Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy: A Meta-Analysis of Prevalence and Impact on Left Ventricular Function and Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Abstraction and Quality Assessment
2.3. Statistical Analysis
3. Results
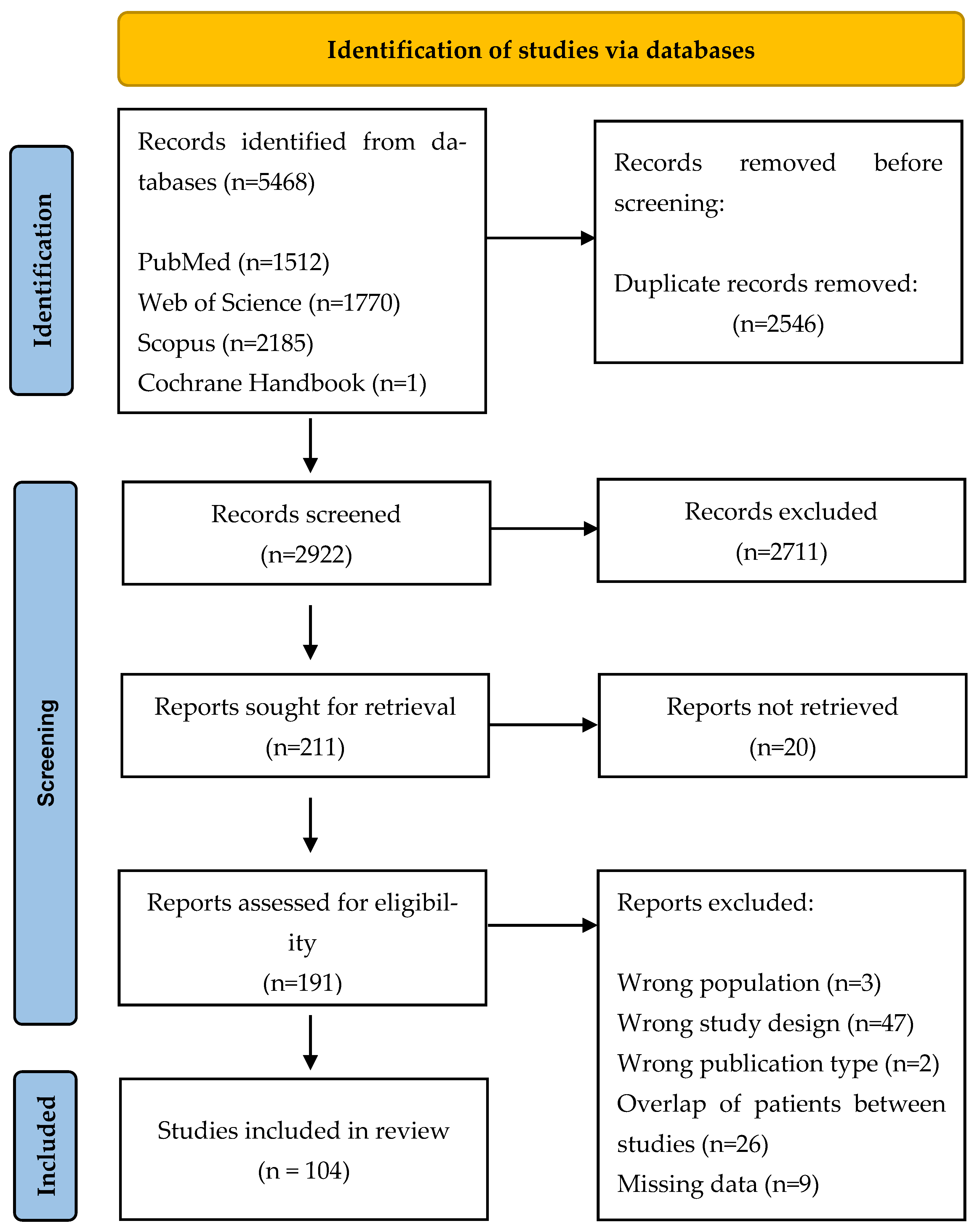
3.1. Search Results
3.2. Characteristics of Eligible Studies
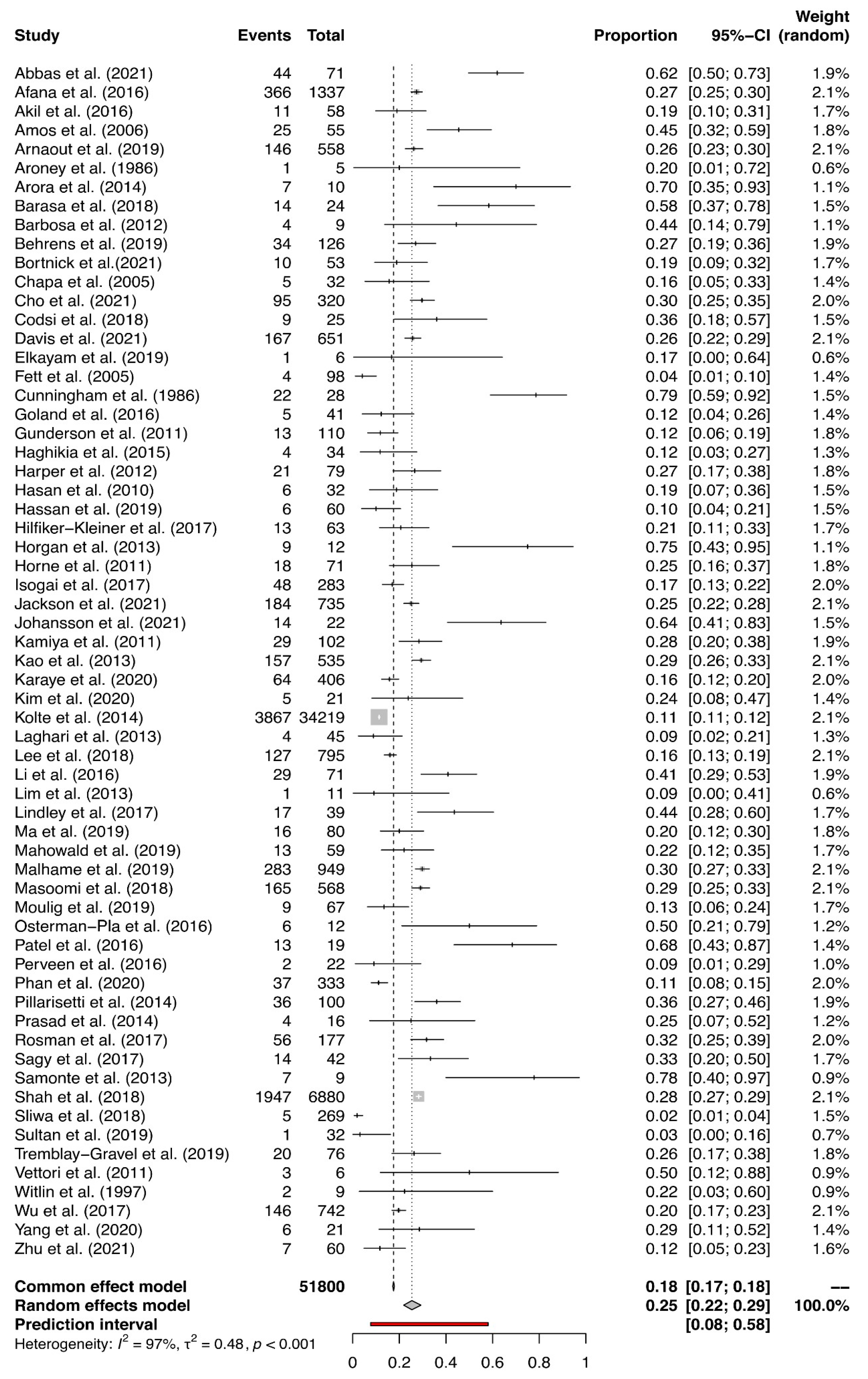
3.3. Prevalence of HDPs and PE in PPCM-Affected Pregnancies
3.4. Results of the Meta-Analysis
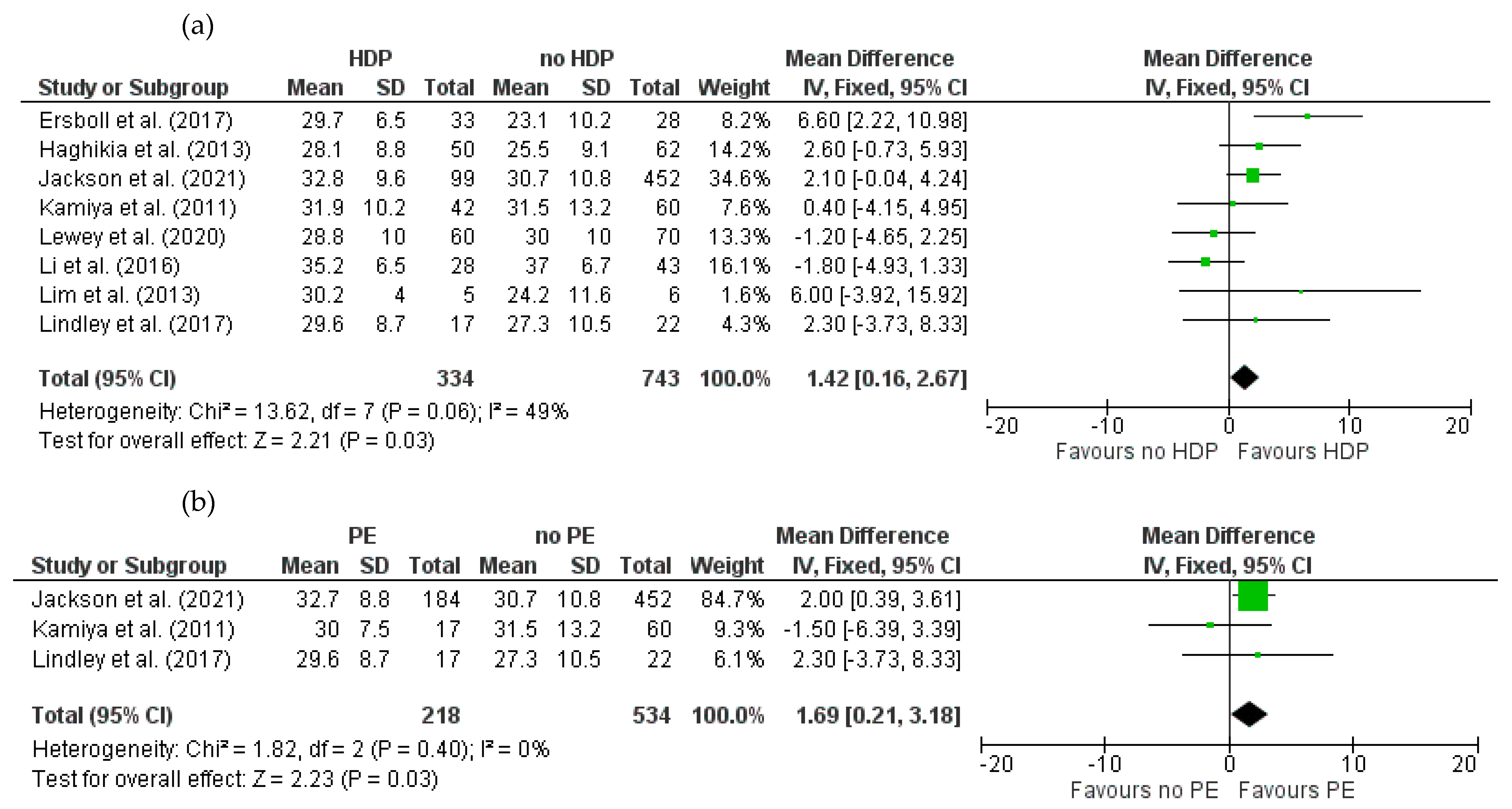
3.4.1. Baseline LVEF for HDPs and PE in Women with PPCM
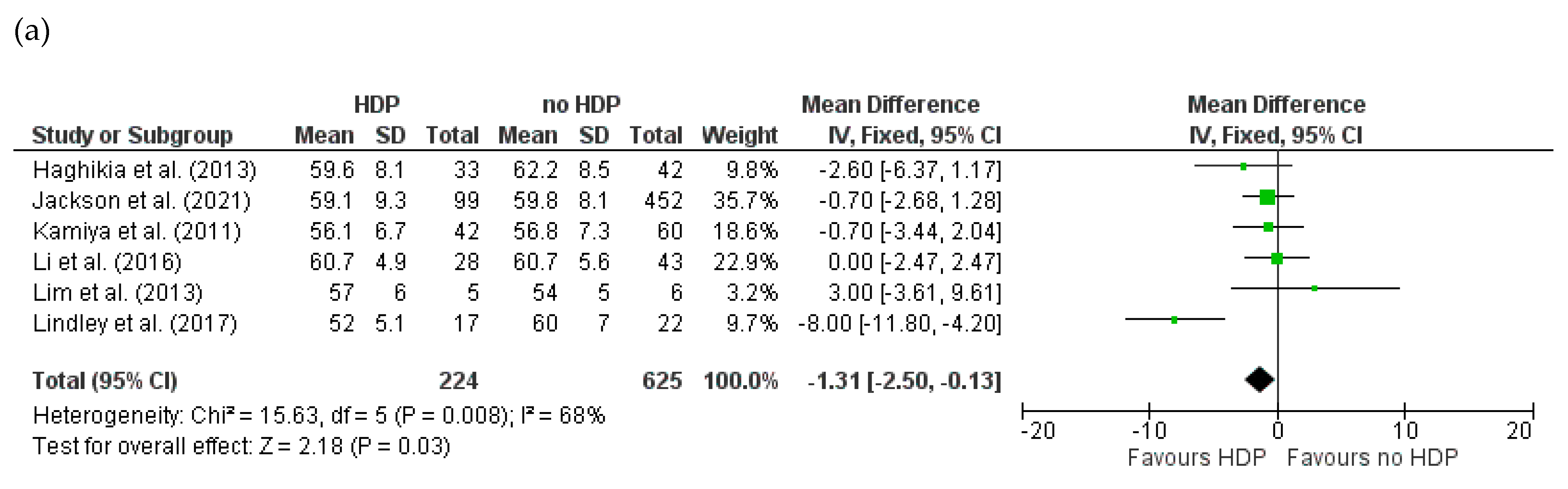
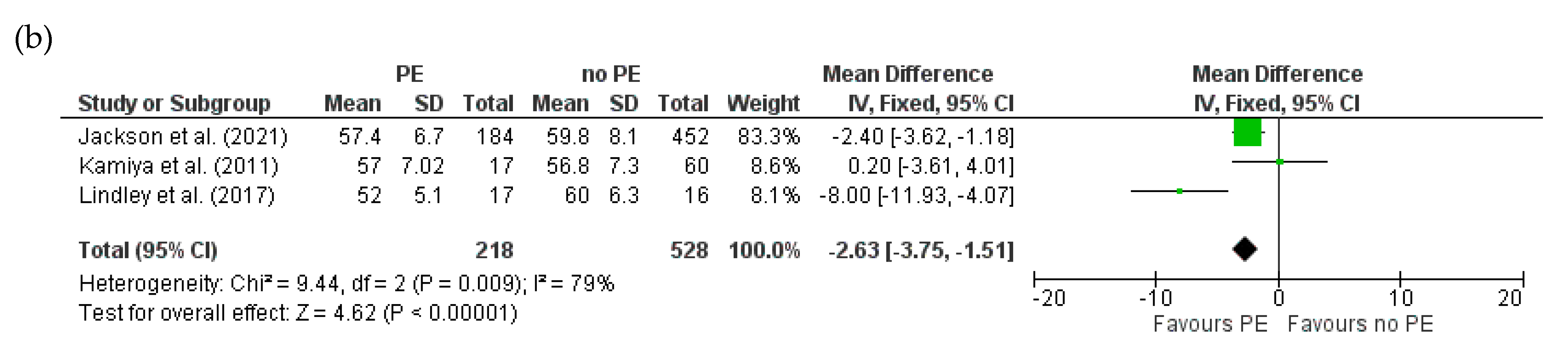
3.4.2. Baseline LVEDD for HDPs and PE in Women with PPCM
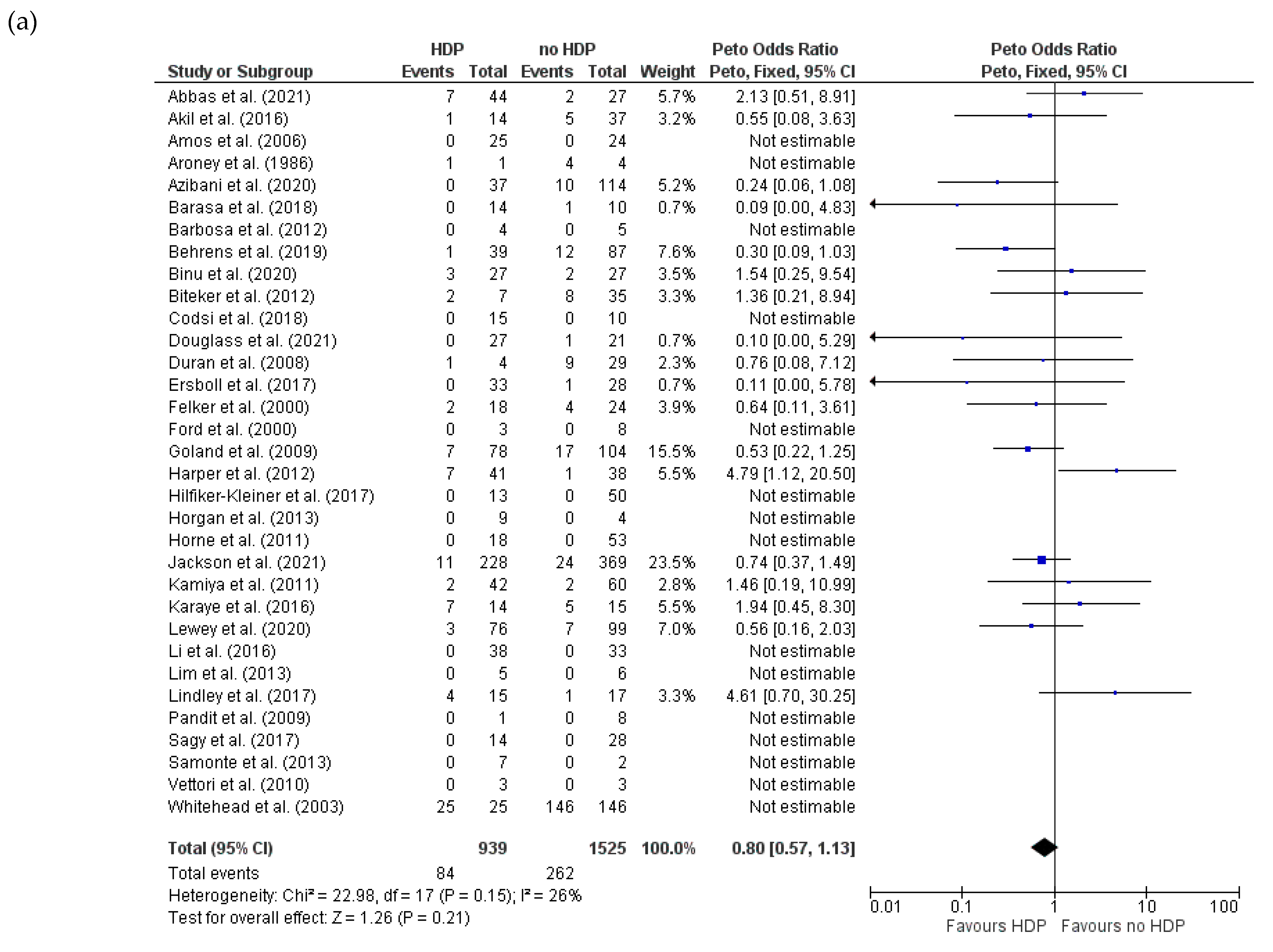
3.4.3. Mortality for HDPs and PE in Women with PPCM
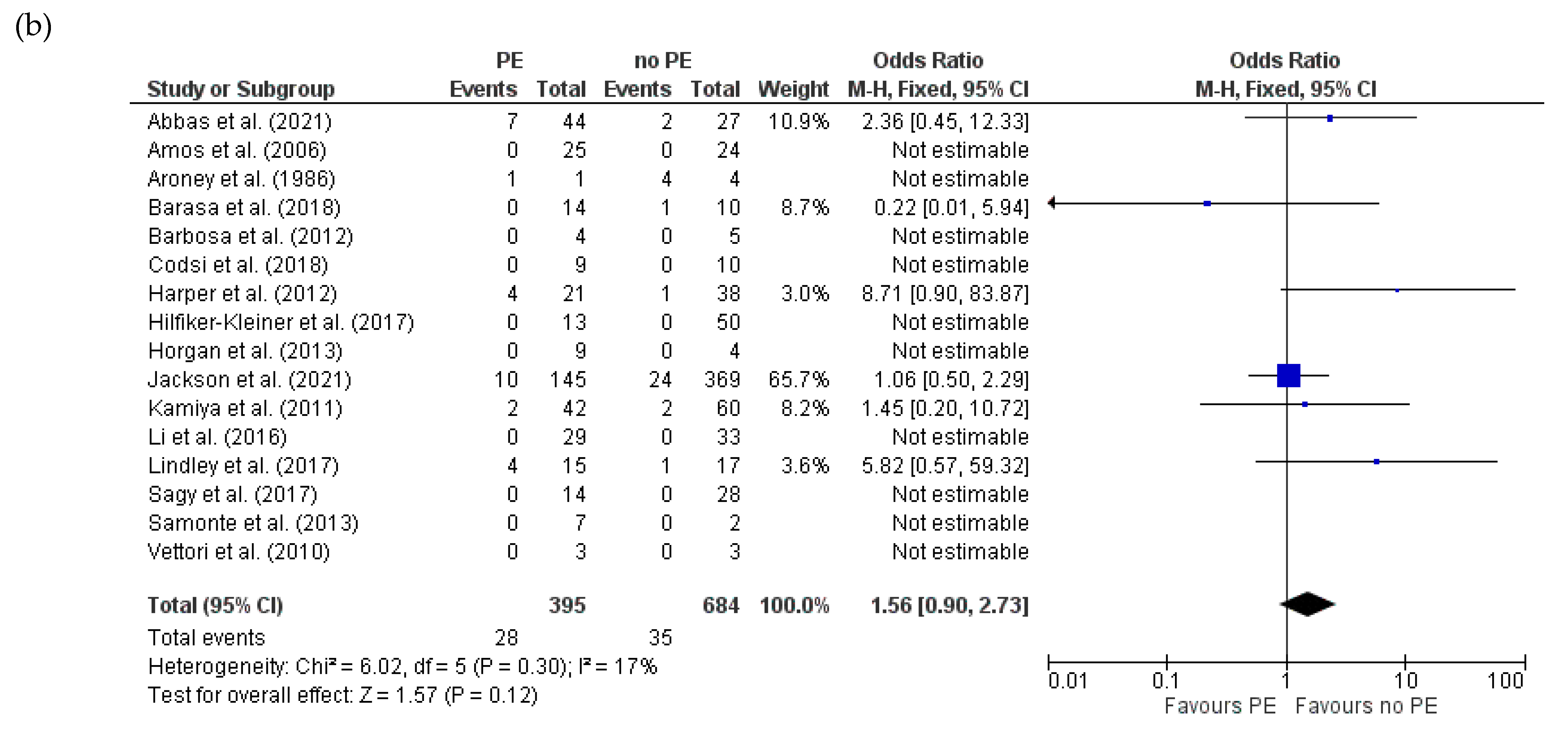
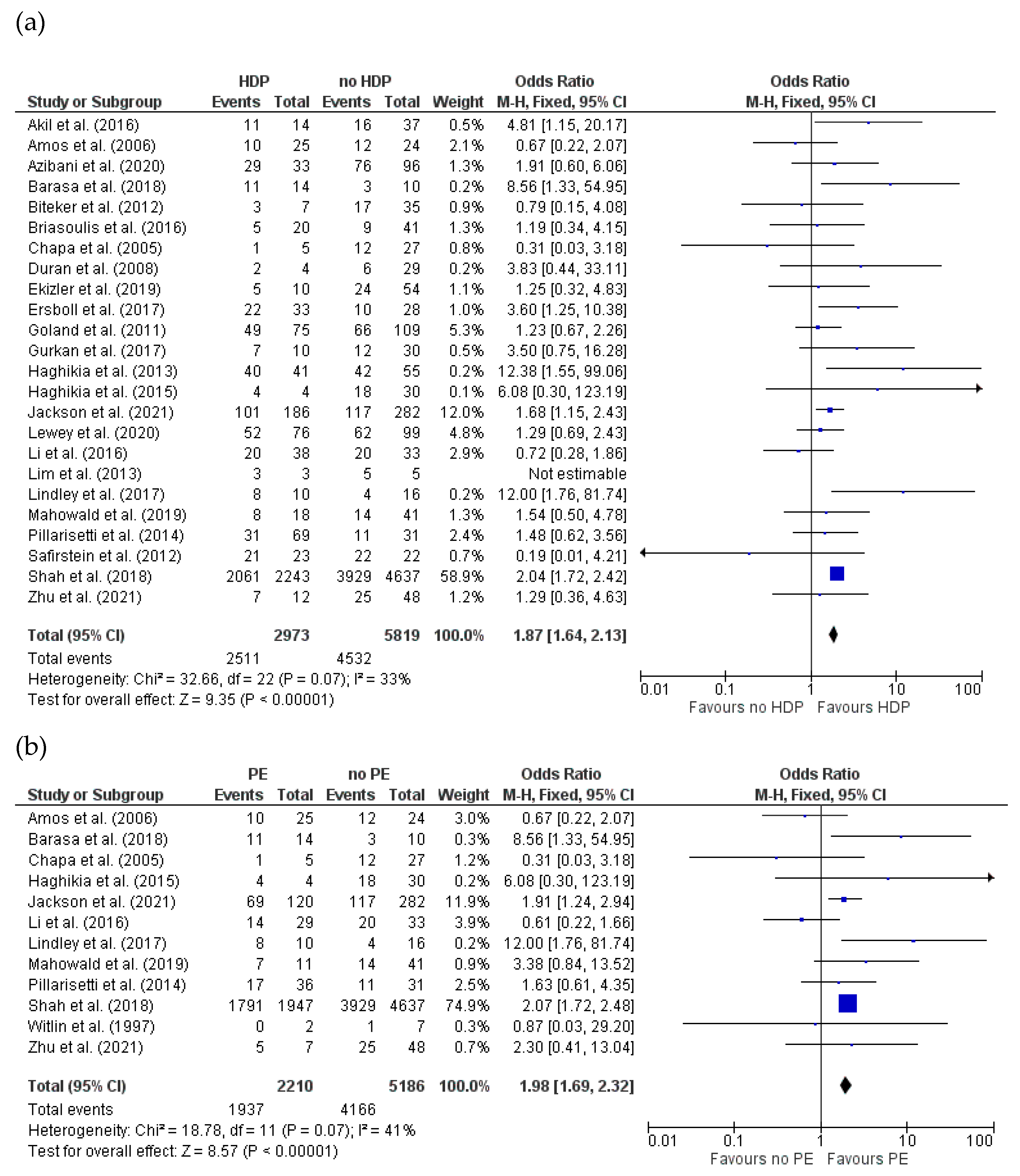
3.4.4. Recovery of LVEF (>50%) for HDPs and PE in Women with PPCM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Demakis, J.G.; Rahimtoola, S.H.; Sutton, G.C.; Meadows, W.R.; Szanto, P.B.; Tobin, J.R.; Gunnar, R.M. Natural course of peripartum cardiomyopathy. Circulation 1971, 44, 1053–1061. [Google Scholar] [CrossRef]
