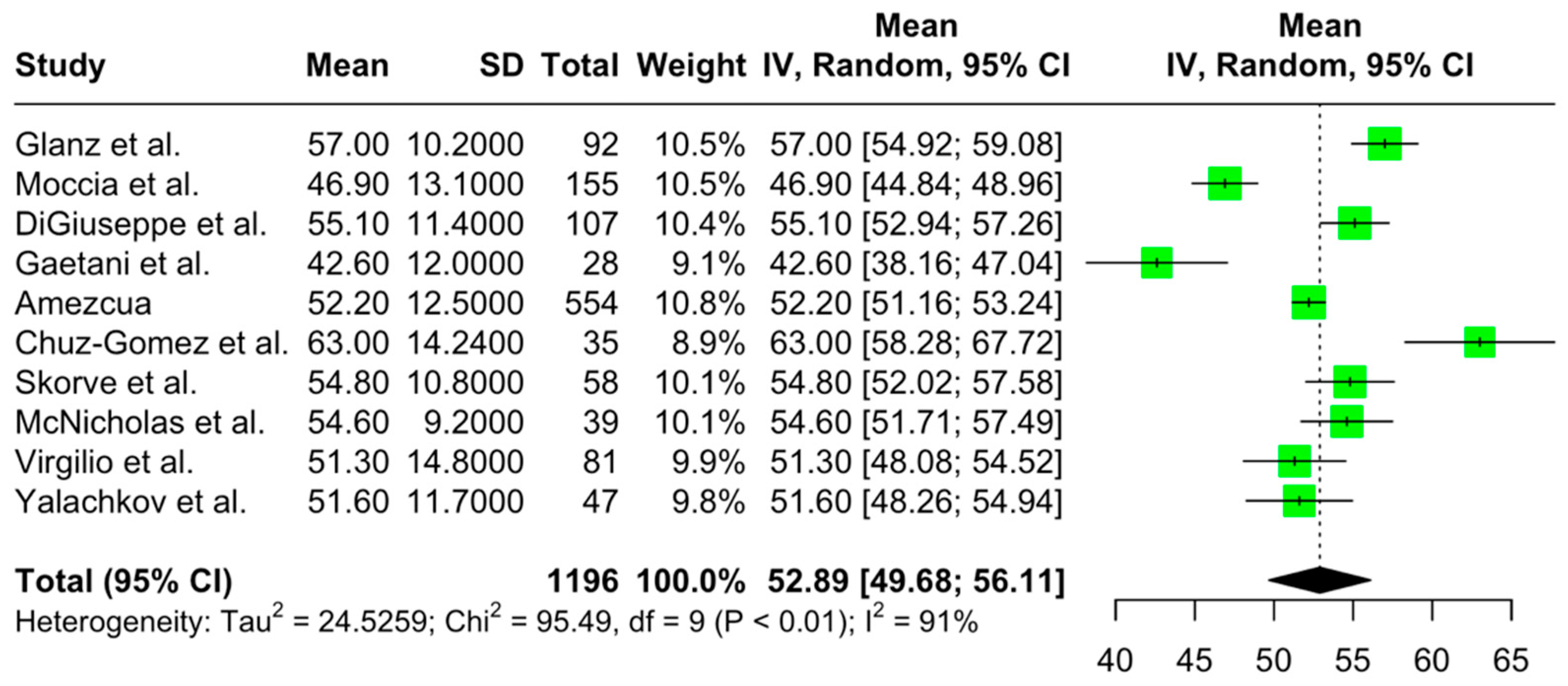
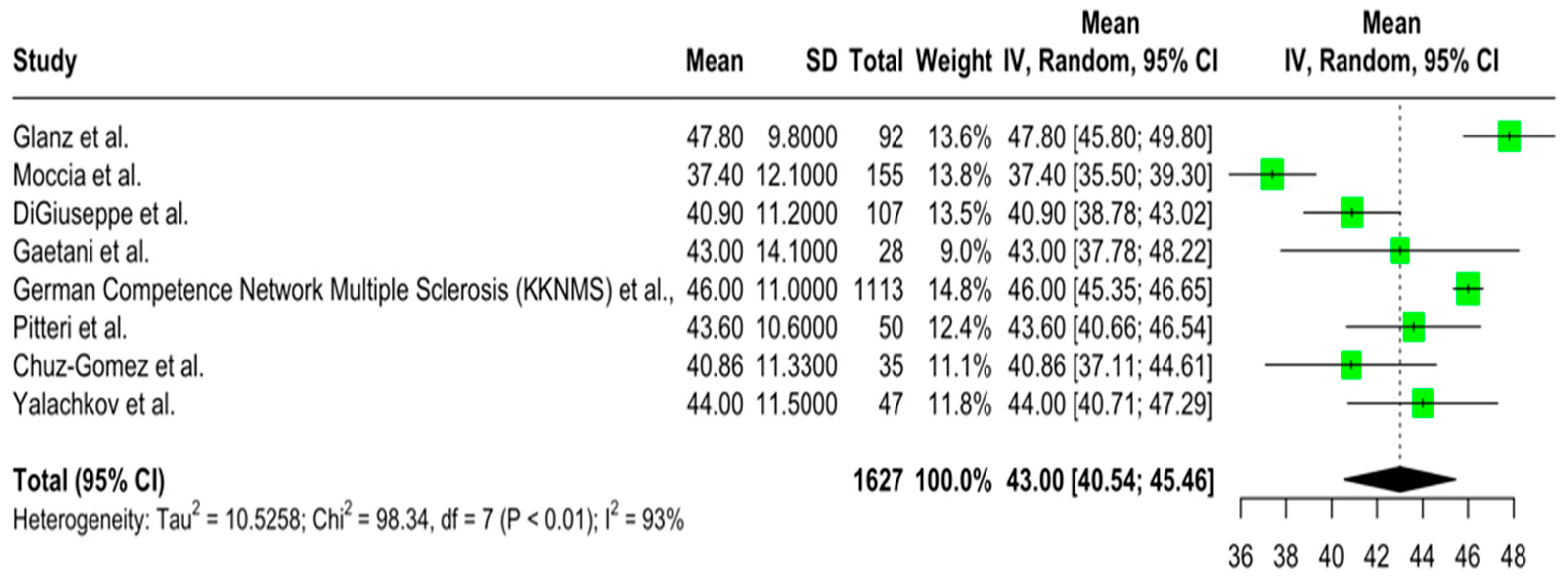
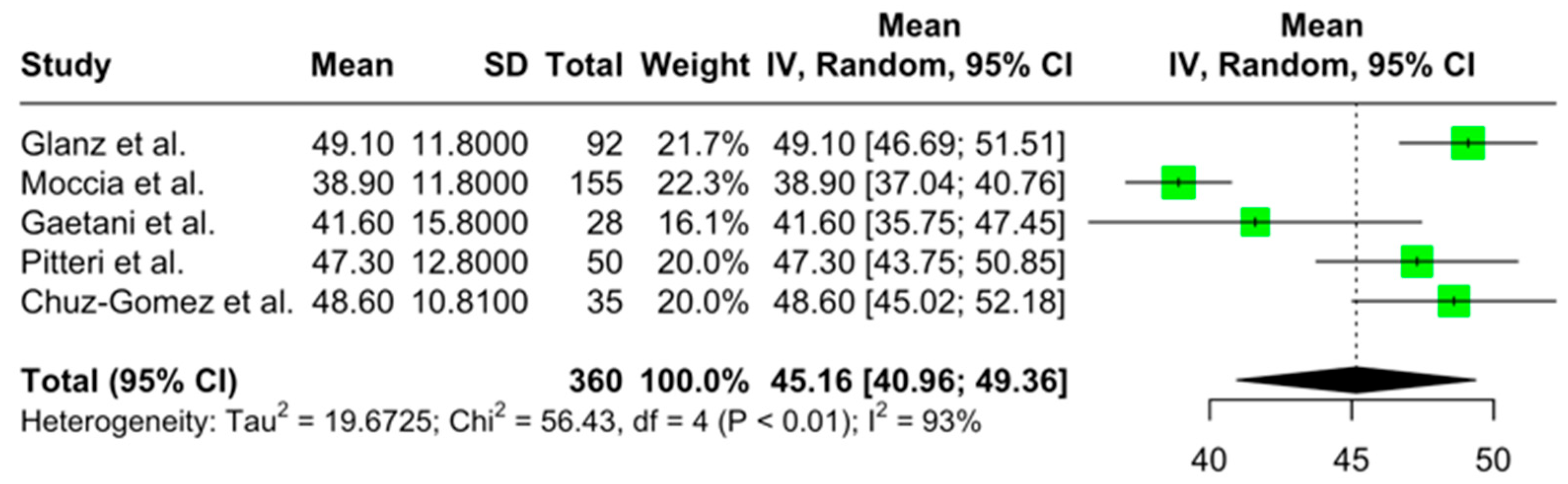
3.4. The Quantitative Results of the Meta-Analysis on Cognitive Performance and Its Associations in Newly Diagnosed pwMS
3.4.1. Primary Outcomes
3.4.2. Secondary Outcomes
3.4.3. Publication Bias
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Ross, A.P. Management of multiple sclerosis. Am. J. Manag. Care 2013, 19 (Suppl. S16), S301–S306. [Google Scholar]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Ferreira, M.L. Cognitive deficits in multiple sclerosis: A systematic review. Arq. Neuro-Psiquiatr. 2010, 68, 632–641. [Google Scholar] [CrossRef]
- Giazkoulidou, A.; Messinis, L.; Nasios, G. Cognitive functions and social cognition in multiple sclerosis: An overview. Hell. J. Nucl. Med. 2019, 22, 102–110. [Google Scholar]
- DeLuca, G.C.; Yates, R.L.; Beale, H.; Morrow, S.A. Cognitive impairment in multiple sclerosis: Clinical, radiologic and pathologic insights. Brain Pathol. 2015, 25, 79–98. [Google Scholar] [CrossRef]
- Jønsson, A.; Andresen, J.; Storr, L.; Tscherning, T.; Soelberg Sørensen, P.; Ravnborg, M. Cognitive impairment in newly diagnosed multiple sclerosis patients: A 4-year follow-up study. J. Neurol. Sci. 2006, 245, 77–85. [Google Scholar] [CrossRef]
- Oset, M.; Stasiolek, M.; Matysiak, M. Cognitive Dysfunction in the Early Stages of Multiple Sclerosis—How Much and How Important? Curr. Neurol. Neurosci. Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef]
- Esmaeili, S.; Obeidat, A.Z.; Zabeti, A. Molecular biomarkers and cognitive impairment in multiple sclerosis: A review. Biomark. Neuropsychiatry 2023, 9, 100077. [Google Scholar] [CrossRef]
- Jakimovski, D.; Zivadinov, R.; Ramanthan, M.; Hagemeier, J.; Weinstock-Guttman, B.; Tomic, D.; Kropshofer, H.; Fuchs, T.A.; Barro, C.; Leppert, D.; et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult. Scler. 2020, 26, 1670–1681. [Google Scholar] [CrossRef]
