Biofilm Growth on Different Materials Used in Contemporary Femoral Head Prosthesis: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Femoral Head Prostheses
2.2. Bacterial Strains
2.3. Study Design
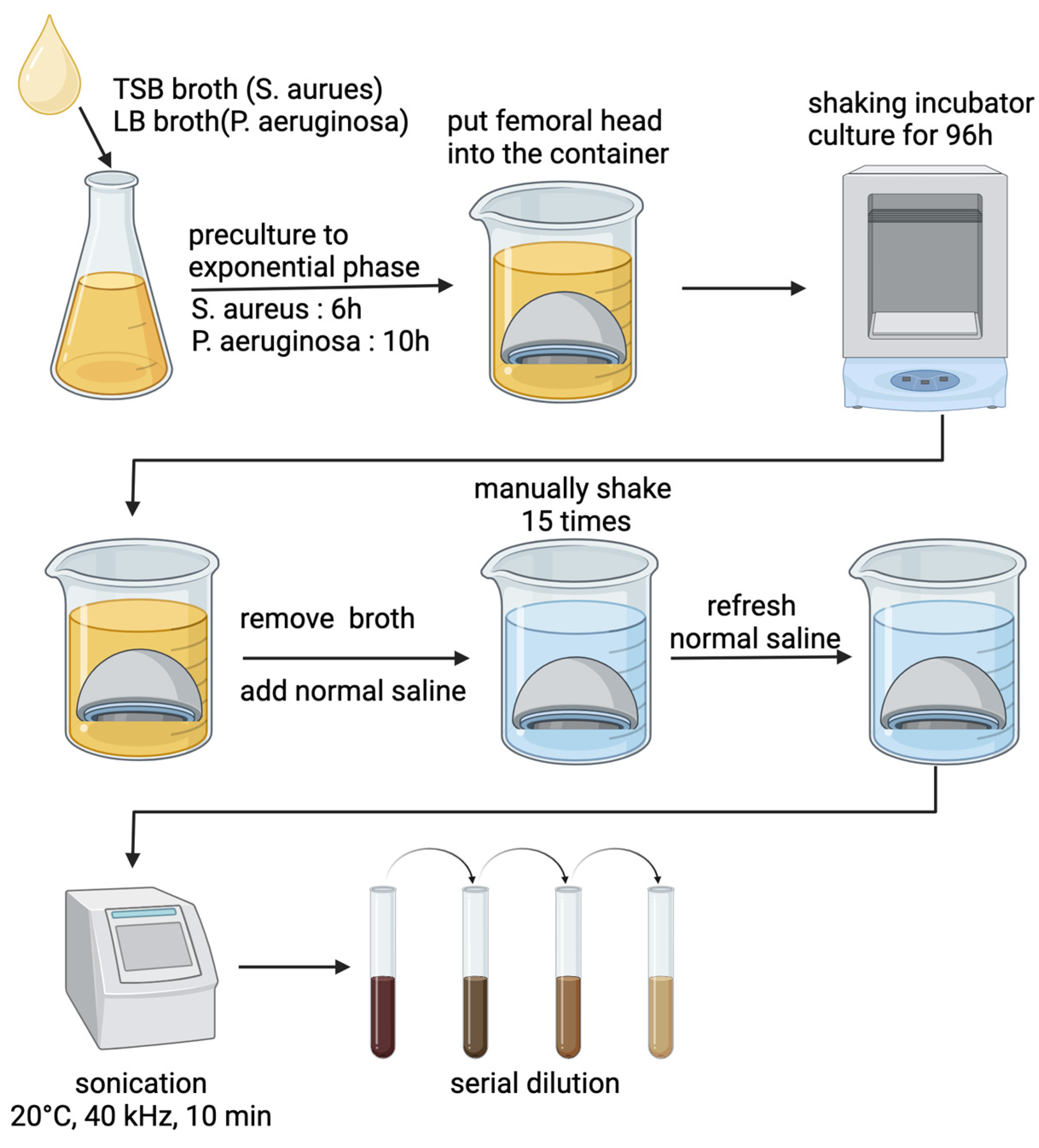
2.4. Biofilm Formation on Femoral Heads
2.5. Adherent Bacterial Quantification
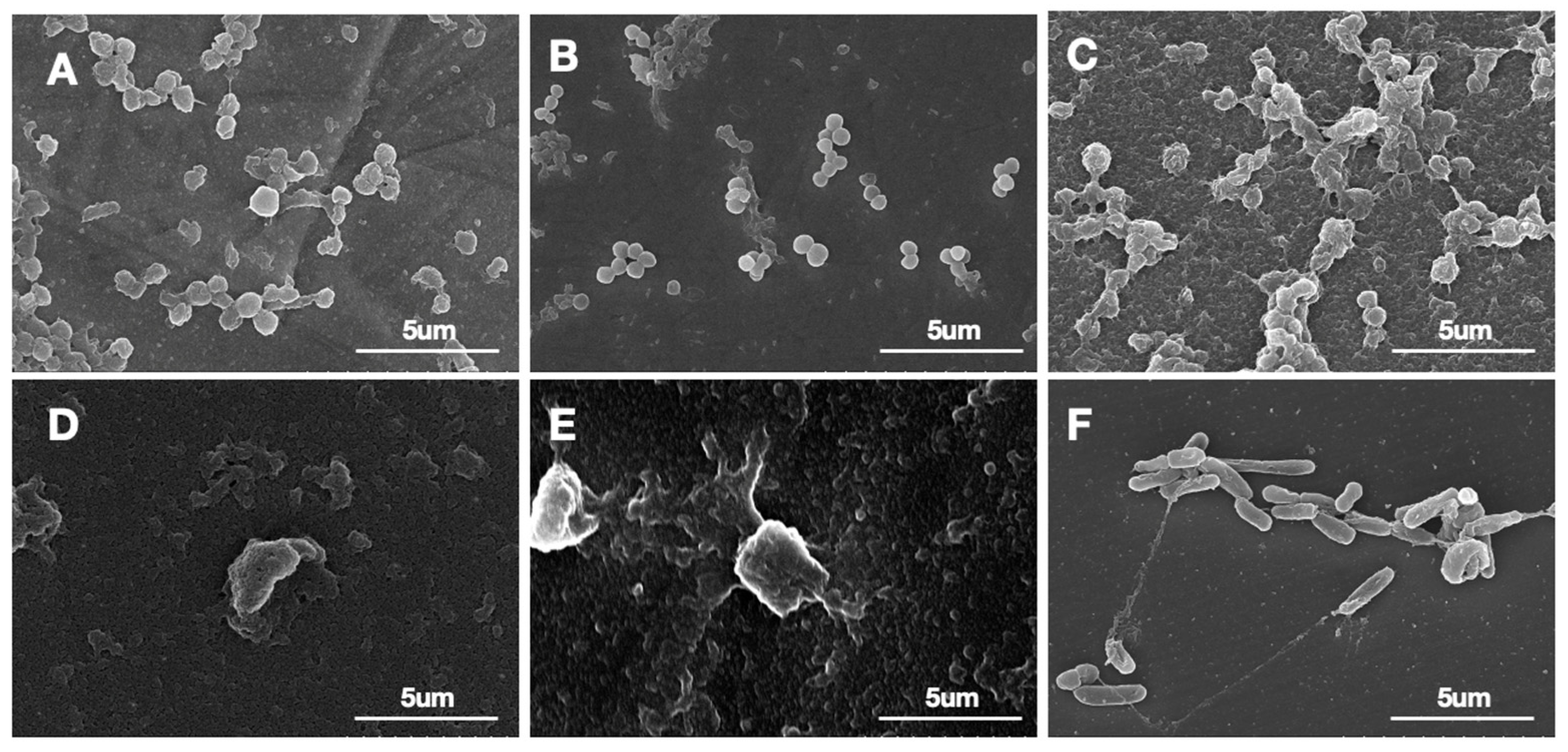
2.6. Scanning Electron Microscopy (SEM) Evaluation
2.7. Crystal Violet Staining
2.8. Statistical Analysis
3. Results
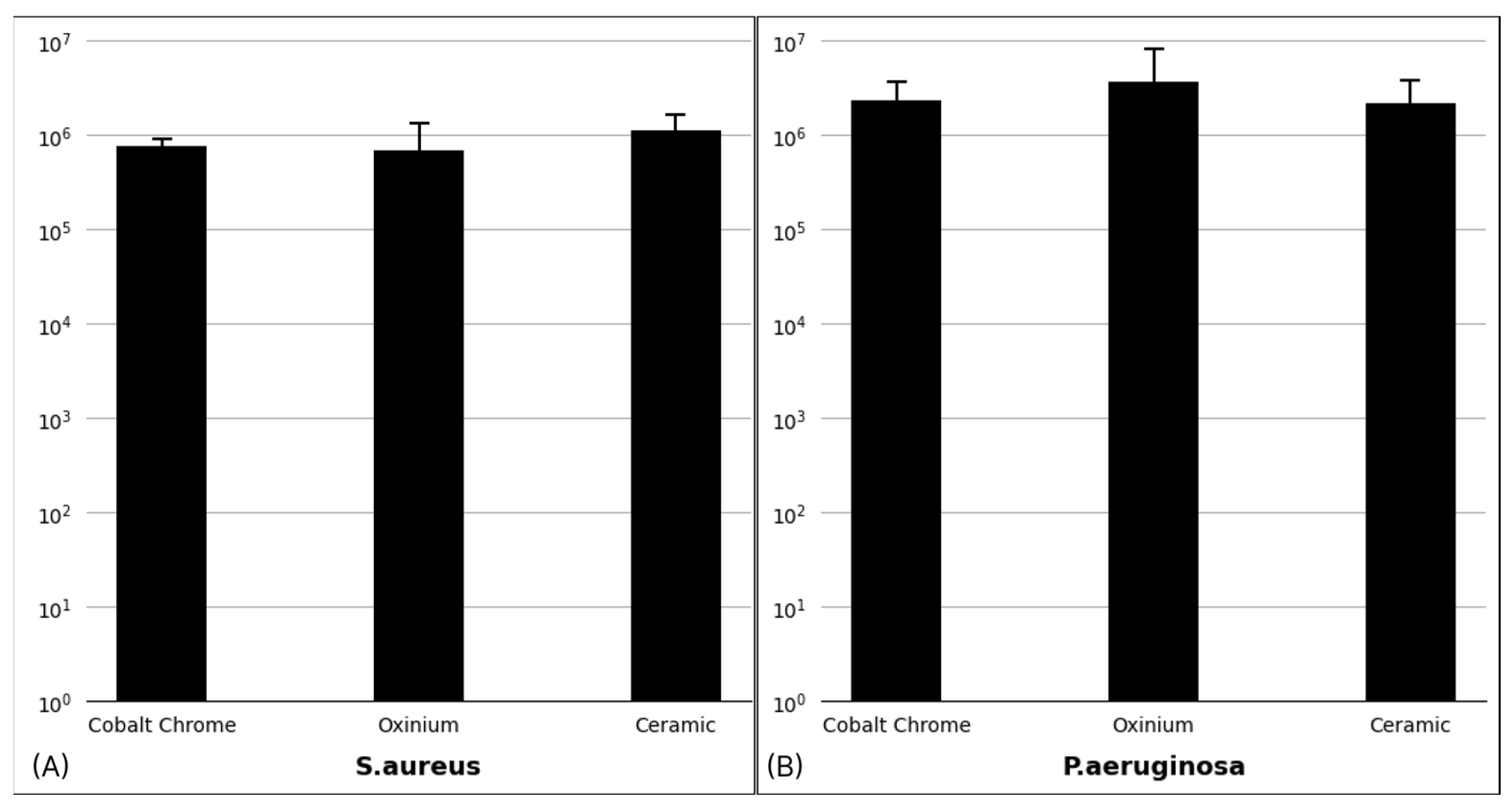
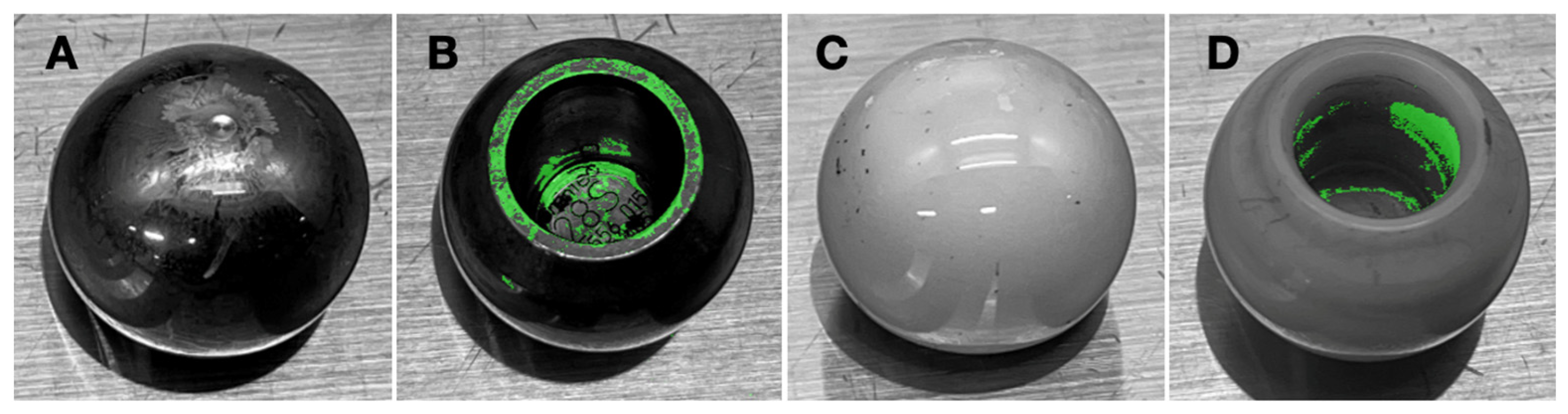
3.1. Biofilm Confirmation
3.2. Biofilm Distribution
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, S.B.; Pinkney, J.A.; Chen, A.F.; Tande, A.J. Periprosthetic joint infection: Current clinical challenges. Clin. Infect. Dis. 2023, 77, e34–e45. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.P.; Stambough, J.B.; Huddleston, J.I., III; Levine, B.R. Highlights of the 2023 American Joint Replacement Registry Annual Report. Arthroplast. Today 2024, 26, 101325. [Google Scholar] [CrossRef] [PubMed]
- Mundi, R.; Pincus, D.; Schemitsch, E.; Ekhtiari, S.; Paterson, J.M.; Chaudhry, H.; Leis, J.A.; Redelmeier, D.A.; Ravi, B. Association between periprosthetic joint infection and mortality following primary total hip arthroplasty. JBJS 2024, 106, 1546–1552. [Google Scholar] [CrossRef]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Le