Postoperative Surveillance in the Postoperative vs. Intensive Care Unit for Patients Undergoing Elective Supratentorial Brain Tumor Removal: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
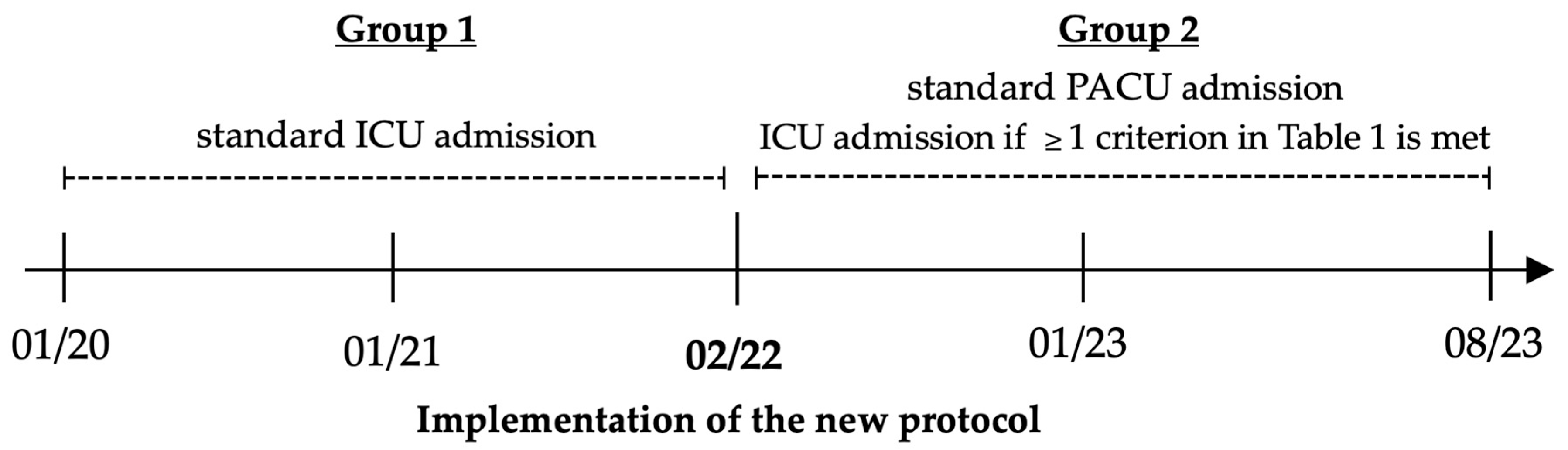
2.1. Study Design, Settings, and Patients
2.2. New Perioperative Regimen
2.3. Patient Allocation to ICU and PACU
2.4. Care Structure in the ICU and PACU
2.5. Variables and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Patients
3.2. Tumor Diagnosis and Surgical Process
3.3. Postoperative Length of Stay
3.4. Cost Analysis
3.5. Complications
4. Discussion
4.1. Current Standard of Practice and Recent Developments
4.2. Clinical and Economic Benefits of PACU Admission
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| COVID-19 | Coronavirus Disease 2019 |
| DRG | Diagnosis-Related Group System |
| ERAS | Enhanced Recovery After Surgery |
| MDPI | Multidisciplinary Digital Publishing Institute |
| ICU | Intensive Care Unit |
| IMC | Intermediate Care Unit |
| IPWT | Inversed Probability Weighting of Treatment |
| LOS | Length of Stay |
| PACU | Postoperative Care Unit |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Ziai, W.C.; Varelas, P.N.; Zeger, S.L.; Mirski, M.A.; Ulatowski, J.A. Neurologic intensive care resource use after brain tumor surgery: An analysis of indications and alternative strategies. Crit. Care Med. 2003, 31, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; Ruppe, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensiv. Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, C.C.; Boone, M.D.; Laviv, Y.; Kasper, B.S.; Chen, C.C.; Kasper, E.M. The Utility of Routine Intensive Care Admission for Patients Undergoing Intracranial Neurosurgical Procedures: A Systematic Review. Neurocritical Care 2018, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lonjaret, L.; Guyonnet, M.; Berard, E.; Vironneau, M.; Peres, F.; Sacrista, S.; Ferrier, A.; Ramonda, V.; Vuillaume, C.; Roux, F.E.; et al. Postoperative complications after craniotomy for brain tumor surgery. Anaesth. Crit. Care Pain Med. 2017, 36, 213–218. [Google Scholar] [CrossRef]
- Henker, C.; Schmelter, C.; Piek, J. Complications and monitoring standards after elective craniotomy in Germany. Anaesthesist 2017, 66, 412–421. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Junker, C.D.; Becker, R.B.; Draper, E.A.; Wagner, D.P.; Knaus, W.A. Neurological intensive care admissions: Identifying candidates for intermediate care and the services they receive. Neurosurgery 1998, 42, 91–101; discussion 101–102. [Google Scholar] [CrossRef]
- Beauregard, C.L.; Friedman, W.A. Routine use of postoperative ICU care for elective craniotomy: A cost-benefit analysis. Surg. Neurol. 2003, 60, 483–489; discussion 489. [Google Scholar] [CrossRef]
- Au, K.; Bharadwaj, S.; Venkatraghavan, L.; Bernstein, M. Outpatient brain tumor craniotomy under general anesthesia. J. Neurosurg. 2016, 125, 1130–1135. [Google Scholar] [CrossRef]
- Florman, J.E.; Cushing, D.; Keller, L.A.; Rughani, A.I. A protocol for postoperative admission of elective craniotomy patients to a non-ICU or step-down setting. J. Neurosurg. 2017, 127, 1392–1397. [Google Scholar] [CrossRef]