- Rademacher, T.D.; Meuth, S.G.; Wiendl, H.; Johnen, A.; Landmeyer, N.C. Molecular biomarkers and cognitive impairment in multiple sclerosis: State of the field, limitations, and future direction—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 146, 105035. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 1st ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Bohning, D.; Lerdsuwansri, R.; Holling, H. Some general points on the I2—Measure of heterogeneity in meta-analysis. Metrika 2017, 80, 685–695. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying Publication Bias in Meta-Analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Giannopapas, V.; Palaiodimou, L.; Kitsos, D.; Papagiannopoulou, G.; Stavrogianni, K.; Chasiotis, A.; Kosmidou, M.; Tzartos, J.S.; Paraskevas, G.P.; Bakalidou, D.; et al. The Prevalence of Diabetes Mellitus Type II (DMII) in the Multiple Sclerosis Population: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4948. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Amezcua, L.; Smith, J.B.; Gonzales, E.G.; Haraszti, S.; Langer-Gould, A. Race, ethnicity, and cognition in persons newly diagnosed with multiple sclerosis. Neurology 2020, 94, e1548–e1556. [Google Scholar] [CrossRef] [PubMed]
- Brummer, T.; Muthuraman, M.; Steffen, F.; Uphaus, T.; Minch, L.; Person, M.; Zipp, F.; Groppa, S.; Bittner, S.; Fleischer, V. Improved prediction of early cognitive impairment in multiple sclerosis combining blood and imaging biomarkers. Brain Commun. 2022, 4, fcac153. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gomez, Á.J.; Forero, L.; Lozano-Soto, E.; Cano-Cano, F.; Sanmartino, F.; Rashid-López, R.; Paz-Expósito, J.; Ramirez, J.D.G.; Espinosa-Rosso, R.; González-Rosa, J.J. Cortical Thickness and Serum NfL Explain Cognitive Dysfunction in Newly Diagnosed Patients With Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1074. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, G.; Blair, M.; Morrow, S.A. Short Report: Prevalence of Cognitive Impairment in Newly Diagnosed Relapsing-Remitting Multiple Sclerosis. Int. J. MS Care 2018, 20, 153–157. [Google Scholar] [CrossRef]
- Engel, S.; Graetz, C.; Salmen, A.; Muthuraman, M.; Toenges, G.; Ambrosius, B.; Bayas, A.; Berthele, A.; Heesen, C.; Klotz, L.; et al. Is APOE ε4 associated with cognitive performance in early MS? Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e728. [Google Scholar] [CrossRef]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J. Neurol. 2019, 266, 2157–2163. [Google Scholar] [CrossRef]