Vavasseur, B.; Zeller, V. Antibiotic therapy for prosthetic joint infections: An overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Nag, T.C. A comparison of bacterial adhesion and biofilm formation on commonly used orthopaedic metal implant materials: An in vitro study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Koseki, H.; Yonekura, A.; Shida, T.; Yoda, I.; Horiuchi, H.; Morinaga, Y.; Yanagihara, K.; Sakoda, H.; Osaki, M.; Tomita, M. Early staphylococcal biofilm formation on solid orthopaedic implant materials: In vitro study. PLoS ONE 2014, 9, e107588. [Google Scholar] [CrossRef] [PubMed]
- Karbysheva, S.; Grigoricheva, L.; Golnik, V.; Popov, S.; Renz, N.; Trampuz, A. Influence of retrieved hip-and knee-prosthesis biomaterials on microbial detection by sonication. Eur. Cell Mater. 2019, 37, 16–22. [Google Scholar] [CrossRef]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorli, L.; Cobo, J.; Fernandez-Sampedro, M.; Del Toro, M.D.; Guío, L. The different microbial etiology of prosthetic joint infections according to route of acquisition and time after prosthesis implantation, including the role of multidrug-resistant organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Ricker, E.B.; Aljaafari, H.A.; Bader, T.M.; Hundley, B.S.; Nuxoll, E. Thermal shock susceptibility and regrowth of Pseudomonas aeruginosa biofilms. Int. J. Hyperth. 