- Pearson, G.D.; Veille, J.-C.; Rahimtoola, S.; Hsia, J.; Oakley, C.M.; Hosenpud, J.D.; Ansari, A.; Baughman, K.L. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000, 283, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; Konig, T.; Meer, P.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mbakwem, A.; Hamdan, R.; Jackson, A.M.; Forsyth, P.; Boer, R.A.; et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 827. [Google Scholar] [CrossRef]
- Viljoen, C.; Hoevelmann, J.; Sliwa, K. Peripartum cardiomyopathy: Risk factors and predictors of outcome. Curr. Opin. Cardiol. 2023, 38, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Fett, J.D.; Christie, L.G.; Carraway, R.D.; Murphy, J.G. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin. Proc. 2005, 80, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Karaye, K.M.; Ishaq, N.A.; Sa’idu, H.; Balarabe, S.A.; Talle, M.A.; Isa, M.S.; Adamu, U.G.; Umar, H.; Okolie, H.I.; Shehu, M.N.; et al. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: Results from the PEACE Registry. ESC Heart Fail. 2020, 7, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Ahn, C.; Jain, D.; Gass, A.; Ahmed, A.; Panza, J.A.; et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: A nationwide population-based study. J. Am. Heart Assoc. 2014, 3, e001056. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Croen, L.A.; Chiang, V.; Yoshida, C.K.; Walton, D.; Go, A.S. Epidemiology of peripartum cardiomyopathy: Inci-dence, predictors, and outcomes. Obstet. Gynecol. 2011, 118, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.; Rendon, I.S.H.; Arany, Z. The relationship between preeclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 1715–1723. [Google Scholar] [CrossRef]
- Garovic, V.D.; White, W.M.; Vaughan, L.; Saiki, M.; Parashuram, S.; Garcia-Valencia, O.; Weissgerber, T.L.; Milic, N.; Weaver, A.; Mielke, M.M. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J. Am. Coll. Cardiol. 2020, 75, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Roh, J.D.; Castro, C.; Yu, A.; Rana, S.; Shahul, S.; Gray, K.J.; Honigberg, M.C.; Ricke-Hoch, M.; Iwamoto, Y.; Yeri, A.; et al. Placental senescence pathophysiology is shared between peripartum cardiomyopathy and preeclampsia in mouse and human. Sci. Transl. Med. 2024, 16, eadi0077. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Blauwet, L. Peripartum Cardiomyopathy and Preeclampsia: Overlapping Diseases of Pregnancy. Curr. Hypertens. Rep. 2018, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Seronde, M.