- German Competence Network Multiple Sclerosis (KKNMS); Johnen, A.; Bürkner, P.C.; Landmeyer, N.C.; Ambrosius, B.; Calabrese, P.; Motte, J.; Hessler, N.; Antony, G.; König, I.R.; et al. Can we predict cognitive decline after initial diagnosis of multiple sclerosis? Results from the German National early MS cohort (KKNMS). J. Neurol. 2019, 266, 386–397. [Google Scholar] [CrossRef]
- Glanz, B.I.; Holland, C.M.; Gauthier, S.A.; Amunwa, E.L.; Liptak, Z.; Houtchens, M.K.; Sperling, R.A.; Khoury, S.J.; Guttmann, C.R.G.; Weiner, H.L. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult. Scler. 2007, 13, 1004–1010. [Google Scholar] [CrossRef]
- Hankomäki, E.; Multanen, J.; Kinnunen, E.; Hämäläinen, P. The progress of cognitive decline in newly diagnosed MS patients. Acta Neurol. Scand. 2014, 129, 184–191. [Google Scholar] [CrossRef]
- McNicholas, N.; Tubridy, N.; Hutchinson, M.; McGuigan, C. Perceived and objective cognitive impairment in newly diagnosed versus established multiple sclerosis: Impact of disease duration. Ir. J. Med. Sci. 2021, 190, 1149–1154. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Chang, K.C.-M.; Costabile, T.; Russo, C.; De Rosa, A.; Carotenuto, A.; Sacca, F.; Maniscalco, G.T.; et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. 2016, 22, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, M.; Magliozzi, R.; Nicholas, R.; Ziccardi, S.; Pisani, A.I.; Pezzini, F.; Marastoni, D.; Calabrese, M. Cerebrospinal fluid inflammatory profile of cognitive impairment in newly diagnosed multiple sclerosis patients. Mult. Scler. 2022, 28, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, M.; Ziccardi, S.; Dapor, C.; Guandalini, M.; Calabrese, M. Lost in Classification: Lower Cognitive Functioning in Apparently Cognitive Normal Newly Diagnosed RRMS Patients. Brain Sci. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Prokopova, B.; Hlavacova, N.; Vlcek, M.; Penesova, A.; Grunnerova, L.; Garafova, A.; Turcani, P.; Kollar, B.; Jezova, D. Early cognitive impairment along with decreased stress-induced BDNF in male and female patients with newly diagnosed multiple sclerosis. J. Neuroimmunol. 2017, 302, 34–40. [Google Scholar] [CrossRef]