2018, 34, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Aruni, W.; Inceoglu, S.; Akpolat, Y.T.; Botimer, G.D.; Cheng, W.K.; Danisa, O.A. A comparison of Staphylococcus aureus biofilm formation on cobalt-chrome and titanium-alloy spinal implants. J. Clin. Neurosci. 2016, 31, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.; Veena, K.; Senthil, R.; Iswamy, K.; Ponmalar, E.M.; Mariappan, V.; Girija, A.S.; Vadivelu, J.; Nagarajan, S.; Challabathula, D. Biofilm-associated Agr and Sar quorum sensing systems of Staphylococcus aureus are inhibited by 3-hydroxybenzoic acid derived from Illicium verum. ACS Omega 2022, 7, 14653–14665. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D. Activity of antimicrobial peptides and ciprofloxacin against Pseudomonas aeruginosa biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef]
- Bjerkan, G.; Witsø, E.; Bergh, K. Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop. 2009, 80, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.; Canova, R.; Esper, L.M.; Perdoncini, G.; Nascimento, V.d.; Pilotto, F.; Santos, L.D.; Rodrigues, L.B. The use of vortex and ultrasound techniques for the in vitro removal of Salmonella spp. biofilms. Acta Sci. Vet. 2015, 43, 1332. [Google Scholar]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy methods for biofilm imaging: Focus on SEM and VP-SEM pros and cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protocols 2016, 2016, pdb-prot087379. [Google Scholar] [CrossRef]