-F.; Gayat, E.; Tibazarwa, K.; Anumba, D.O.; Akrout, N.; Sadoune, M.; Sarb, J.; Arrigo, M.; Motiejunaite, J.; et al. Imbalanced Angiogenesis in Peripartum Cardiomyopathy—Diagnostic Value of Placenta Growth Factor. Circ. J. 2017, 81, 1654–1661. [Google Scholar] [CrossRef]
- Barasa, A.; Goloskokova, V.; Ladfors, L.; Patel, H.; Schaufelberger, M. Symptomatic recovery and pharmacological management in a clinical cohort with peripartum cardiomyopathy. J. Matern.-Fetal Neonatal Med. 2018, 31, 1342–1349. [Google Scholar] [CrossRef]
- Jackson, A.M.; Petrie, M.C.; Frogoudaki, A.; Laroche, C.; Gustafsson, F.; Ibrahim, B.; Mebazaa, A.; Johnson, M.R.; Seferovic, P.M.; Regitz-Zagrosek, V.; et al. Hypertensive disorders in women with peripartum cardiomyopathy: Insights from the ESC EORP PPCM Registry. Eur. J. Heart Fail. 2021, 23, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Ntusi, N.B.A.; Badri, M.; Gumedze, F.; Sliwa, K.; Mayosi, B.M. Pregnancy-Associated Heart Failure: A Comparison of Clinical Presentation and Outcome between Hypertensive Heart Failure of Pregnancy and Idiopathic Peripartum Cardiomyopathy. PLoS ONE 2015, 10, e0133466. [Google Scholar] [CrossRef]