- Quintana, E.; Coll, C.; Salavedra-Pont, J.; Martín, M.M.; Robles-Cedeño, R.; Tomàs-Roig, J.; Buxó, M.; Matute-Blanch, C.; Villar, L.M.; Montalban, X.; et al. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur. J. Neurol. 2018, 25, 1189–1191. [Google Scholar] [CrossRef]
- Ruet, A.; Deloire, M.; Hamel, D.; Ouallet, J.C.; Petry, K.; Brochet, B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: A 7-year longitudinal study. J. Neurol. 2013, 260, 776–784. [Google Scholar] [CrossRef]
- Skorve, E.; Lundervold, A.J.; Torkildsen, Ø.; Myhr, K.M. A two-year longitudinal follow-up of cognitive performance assessed by BICAMS in newly diagnosed patients with MS. Mult. Scler. Relat. Disord. 2020, 46, 102577. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Barbero, P.; Caloni, B.; Naldi, P.; Cantello, R.; Dianzani, U.; Comi, C. Serum Vitamin D as a Marker of Impaired Information Processing Speed and Early Disability in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1521. [Google Scholar] [CrossRef]
- Yalachkov, Y.; Anschütz, V.; Jakob, J.; Schaller-Paule, M.A.; Schäfer, J.H.; Reiländer, A.; Friedauer, L.; Behrens, M.; Steffen, F.; Bittner, S.; et al. Brain-derived neurotrophic factor and neurofilament light chain in cerebrospinal fluid are inversely correlated with cognition in Multiple Sclerosis at the time of diagnosis. Mult. Scler. Relat. Disord. 2022, 63, 103822. [Google Scholar] [CrossRef]
- Valentine, J.C.; Pigott, T.D.; Rothstein, H.R. How Many Studies Do You Need?: A Primer on Statistical Power for Meta-Analysis. J. Educ. Behav. Stat. 2010, 35, 215–247. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- delCacho-Tena, A.; Christ, B.R.; Arango-Lasprilla, J.C.; Perrin, P.B.; Rivera, D.; Olabarrieta-Landa, L. Normative Data Estimation in Neuropsychological Tests: A Systematic Review. Arch. Clin. Neuropsychol. 2024, 39, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Granberg, T.; Martola, J.; Bergendal, G.; Shams, S.; Damangir, S.; Aspelin, P.; Fredrikson, S.; Kristoffersen-Wiberg, M. Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: Results of a 17-year longitudinal study. Mult. Scler. 2015, 21, 1151–1158. [Google Scholar] [CrossRef]