- Kamimura, R.; Kanematsu, H.; Ogawa, A.; Kogo, T.; Miura, H.; Kawai, R.; Hirai, N.; Kato, T.; Yoshitake, M.; Barry, D.M. Quantitative analyses of biofilm by using crystal violet staining and optical reflection. Materials 2022, 15, 6727. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Chao, C.; Khilnani, T.K.; Shenoy, A.; Bostrom, M.P.; Carli, A.V. The Infected Polypropylene Mesh: When Does Biofilm Form and Which Antiseptic Solution Most Effectively Removes It? J. Arthroplast. 2024, 39, S294–S299. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.A.; Bakr, H.M. Updates in biomaterials of bearing surfaces in total hip arthroplasty. Arthroplasty 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Ruggiero, A.; Merola, M. Advanced biomaterials in hip joint arthroplasty. A review on polymer and ceramics composites as alternative bearings. Compos. Part B Eng. 2015, 83, 276–283. [Google Scholar] [CrossRef]
- McEntire, B.; Bal, B.S.; Rahaman, M.; Chevalier, J.; Pezzotti, G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2015, 35, 4327–4369. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Milan, A.; Del Lupo, V.; Maglione, M.; Dolzani, L. Biofilms developed on dental implant titanium surfaces with different roughness: Comparison between in vitro and in vivo studies. Curr. Microbiol. 2018, 75, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.; Kim, H.-Y.; Lim, K.S.; Han, J.