- Pillarisetti, J.; Kondur, A.; Alani, A.; Reddy, M.; Reddy, M.; Vacek, J.; Weiner, C.P.; Ellerbeck, E.; Schreiber, T.; Lakkireddy, D. Peripartum Cardiomyopathy: Predictors of Recovery and Current State of Implantable Cardioverter-Defibrillator Use. J. Am. Coll. Cardiol. 2014, 63 Pt A, 2831–2839. [Google Scholar] [CrossRef]
- Lewey, J.; Levine, L.D.; Elovitz, M.A.; Irizarry, O.C.; Arany, Z. Importance of Early Diagnosis in Peripartum Cardiomyopathy. Hypertension 2020, 75, 91–97. [Google Scholar] [CrossRef]
- Nugrahani, A.D.; Maulana, S.; Tjandraprawira, K.D.; Santoso, D.P.J.; Setiawan, D.; Pribadi, A.; Siddiq, A.; Pramatirta, A.Y.; Aziz, M.A.; Irianti, S. Analysis of Clinical Profiles and Echocardiographic Cardiac Outcomes in Peripartum Cardiomyopathy (PPCM) vs. PPCM with Co-Existing Hypertensive Pregnancy Disorder (HPD-PPCM) Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5303. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; A Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Me-ta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis: Ottawa Hospital Research Institute Website. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 January 2025).