- Riccitelli, G.C.; Pagani, E.; Rodegher, M.; Colombo, B.; Preziosa, P.; Falini, A.; Comi, G.; Filippi, M.; Rocca, M.A. Imaging patterns of gray and white matter abnormalities associated with PASAT and SDMT performance in relapsing-remitting multiple sclerosis. Mult. Scler. 2019, 25, 204–216. [Google Scholar] [CrossRef]
- Matias-Guiu, J.A.; Cortés-Martínez, A.; Montero, P.; Pytel, V.; Moreno-Ramos, T.; Jorquera, M.; Yus, M.; Arrazola, J. Structural MRI correlates of PASAT performance in multiple sclerosis. BMC Neurol. 2018, 18, 214. [Google Scholar] [CrossRef]
- Borkowska, A.R.; Daniluk, B.; Adamczyk, K. Significance of the Diagnosis of Executive Functions in Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Environ. Res. Public Health 2021, 18, 10527. [Google Scholar] [CrossRef]
- Possa, M.F.; Minacapelli, E.; Canale, S.; Comi, G.; Martinelli, V.; Falautano, M. The first year after diagnosis: Psychological impact on people with multiple sclerosis. Psychol. Health Med. 2017, 22, 1063–1071. [Google Scholar] [CrossRef]
- Plieger, T.; Reuter, M. Stress & executive functioning: A review considering moderating factors. Neurobiol. Learn. Mem. 2020, 173, 107254. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Anhoque, C.F.; Domingues, S.C.A.; Teixeira, A.L.; Domingues, R.B. Cognitive impairment in clinically isolated syndrome: A systematic review. Dement. Neuropsychol. 2010, 4, 86–90. [Google Scholar] [CrossRef]
| Author | Year | Region | Study Design | Sample Size | MS/CIS | Disease Duration 1 | Male/ Female |
|---|---|---|---|---|---|---|---|
| Amezcua et al. [22] | 2020 | USA | Cross-sectional | 554 | <1 | 160/394 | |
| Brummer et al. [23] | 2022 | Germany | Cross-sectional | 152 | 118/34 | 1.4 | 45/107 |
| Cruz-Gomez et al. [24] | 2020 | Spain | Cross-sectional | 35 | 35 | 3 | 20/15 |
| DiGiuseppe et al. [25] | 2018 | England | Cross-sectional | 107 | 107 | <1 | 25/82 |
| Engel et al. [26] | 2020 | Germany | Cross-sectional | 552 | 308/244 | 4 | 157/395 |
| Gaetani et al. [27] | 2019 | Italy | Cross-sectional | 28 | 25/3 | 2.5 | 8/20 |
| German Competence Network of Multiple Sclerosis et al. [28] | 2019 | Germany | Longitudinal | 1113 | 622/501 | <1 | 338/775 |
| Glanz et al. [29] | 2007 | USA | Cross-sectional | 92 | 77/15 | <1 | 21/71 |
| Hankomäki et al. [30] | 2014 | Finland | Longitudinal | 36 | 36 | 12/24 | |