- Hanak, B.W.; Walcott, B.P.; Nahed, B.V.; Muzikansky, A.; Mian, M.K.; Kimberly, W.T.; Curry, W.T. Postoperative intensive care unit requirements after elective craniotomy. World Neurosurg. 2014, 81, 165–172. [Google Scholar] [CrossRef]
- Laan, M.T.; Roelofs, S.; Van Huet, I.; Adang, E.M.M.; Bartels, R. Selective Intensive Care Unit Admission After Adult Supratentorial Tumor Craniotomy: Complications, Length of Stay, and Costs. Neurosurgery 2020, 86, E54–E59. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Chan, A.K.; Viner, J.A.; Sankaran, S.; Chan, A.Y.; Imershein, S.; Meary-Miller, A.; Theodosopoulos, P.V.; Jacques, L.; Aghi, M.K.; et al. A Safe Transitions Pathway for post-craniotomy neurological surgery patients: High-value care that bypasses the intensive care unit. J. Neurosurg. 2020, 134, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, W.; Guan, J.; Langevin, J.P.; Barkhoudarian, G.; Kelly, D.F.; Martin, N. Enhanced recovery after brain tumor surgery: Pilot protocol implementation in a large healthcare system. Neurosurg. Focus 2023, 55, E5. [Google Scholar] [CrossRef] [PubMed]
- Supbumrung, S.; Kaewborisutsakul, A.; Kitsiripant, C.; Kaewborisutsakul, W.K.; Churuangsuk, C. Effect of the enhanced recovery protocol in patients with brain tumors undergoing elective craniotomies: A systematic review and meta-analysis. Neurosurg. Focus 2023, 55, E7. [Google Scholar] [CrossRef]
- Stumpo, V.; Staartjes, V.E.; Quddusi, A.; Corniola, M.V.; Tessitore, E.; Schroder, M.L.; Anderer, E.G.; Stienen, M.N.; Serra, C.; Regli, L. Enhanced Recovery After Surgery strategies for elective craniotomy: A systematic review. J. Neurosurg. 2021, 135, 1857–1881. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Gaudino, M.; Girola, F.; Piscitelli, M.; Martinelli, L.; Anselmi, A.; Della Vella, C.; Schiavello, R.; Possati, G. Long-term survival and quality of life of patients with prolonged postoperative intensive care unit stay: Unmasking an apparent success. J. Thorac. Cardiovasc. Surg. 2007, 134, 465–469. [Google Scholar] [CrossRef][Green Version]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Linzey, J.R.; Foshee, R.; Moriguchi, F.; Adapa, A.R.; Koduri, S.; Kahn, E.N.; Williamson, C.A.; Sheehan, K.; Rajajee, V.; Thompson, B.G.; et al. Length of Stay Beyond Medical Readiness in a Neurosurgical Patient Population and Associated Healthcare Costs. Neurosurgery 2021, 88, E259–E264. [Google Scholar] [CrossRef]
- Hoffman, S.E.; Gupta, S.; O’Connor, M.; Jarvis, C.A.; Zhao, M.; Hauser, B.M.; Bernstock, J.D.; Murphy, S.; Raftery, S.M.; Lane, K.; et al. Reduced time to imaging, length of stay, and hospital charges following implementation of a novel postoperative pathway for craniotomy. J. Neurosurg. 2023, 139, 373–384. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals—2022–2023; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- Bearman, G.M.; Munro, C.; Sessler, C.N.; Wenzel, R.P. Infection control and the prevention of nosocomial infections in the intensive care unit. Semin. Respir. Crit. Care Med. 2006, 27, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Kirkland, K.B.; Kaye, K.S.; Thacker, P.A., 2nd; Kanafani, Z.A.; Auten, G.; Sexton, D.J. Underresourced hospital infection control and prevention programs: Penny wise, pound foolish? Infect. Control Hosp. Epidemiology 2007, 28, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Graves, N.; Dancer, S.J.; et al. Impact of healthcare-associated infection on length of stay. J. Hosp. Infect. 