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Abbas, S.; Yousfani, S.; Shaikh, F.; Sultana, F.; Shaikh, N.; Tahira, S. Association of Peripartum Cardiomyopathy with Pre-eclampsia and Maternal Outcome. J. Pharm. Res. Int. 2021, 33, 110–115. [Google Scholar] [CrossRef]
- Afana, M.; Brinjikji, W.; Kao, D.; Jackson, E.; Maddox, T.M.; Childers, D.; Eagle, K.A.; Davis, M.B. Characteristics and In-Hospital Outcomes of Peripartum Cardiomyopathy Diagnosed During Delivery in the United States from the Nationwide Inpatient Sample (NIS) Database. J. Card. Fail. 2016, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Akil, M.A.; Bilik, M.Z.; Yildiz, A.; Acet, H.; Ertas, F.; Simsek, H.; Polat, N.; Zengin, H.; Akilli, R.; Agacayak, E.; et al. Peripartum cardiomyopathy in Turkey: Experience of three tertiary centres. J. Obstet. Gynaecol. 2016, 36, 574–580. [Google Scholar] [CrossRef]
- Amos, A.M.; Jaber, W.A.; Russell, S.D. Improved outcomes in peripartum cardiomyopathy with contemporary. Am. Heart J. 2006, 152, 509–513. [Google Scholar] [CrossRef]
- Arnaout, R.; Nah, G.; Marcus, G.; Tseng, Z.; Foster, E.; Harris, I.S.; Divanji, P.; Klein, L.; Gonzalez, J.; Parikh, N. Pregnancy com-plications and premature cardiovascular events among 1.6 million California pregnancies. Open Heart 2019, 6, e000927. [Google Scholar] [CrossRef] [PubMed]
- Aroney, C.; Khafagi, F.; Boyle, C.; Bett, N. Peripartum cardiomyopathy: Echocardiographic features in five cases. Am. J. Obstet. Gynecol. 1986, 155, 103–106. [Google Scholar] [CrossRef]
- Arora, N.P.; Mohamad, T.; Mahajan, N.; Danrad, R.; Kottam, A.; Li, T.; Afonso, L.C. Cardiac magnetic resonance imaging in per-ipartum cardiomyopathy. Am. J. Med. Sci. 2014, 347, 112–117. [Google Scholar] [CrossRef]
- Azibani, F.; Pfeffer, T.J.; Ricke-Hoch, M.; Dowling, W.; Pietzsch, S.; Briton, O.; Baard, J.; Abou Moulig, V.; König, T.; Berliner, D.; et al. Outcome in German and South African peripartum cardiomyopathy cohorts associates with medical therapy and fibrosis markers. ESC Heart Fail. 2020, 7, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.M.; Freire, C.M.; Nascimento, B.R.; Rochitte, C.E.; Silva, M.C.; Siqueira, M.H.; Nunes, M.C. Rest left ventricular function and contractile reserve by dobutamine stress echocardiography in peripartum cardiomyopathy. Rev. Port. Cardiol. 2012, 31, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Basit, S.; Lykke, J.A.; Ranthe, M.F.; Wohlfahrt, J.; Bundgaard, H.; Melbye, M.; Boyd, H.A. Hypertensive disorders of pregnancy and peripartum cardiomyopathy: A nationwide cohort study. PLoS ONE 2019, 14, e0211857. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Magriples, U. Cardiomyopathy in pregnancy: A retrospective study. Am. J. Perinatol. 2001, 18, 163–168. [Google Scholar] [CrossRef]
- Binu, A.J.; Rajan, S.J.; Rathore, S.; Beck, M.; Regi, A.; Thomson, V.S.; Sathyendra, S. Peripartum cardiomyopathy: An analysis of clinical profiles and outcomes from a tertiary care centre in southern India. Obstet. Med. 2020, 13, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Biteker, M.; Duran, N.E.; Kaya, H.; Gündüz, S.; Tanboğa, H.Î.; Gökdeniz, T.; Kahveci, G.; Akgün, T.; Yildiz, M.; Õzkan, M. Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin. Res. Cardiol. 2011, 100, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Biteker, M.; Özlek, B.; Özlek, E.; Çil, C.; Çelik, O.; Doğan, V.; Başaran, Ö. Predictors of early and delayed recovery in peripartum cardiomyopathy: A prospective study of 52 Patients. J. Matern. Fetal Neonatal Med. 2020, 33, 390–397. [Google Scholar] [CrossRef]
- Bortnick, A.E.; Lama von Buchwald, C.; Hasani, A.; Liu, C.; Berkowitz, J.L.; Vega, S.; Mustehsan, M.H.; Wolfe, D.S.; Taub, C. Per-sistence of abnormal global longitudinal strain in women with peripartum cardiomyopathy. Echocardiography 2021, 38, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Mocanu, M.; Marinescu, K.; Qaqi, O.; Palla, M.; Telila, T.; Afonso, L. Longitudinal systolic strain profiles and outcomes in peripartum cardiomyopathy. Echocardiography 2016, 33, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Brandao, A.; Martinez, E.E.; Alexopoulos, D.; Lima, V.C.; Andrade, J.L.; Ambrose, J.A. Prognosis in peripartum cardiomyopathy. Am. J. Cardiol. 1989, 64, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Ho, C.H.; Chen, J.Y.; Wu, M.P.; Yu, C.H.; Wang, J.J.; Chen, C.M.; Chu, C.C. Epidemiological profile and obstetric outcomes of patients with peripartum congestive heart failure in Taiwan: A retrospective nationwide study. BMC Pregnancy Childbirth 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Chapa, J.B.; Heiberger, H.B.; Weinert, L.; Decara, J.; Lang, R.M.; Hibbard, J.U. Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet. Gynecol. 2005, 105, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Leonard, S.A.; Lyndon, A.; Main, E.K.; Abrams, B.; Hameed, A.B.; Carmichael, S.L. Pre-pregnancy Obesity and the Risk of Peripartum Cardiomyopathy. Am. J. Perinatol. 2021, 38, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Codsi, E.; Rose, C.H.; Blauwet, L.A. Subsequent Pregnancy Outcomes in Patients With Peripartum Cardiomyopathy. Obstet. Gynecol. 2018, 131, 322–327. [Google Scholar] [CrossRef]