| Jønsson et al. [7] | 2006 | Denmark | Longitudinal | 80 | 80 | <1 | |
| McNicholas et al. [31] | 2021 | Ireland | Cross-sectional | 39 | 39 | <1 | |
| Moccia et al. [32] | 2016 | Italy | Longitudinal | 155 | 155 | <1 | 56/99 |
| Pitteri et al. [33] | 2022 | Italy | Cross-sectional | 69 | 64/5 | 3.4 | 15/54 |
| Pitteri et al. [34] | 2019 | Italy | Cross-sectional | 50 | 50 | 3.5 | 13/37 |
| Prokopova et al. [35] | 2017 | Slovakia | Cross-sectional | 19 | 19 | 9/10 | |
| Quintana et al. [36] | 2018 | Spain | Cross-sectional | 51 | 51 | 12/39 | |
| Ruet et al. [37] | 2013 | France | Longitudinal | 69 | 69 | 2.6 | 24/45 |
| Skorve et al. [38] | 2020 | Norway | Longitudinal | 58 | 58 | 1.2 | 14/44 |
| Virgilio et al. [39] | 2021 | Switzerland | Cross-sectional | 81 | 79/2 | <1 | 27/54 |
| Yalachkov et al. [40] | 2022 | Germany | Cross-sectional | 47 | 38/9 | 12/34 |
| Author | Mean Age | EDSS | Neuropsychological Assessment | Psychological Assessment | Biomarker | Male/Female |
|---|---|---|---|---|---|---|
| Amezcua et al. [22] | 40.3 | SDMT | 160/394 | |||
| Brummer et al. [23] | 33.0 | 1.3 | SDMT, PASAT, and VLMT | HADS | sNfL | 45/107 |
| Cruz-Gomez et al. [24] | 38.4 | 1 | BRB-N | STAI, BDI | sNfL | 20/15 |
| DiGiuseppe et al. [25] | 35.8 | 1.8 | MACFIMS | HADS | 25/82 | |
| Engel et al. [26] | 32 | 1.5 | MUSIC, PASAT-3 | BDI-II | APOE e4 | 157/395 |
| Gaetani et al. [27] | 39.1 | 2 | BRB-N | CSF-NfL | 8/20 | |
| German Competence Network of Multiple Sclerosis et al. [28] | 34.1 | 1.5 | MUSIC, PASAT-3 | BDI-II | 338/775 | |
| Glanz et al. [29] | 36.5 | 1.3 | BRB-N | CES-D | 21/71 | |
| Hankomäki et al. [30] | 36 | CS, PASAT, SDMT, dual task, WMS-R Logical Memory, SRT, Benton VRT, WAIS-R Similarities and Block Design, VF | BDI-II | 12/24 | ||
| Jønsson et al. [7] | 35 | 2.7 | WAIS Vocabulary and Similarities, SSST, Digits Forward and Backward, Design Fluency, SCNT, RPM A-B, SDMT, RCFT, ToL, SRT, Animals, MCT, SGCT, BN, and Famous Faces | |||
| McNicholas et al. [31] | 35.3 | 0.8 | PDQ, BICAMS | HADS | ||
| Moccia et al. [32] | 32.1 | 1.8 | BRB-N | 56/99 | ||
| Pitteri et al. [33] | 37.3 | 2.0 | BRB-N, Stroop test, Phonological, Semantic, and Alternate VF, MFPT | DASS-21 | Inflammatory mediators, such as IL11, IL34, IL35, CHI3L1, CXCL12, CCL22, CCL13, CCL8, CXCL10, CXCL12, MIF, APRIL, and CSF-NfL | 15/54 |