-S. Implant surface factors and bacterial adhesion: A review of the literature. Int. J. Artif. Organs 2012, 35, 762–772. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Mai, K.T.; Verioti, C.; D’Lima, D.; Colwell Jr, C.W.; Ezzett, K.A. Surface roughness of femoral head prostheses after dislocation. Am. J. Orthop. 2010, 39, 495. [Google Scholar]
- Villapun Puzas, V.M.; Carter, L.N.; Schröder, C.; Colavita, P.E.; Hoey, D.A.; Webber, M.A.; Addison, O.; Shepherd, D.E.T.; Attallah, M.M.; Grover, L.M.; et al. Surface Free Energy Dominates the Biological Interactions of Postprocessed Additively Manufactured Ti-6Al-4V. ACS Biomater. Sci. Eng. 2022, 8, 4311–4326. [Google Scholar] [CrossRef]
- Hays, M.R.; Kildow, B.J.; Hartman, C.W.; Lyden, E.R.; Springer, B.D.; Fehring, T.K.; Garvin, K.L. Increased incidence of methicillin-resistant Staphylococcus aureus in knee and hip prosthetic joint infection. J. Arthroplast. 2023, 38, S326–S330. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Gupta, P. Polymicrobial interaction in biofilm: Mechanistic insights. Pathog. Dis. 2022, 80, ftac010. [Google Scholar] [CrossRef] [PubMed]
- Najjar, D.; Bigerelle, M.; Migaud, H.; Iost, A. Identification of scratch mechanisms on a retrieved metallic femoral head. Wear 2005, 258, 240–250. [Google Scholar] [CrossRef]
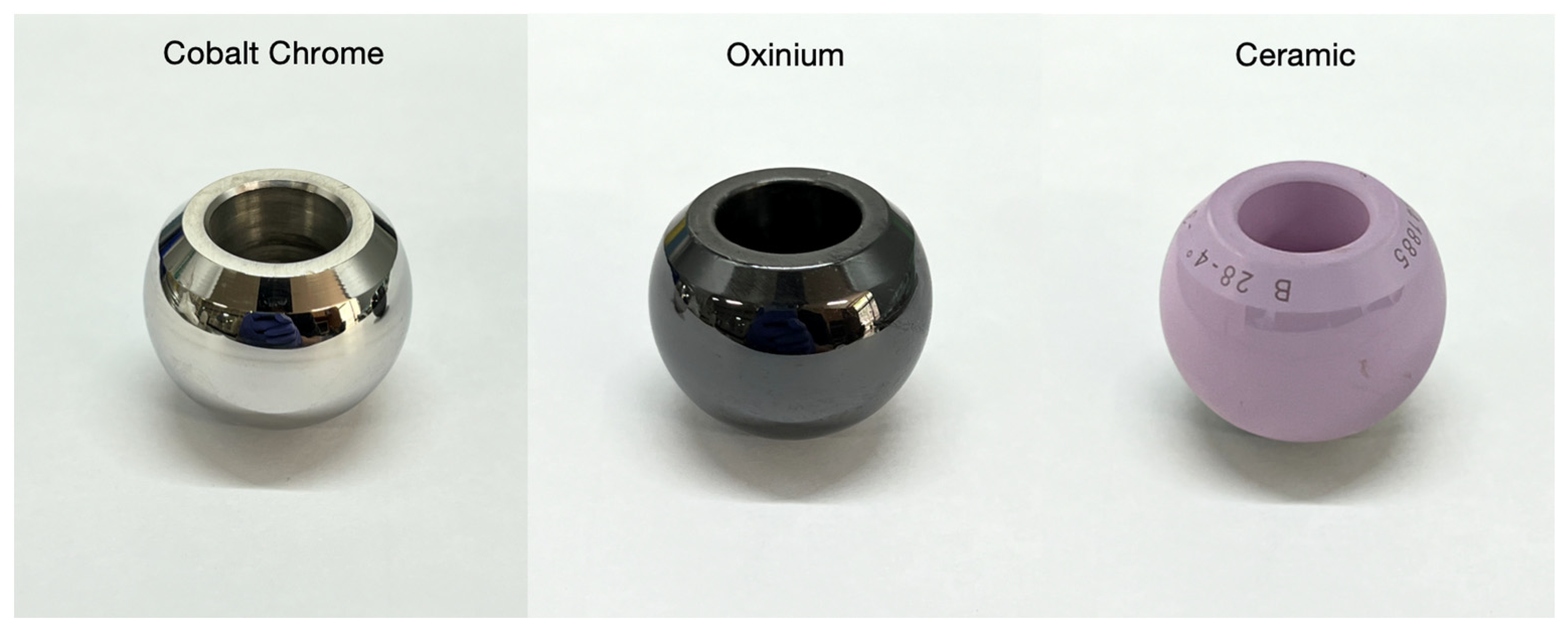
| Material | Reasons to Use | Reasons to Avoid |
|---|---|---|