- Cuenza, L.R.; Manapat, N.; Jalique, J.R. Clinical Profi le and Predictors of Outcomes of Patients with Peripartum Cardiomyopathy: The Philippine Heart Center Experience. ASEAN Heart J. 2016, 24, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunningham, F.G.; Pritchard, J.A.; Hankins, G.D.; Anderson, P.L.; Lucas, M.J.; Armstrong, K.F. Peripartum heart failure: Idiopathic cardiomyopathy or compounding cardiovascular events? Obstet. Gynecol. 1986, 67, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Ewald, G.; Givertz, M.M.; Rajagopalan, N.; Cooper, L.T., Jr.; Briller, J.; Felker, G.M.; Bozkurt, B.; Drazner, M.H.; Hanley-Yanez, K.; et al. Maternal Obesity Affects Cardiac Remodeling and Recovery in Women with Peripartum Cardiomyopathy. Am. J. Perinatol. 2019, 36, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Jarvie, J.; Gambahaya, E.; Lindenfeld, J.; Kao, D. Risk Prediction for Peripartum Cardiomyopathy in Delivering Mothers: A Validated Risk Model: PPCM Risk Prediction Model. J. Card. Fail. 2021, 27, 159–167. [Google Scholar] [CrossRef]
- Dhesi, S.; Savu, A.; Ezekowitz, J.A.; Kaul, P. Association Between Diabetes During Pregnancy and Peripartum Cardiomyopathy: A Population-Level Analysis of 309,825 Women. Can. J. Cardiol. 2017, 33, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Douglass, E.J.; Cooper, L.T., Jr.; Morales-Lara, A.C.; Adedinsewo, D.A.; Rozen, T.D.; Blauwet, L.A.; Fairweather, D. A Case-Control Study of Peripartum Cardiomyopathy Using the Rochester Epidemiology Project. J. Card. Fail. 2021, 27, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Günes, H.; Duran, I.; Biteker, M.; Ozkan, M. Predictors of prognosis in patients with peripartum cardiomyopathy. Int. J. Gynaecol. Obstet. 2008, 101, 137–140. [Google Scholar] [CrossRef]
- Ekizler, F.A.; Cay, S. A novel marker of persistent left ventricular systolic dysfunction in patients with peripartum cardiomyopathy: Monocyte count- to- HDL cholesterol ratio. BMC Cardiovasc. Disord. 2019, 19, 114. [Google Scholar] [CrossRef]
- Elkayam, U.; Schäfer, A.; Chieffo, A.; Lansky, A.; Hall, S.; Arany, Z.; Grines, C. Use of Impella heart pump for management of women with peripartum cardiogenic shock. Clin. Cardiol. 2019, 42, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, A.S.; Johansen, M.; Damm, P.; Rasmussen, S.; Vejlstrup, N.G.; Gustafsson, F. Peripartum cardiomyopathy in Denmark: A retrospective, population-based study of incidence, management and outcome. Eur. J. Heart Fail. 2017, 19, 1712–1720. [Google Scholar] [CrossRef]
- Farhan, H.A.; Yaseen, I.F. Peripartum cardiomyopathy in Iraq: Initial registry-based data and 6 month outcomes. ESC Heart Fail. 2021, 8, 4048–4054. [Google Scholar] [CrossRef]
- Felker, G.M.; Jaeger, C.J.; Klodas, E.; Thiemann, D.R.; Hare, J.M.; Hruban, R.H.; Kasper, E.K.; Baughman, K.L. Myocarditis and long-term survival in peripartum cardiomyopathy. Am. Heart J. 2000, 140, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.F.; Barton, J.R.; O’brien, J.M.; Hollingsworth, P.W. Demographics, management, and outcome of peripartum cardiomyo-pathy in a community hospital. Am. J. Obstet. Gynecol. 2000, 182, 1036–1038. [Google Scholar] [CrossRef]
- Gambahaya, E.T.; Hakim, J.; Kao, D.; Munyandu, N.; Matenga, J. Peripartum cardiomyopathy among cardiovascular patients referred for echocardiography at Parirenyatwa Teaching Hospital, Harare, Zimbabwe. Cardiovasc. J. Afr. 2017, 28, 8–13. [Google Scholar] [CrossRef]
- Goland, S.; Bitar, F.; Modi, K.; Safirstein, J.; Ro, A.; Mirocha, J.; Khatri, N.; Elkayam, U. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J. Card. Fail. 2011, 17, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Goland, S.; Weinstein, J.M.; Zalik, A.; Kuperstein, R.; Zilberman, L.; Shimoni, S.; Arad, M.; Ben Gal, T.; George, J. Angiogenic Im-balance and Residual Myocardial Injury in Recovered Peripartum Cardiomyopathy Patients. Circ. Heart Fail. 2016, 9, e003349. [Google Scholar] [CrossRef] [PubMed]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; DePalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.E.; et al. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef]
- Guldbrandt Hauge, M.; Johansen, M.; Vejlstrup, N.; Gustafsson, F.; Damm, P.; Ersbøll, A.S. Subsequent reproductive outcome among women with peripartum cardiomyopathy: A nationwide study. BJOG 2018, 125, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, U.; Akgöz, H.; Aksoy, Ş.; Can Gürkan, Ö.; Osken, A.; Unal Dayi, S.; Oz, D.; Haci, R. Value of the neutrophil-to-lymphocyte ratio in predicting left ventricular recovery in patients with peripartum cardiomyopathy. Wien. Klin. Wochenschr. 2017, 129, 893–899. [Google Scholar] [CrossRef]
- Haghikia, A.; Podewski, E.; Libhaber, E.; Labidi, S.; Fischer, D.; Roentgen, P.; Tsikas, D.; Jordan, J.; Lichtinghagen, R.; von Kaisenberg, C.S.; et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic. Res. Cardiol. 2013, 108, 366. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Röntgen, P.; Vogel-Claussen, J.; Schwab, J.; Westenfeld, R.; Ehlermann, P.; Berliner, D.; Podewski, E.; Hilfiker-Kleiner, D.; Bauersachs, J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: A cardiovascular magnetic resonance study. ESC Heart Fail. 2015, 2, 139–149. [Google Scholar] [CrossRef]
- Harper, M.A.; Meyer, R.E.; Berg, C.J. Peripartum cardiomyopathy: Population-based birth prevalence and 7-year mortality. Obstet. Gynecol. 2012, 120, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.A.; Qureshi, A.; Ramejo, B.B.; Kamran, A. Peripartum cardiomyopathy characteristics and outcome in a tertiary care hospital. J. Pak. Med. Assoc. 2010, 60, 377–380. [Google Scholar]
- Hassan, M.U.; Shah, B.; Rauf, B.; Hussain, C. Peripartum cardiomyopathy-an inhospital study. Pak. Heart J. 2019, 52, 219–223. [Google Scholar]
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart J. 2017, 38, 2671–2679. [Google Scholar] [CrossRef]