| Pitteri et al. [34] | 38.2 | 1.5 | BRB-N, Stroop test, Phonological, Semantic, Alternate VF, MFPT | DASS-21 | 13/37 | |
| Prokopova et al. [35] | 30.6 | 1.1 | Stroop test | STAI, BDI, 8SQ | P cortisol, copeptin, aldosterone, BDNF | 9/10 |
| Quintana et al. [36] | 35.7 | 2 | BRB-N, TMT-A | HADS-A | CHI3L1, NfL | 12/39 |
| Ruet et al. [37] | 39.0 | 2.0 | The WAIS-R Similarities subtest, BRB-N | 24/45 | ||
| Skorve et al. [38] | 37.6 | 1.4 | BICAMS | HADS | 14/44 | |
| Virgilio et al. [39] | 37.6 | SDMT | Vitamin D | 27/54 | ||
| Yalachkov et al. [40] | 35.4 | 2.1 | SDMT, PASAT-3, RCFT, VLMT | BDI-II | sNfL, CSF-NfL, sBDNF, CSF-BDNF | 12/34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrogianni, K.; Giannopapas, V.; Kitsos, D.K.; Christouli, N.; Smyrni, V.; Chasiotis, A.K.; Akrivaki, A.; Dimitriadou, E.-M.; Tzartos, J.S.; Tsivgoulis, G.; et al. Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics. J. Clin. Med. 2025, 14, 2630. https://doi.org/10.3390/jcm14082630
Stavrogianni K, Giannopapas V, Kitsos DK, Christouli N, Smyrni V, Chasiotis AK, Akrivaki A, Dimitriadou E-M, Tzartos JS, Tsivgoulis G, et al. Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics. Journal of Clinical Medicine. 2025; 14(8):2630. https://doi.org/10.3390/jcm14082630
Chicago/Turabian StyleStavrogianni, Konstantina, Vasileios Giannopapas, Dimitrios K. Kitsos, Niki Christouli, Vassiliki Smyrni, Athanasios K. Chasiotis, Alexandra Akrivaki, Evangelia-Makrina Dimitriadou, John S. Tzartos, Georgios Tsivgoulis, and et al. 2025. "Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics" Journal of Clinical Medicine 14, no. 8: 2630. https://doi.org/10.3390/jcm14082630
APA StyleStavrogianni, K., Giannopapas, V., Kitsos, D. K., Christouli, N., Smyrni, V., Chasiotis, A. K., Akrivaki, A., Dimitriadou, E.-M., Tzartos, J. S., Tsivgoulis, G., Paraskevas, G. P., Peschos, D., Tsamis, K. I., & Giannopoulos, S. (2025). Cognitive Impairment in Newly Diagnosed Patients with Multiple Sclerosis: A Systematic Review of Related Molecular Biomarkers and a Meta-Analysis of Associated Demographic and Disease-Related Characteristics. Journal of Clinical Medicine, 14(8), 2630. https://doi.org/10.3390/jcm14082630