2021, 114, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Page, K.; Campbell, M.; Martin, E.; Rashleigh-Rolls, R.; Halton, K.; Paterson, D.L.; Hall, L.; Jimmieson, N.; White, K.; et al. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: A case-control study. BMJ Open 2013, 3, e003587. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Bandila, S.R.; Onisor, D.; Laszlo, S.S.; Lupu, G.; Danielescu, A.; Baba, D.F.; Vasiesiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. [Google Scholar] [CrossRef]
- Osorio, J.A.; Safaee, M.M.; Viner, J.; Sankaran, S.; Imershein, S.; Adigun, E.; Weigel, G.; Berger, M.S.; McDermott, M.W. Cost-effectiveness development for the postoperative care of craniotomy patients: A safe transitions pathway in neurological surgery. Neurosurg. Focus 2018, 44, E19. [Google Scholar] [CrossRef]
- Bernstein, M. Outpatient craniotomy for brain tumor: A pilot feasibility study in 46 patients. Can. J. Neurol. Sci. 2001, 28, 120–124. [Google Scholar] [CrossRef]
- Mirza, F.A.; Wang, C.; Pittman, T. Can patients safely be admitted to a ward after craniotomy for resection of intra-axial brain tumors? Br. J. Neurosurg. 2018, 32, 201–205. [Google Scholar] [CrossRef]
- Bui, J.Q.; Mendis, R.L.; van Gelder, J.M.; Sheridan, M.M.; Wright, K.M.; Jaeger, M. Is postoperative intensive care unit admission a prerequisite for elective craniotomy? J. Neurosurg. 2011, 115, 1236–1241. [Google Scholar] [CrossRef]
- Rhondali, O.; Genty, C.; Halle, C.; Gardellin, M.; Ollinet, C.; Oddoux, M.; Carcey, J.; Francony, G.; Fauvage, B.; Gay, E.; et al. Do patients still require admission to an intensive care unit after elective craniotomy for brain surgery? J. Neurosurg. Anesthesiol. 2011, 23, 118–123. [Google Scholar] [CrossRef]
| Preoperative Criteria | |
|---|---|
| Anesthesia | |
| Presence of patient-related risk factors based on preexisting health concerns | |
| Neurosurgical | |
| Thalamic or hypothalamic tumors | |
| Hormone-active pituitary gland tumors | |
| Skull base tumors | |
| Posterior falx meningiomas | |
| Perioperative Criteria | |
| Awake craniotomy | |
| Intraoperative complications such as brain edema or massive hemorrhage | |
| High risk for postoperative bleeding, dysphagia, or brain edema | |
| Blood loss > 1500 mL | |
| Duration of surgery > 240 min | |
| Postoperative Criteria | |
| Sufficient oxygenation after extubation in the operating room | |
| Adequate waking response and vigilance after anesthesia emergence | |
| Neurologic | Pulmonary | Cardiac/Metabolic | Hepatic | Nephrological |
|---|---|---|---|---|
| Epilepsy | COPD | Arterial hypertension | Chronic hepatitis B | Chronic kidney failure |
| Stroke/TIA | OSAS | Atrial fibrillation | Liver fibrosis | Renal cell carcinoma |
| Myelopathy | Lung carcinoma | Chronic heart failure | Liver cirrhosis | |
| Neuropathy | Lung emphysema | Coronary artery disease | Hepatomegaly | |