| cobalt–chrome [24] | Resistance to mechanical failure cost effective | Releases metal ion Higher wear particle production |
| oxinium [25] | Wear resistance Reduces release of metal ions | Higher cost Limited long-term data |
| ceramic [24] | Wear and scratch resistance Lower wear particle High biocompatibility | Brittleness May make noise Expensive than CoCr heads |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.; Hong, J.; Choi, S.; Kim, H.; Sohn, H.M.; Jo, S. Biofilm Growth on Different Materials Used in Contemporary Femoral Head Prosthesis: An In Vitro Study. J. Clin. Med. 2025, 14, 1722. https://doi.org/10.3390/jcm14051722
Moon Y, Hong J, Choi S, Kim H, Sohn HM, Jo S. Biofilm Growth on Different Materials Used in Contemporary Femoral Head Prosthesis: An In Vitro Study. Journal of Clinical Medicine. 2025; 14(5):1722. https://doi.org/10.3390/jcm14051722
Chicago/Turabian StyleMoon, Yonggyun, Jaeyoung Hong, Sookyung Choi, Hyoungtae Kim, Hong Moon Sohn, and Suenghwan Jo. 2025. "Biofilm Growth on Different Materials Used in Contemporary Femoral Head Prosthesis: An In Vitro Study" Journal of Clinical Medicine 14, no. 5: 1722. https://doi.org/10.3390/jcm14051722
APA StyleMoon, Y., Hong, J., Choi, S., Kim, H., Sohn, H. M., & Jo, S. (2025). Biofilm Growth on Different Materials Used in Contemporary Femoral Head Prosthesis: An In Vitro Study. Journal of Clinical Medicine, 14(5), 1722. https://doi.org/10.3390/jcm14051722