| Major TBI | Lung fibrosis | CABG/PCI | ||
| Psychosis | Asthma bronchiale | Diabetes mellitus | ||
| Depression | Smoking |
| Group 1: 01/2020–01/2022 | ||
| Total patients | 205 | |
| Included in analysis | ICU admission as per protocol | 199 |
| Excluded from analysis | PACU admission 1 | 6 |
| Group 2: 02/2022–08/2023 | ||
| Total patients | 213 | |
| Included in analysis | 211 | |
| PACU admission as per protocol | 142 | |
| ICU admission | 69 | |
| Transfer to ICU after initial PACU admission | 2 |
| Group 1 (n = 199) | Group 2 (n = 412) | p-Value | ||
|---|---|---|---|---|
| PACU (n = 199) | ICU (n = 213) | |||
| Baseline patient characteristics | ||||
| Sex, female | 106 (53.3) | 214 (51.9) | 0.759 | |
| 105 (52.8) | 109 (51.2) | 0.747 | ||
| Age, years | 65 [55 to 77] | 64 [55 to 74] | 0.379 | |
| 64 [52 to 74] | 66 [55 to 75] | 0.184 | ||
| Body mass index (kg m−2) | 25.6 [22.5 to 29.4] | 26.6 [23.2 to 29.8] | 0.139 | |
| 26.1 [23.7 to 29.7] | 27.0 [22.4 to 29.8] | 0.706 | ||
| ASA physical status | ||||
| II | 67 (33.8) | 139 (33.7) | ||
| III | 120 (60.6) | 259 (62.9) | ||
| IV | 11 (5.6) | 14 (3.4) | ||
| Preexisting health conditions | ||||
| Neurologic | 52 (26.1) | 133 (32.3) | 0.121 | |
| 59 (29.6) | 74 (34.7) | 0.269 | ||
| Pulmonary | 56 (28.1) | 125 (30.3) | 0.577 | |
| 65 (32.7) | 60 (28.2) | 0.321 | ||
| Cardiac | 91 (45.7) | 204 (49.5) | 0.380 | |
| 90 (45.2) | 114 (53.5) | 0.092 | ||
| Nephrological | 15 (7.5) | 28 (6.3) | 0.743 | |
| 13 (6.5) | 15 (7.1) | 0.827 | ||
| Hepatic | 9 (4.5) | 18 (4.4) | 0.931 | |
| 7 (3.5) | 11 (5.2) | 0.414 | ||
| Diabetes | 16 (8.0) | 80 (19.4) | <0.001 | |
| 32 (16.1) | 48 (22.5) | 0.098 | ||
| Rheologic medication | 53 (26.6) | 68 (16.5) | 0.003 | |
| 33 (16.6) | 35 (16.5) | 0.984 | ||
| Group 1 (n = 199) | Group 2 (n = 412) | p-Value | ||
|---|---|---|---|---|
| PACU (n = 199) | ICU (n = 213) | |||
| Surgical details | ||||
| Length of surgery [min] | 147 [112 to 193] | 124 [98 to 163] | <0.001 | |
| 125 [91 to 160] | 124 [105 to 172] | 0.114 | ||
| Blood loss [mL] | 400 [200 to 600] | 400 [200 to 600] | 0.629 | |
| 300 [200 to 400] | 500 [300 to 700] | <0.001 | ||
| Awake craniotomy | 12 (6.0) | 26 (6.3) | 0.887 | |
| 0 (0.0) | 26 (12.3) | <0.001 | ||
| Tumor diagnosis | ||||
| Tumor volume [cm3] | 11.5 [3.6 to 42.4] | 19.0 [8.2 to 46.1] | 0.024 | |
| 15.1 [4.9 to 38.8] | 20.6 [8.2 to 54.4] | 0.026 | ||
| Tumor entity | ||||
| Meningioma | 55 (27.6) | 115 (28.0) | 0.880 | |
| Glioma | 66 (33.2) | 123 (29.9) | 0.519 | |
| Metastasis | 58 (29.1) | 124 (30.2) | 0.795 | |
| Other | 20 (10.1) | 49 (11.9) | 0.736 | |
| Tumor localization | ||||
| Frontal | 114 (57.3) | 220 (53.3) | ||
| Temporal | 41 (20.6) | 99 (24.0) | ||
| Parietal | 28 (14.1) | 62 (15.0) | ||
| Occipital | 10 (5.0) | 14 (3.4) | ||
| Other | 6 (3.0) | 17 (4.1) | ||
| Group 1 (n = 199) | Group 2 (n = 412) | p-Value | ||
|---|---|---|---|---|
| PACU (n = 199) | ICU (n = 213) | |||
| Length of stay | ||||
| Total LOS [d] | 10 [7 to 17] | 11 [7 to 16] | 0.416 | |
| 9 [7 to 15] | 12 [9 to 19] | <0.001 | ||
| Postoperative LOS [d] | 7 [5 to 10] | 6 [5 to 9] | 0.045 | |
| 6 [4 to 7] | 6 [5 to 10] | 0.093 | ||
| Length of stay PACU/ICU [hr] | 20:47 [18:05 to 23:02] | / | <0.001 | |
| 16:45 [14:00 to 18:43] | 24:48 [19:56 to 94:16] | <0.001 | ||
| Readmission and mortality rate | ||||
| 30-day readmission | 37 (18.6) | 59 (14.4) | 0.178 | |
| 23 (11.6) | 36 (17.0) | 0.127 | ||
| 30-day mortality | 6 (3.0) | 5 (1.2) | 0.190 | |
| 4 (2.0) | 1 (0.5) | 0.203 | ||
| 90-day readmission | 27 (13.6) | 55 (13.4) | 0.950 | |
| 23 (11.6) | 33 (15.5) | 0.244 | ||
| 90-day mortality | 7 (3.5) | 4 (1.0) | 0.046 | |
| 4 (2.0) | 0 (0.0) | 0.054 | ||
| Costs | ||||
| Total revenue [EUR (€)] | 13,649 [11,845 to 17,939] | 15,729 [12,903 to 18,672] | <0.001 | |
| 13,551 [12,292 to 17,940] | 16,016 [13,778 to 21,571] | <0.001 | ||
| Nursing revenue [EUR (€)] | 2280 [1520 to 4126] | 2781 [1792 to 5194] | 0.003 | |
| 2286 [1469 to 3534] | 3399 [2318 to 5251] | <0.001 | ||
| Group 1 (n = 199) | Group 2 (n = 412) | p-Value | ||
|---|---|---|---|---|
| PACU (n = 199) | ICU (n = 213) | |||
| Surgical site or intracranial infections | 10 (5.0) | 9 (2.2) | 0.060 | |
| 4 (2.0) | 5 (2.3) | 0.548 | ||
| Cerebral infarction | 4 (2.0) | 17 (4.1) | 0.178 | |
| 8 (4.0) | 9 (4.2) | 0.917 | ||
| Brain edema | 6 (3.0) | 25 (6.1) | 0.106 | |
| 5 (2.5) | 20 (9.4) | 0.002 | ||
| Postoperative bleeding | 16 (8.0) | 30 (7.3) | 0.739 | |
| 7 (3.5) | 23 (10.8) | 0.004 | ||
| Thromboembolism | 2 (1.0) | 2 (0.5) | 0.600 | |
| 2 (1.0) | 0 (0.0) | 0.233 | ||
| Pneumonia | 9 (4.5) | 3 (0.7) | 0.003 | |
| 2 (1.0) | 1 (0.5) | 0.612 | ||
| Urinary tract infection | 9 (4.5) | 9 (2.2) | 0.108 | |
| 2 (1.0) | 7 (3.3) | 0.177 | ||
| Death | 11 (5.5) | 10 (2.4) | 0.049 | |
| 3 (1.5) | 8 (3.8) | 0.157 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nothofer, S.; Geipel, J.; Aehling, K.; Sommer, B.; Heller, A.R.; Shiban, E.; Simon, P. Postoperative Surveillance in the Postoperative vs. Intensive Care Unit for Patients Undergoing Elective Supratentorial Brain Tumor Removal: A Retrospective Observational Study. J. Clin. Med. 2025, 14, 2632. https://doi.org/10.3390/jcm14082632
Nothofer S, Geipel J, Aehling K, Sommer B, Heller AR, Shiban E, Simon P. Postoperative Surveillance in the Postoperative vs. Intensive Care Unit for Patients Undergoing Elective Supratentorial Brain Tumor Removal: A Retrospective Observational Study. Journal of Clinical Medicine. 2025; 14(8):2632. https://doi.org/10.3390/jcm14082632
Chicago/Turabian StyleNothofer, Stefanie, Julia Geipel, Kathrin Aehling, Björn Sommer, Axel Rüdiger Heller, Ehab Shiban, and Philipp Simon. 2025. "Postoperative Surveillance in the Postoperative vs. Intensive Care Unit for Patients Undergoing Elective Supratentorial Brain Tumor Removal: A Retrospective Observational Study" Journal of Clinical Medicine 14, no. 8: 2632. https://doi.org/10.3390/jcm14082632
APA StyleNothofer, S., Geipel, J., Aehling, K., Sommer, B., Heller, A. R., Shiban, E., & Simon, P. (2025). Postoperative Surveillance in the Postoperative vs. Intensive Care Unit for Patients Undergoing Elective Supratentorial Brain Tumor Removal: A Retrospective Observational Study. Journal of Clinical Medicine, 14(8), 2632. https://doi.org/10.3390/jcm14082632