- Horgan, S.J.; Margey, R.; Brennan, D.J.; O’Herlihy, C.; Mahon, N.G. Natural history, management, and outcomes of peripartum cardiomyopathy: An Irish single-center cohort study. J. Matern. Fetal Neonatal Med. 2013, 26, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Rasmusson, K.D.; Alharethi, R.; Budge, D.; Brunisholz, K.D.; Metz, T.; Carlquist, J.F.; Connolly, J.J.; Porter, T.F.; Lappé, D.L.; et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ. Cardiovasc. Genet. 2011, 4, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Zhang, L.Y.; Bai, T.F.; Wang, R.K.; Zhang, X.S. Effect of inflammation and autoimmunity in peripartum cardiomyo-pathy. J. Geriatr. Cardiol. 2010, 7, 106. [Google Scholar]
- Huisman, C.M.; Zwart, J.J.; Roos-Hesselink, J.W.; Duvekot, J.J.; van Roosmalen, J. Incidence and predictors of maternal cardiovas-cular mortality and severe morbidity in The Netherlands: A prospective cohort study. PLoS ONE 2013, 8, e56494. [Google Scholar] [CrossRef]
- Irizarry, O.C.; Levine, L.D.; Lewey, J.; Boyer, T.; Riis, V.; Elovitz, M.A.; Arany, Z. Comparison of Clinical Characteristics and Out-comes of Peripartum Cardiomyopathy Between African American and Non-African American Women. JAMA Cardiol. 2017, 2, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Isogai, T.; Matsui, H.; Tanaka, H.; Fushimi, K.; Yasunaga, H. In-hospital management and outcomes in patients with peripartum cardiomyopathy: A descriptive study using a national inpatient database in Japan. Heart Vessel. 2017, 32, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.C.; Barasa, A.; Basic, C.; Nyberg, G.; Schaufelberger, M. Increased arterial stiffness and reduced left ventricular long-axis function in patients recovered from peripartum cardiomyopathy. Clin. Physiol. Funct. Imaging 2021, 41, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, C.A.; Kitakaze, M.; Ishibashi-Ueda, H.; Nakatani, S.; Murohara, T.; Tomoike, H.; Ikeda, T. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ. J. 2011, 75, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.P.; Hsich, E.; Lindenfeld, J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013, 1, 409–416. [Google Scholar] [CrossRef]
- Karaye, K.M.; Lindmark, K.; Henein, M.Y. One Year Survival in Nigerians with Peripartum Cardiomyopathy. Heart Views 2016, 17, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, S.R.; Park, S.J.; Seo, J.H.; Kim, E.K.; Yang, J.H.; Chang, S.A.; Choi, J.O.; Lee, S.C.; Park, S.W. Clinical characteristics and long-term outcomes of peripartum takotsubo cardiomyopathy and peripartum cardiomyopathy. ESC Heart Fail. 2020, 7, 3644–3652. [Google Scholar] [CrossRef]
- Laghari, A.H.; Khan, A.H.; Kazmi, K.A. Peripartum cardiomyopathy: Ten year experience at a tertiary care hospital in Pakistan. BMC Res. Notes 2013, 6, 495. [Google Scholar] [CrossRef]
- Lee, S.; Cho, G.J.; Park, G.U.; Kim, L.Y.; Lee, T.S.; Kim, D.Y.; Choi, S.W.; Youn, J.C.; Han, S.W.; Ryu, K.H.; et al. Incidence, Risk Factors, and Clinical Characteristics of Peripartum Cardiomyopathy in South Korea. Circ. Heart Fail. 2018, 11, e004134. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Long, Y. Clinical Characteristics and Long-term Predictors of Persistent Left Ventricular Systolic Dysfunction in Peripartum Cardiomyopathy. Can. J. Cardiol. 2016, 32, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.P.; Sim, D.K. Peripartum cardiomyopathy: Experience in an Asian tertiary centre. Singapore Med. J. 2013, 54, 24–27. [Google Scholar] [PubMed]
- Lindley, K.J.; Conner, S.N.; Cahill, A.G.; Novak, E.; Mann, D.L. Impact of Preeclampsia on Clinical and Functional Outcomes in Women With Peripartum Cardiomyopathy. Circ. Heart Fail. 2017, 10, e003797. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Hou, D.; Liu, J.; Zhang, J.; Xu, L.; Wang, H.; Zhao, W.; Zhang, Y.; Zhang, L. Association of autoantibodies against the M2-muscarinic receptor with long-term outcomes in peripartum cardiomyopathy patients: A 5-year prospective study. J. Cardiol. 2019, 74, 251–257. [Google Scholar] [CrossRef]
- Mahowald, M.K.; Basu, N.; Subramaniam, L.; Scott, R.; Davis, M.B. Long-term outcomes in peripartum cardiomyopathy. Open Cardiovasc. Med. J. 2019, 13, 13–23. [Google Scholar] [CrossRef]
- Malhamé, I.; Dayan, N.; Moura, C.S.; Samuel, M.; Vinet, E.; Pilote, L. Peripartum cardiomyopathy with co-incident preeclampsia: A cohort study of clinical risk factors and outcomes among commercially insured women. Pregnancy Hypertens. 2019, 17, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, R.; Shah, Z.; Arany, Z.; Gupta, K. Peripartum cardiomyopathy: An epidemiologic study of early and late presentations. Pregnancy Hypertens. 2018, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.M.; Elkayam, U.; Alharethi, R.; Damp, J.; Hsich, E.; Ewald, G.; Modi, K.; Alexis, J.D.; Ramani, G.V.; Semigran, M.J.; et al. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J. Am. Coll. Cardiol. 2015, 66, 905–914. [Google Scholar] [CrossRef]
- Midei, M.G.; DeMent, S.H.; Feldman, A.M.; Hutchins, G.M.; Baughman, K.L. Peripartum myocarditis and cardiomyopathy. Circulation 1990, 81, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Moulig, V.; Pfeffer, T.J.; Ricke-Hoch, M.; Schlothauer, S.; Koenig, T.; Schwab, J.; Berliner, D.; Pfister, R.; Michels, G.; Haghikia, A.; et al. Long-term follow-up in peripartum cardiomyopathy patients with contemporary treatment: Low mortality, high cardiac recovery, but significant cardiovascular co-morbidities. Eur. J. Heart Fail. 2019, 21, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.B.; Costanzo-Nordin, M.R.; Subramanian, R.; Robinson, J.A.; Wallis, D.E.; Scanlon, P.J.; Gunnar, R.M. Peripartum cardiomyopathy: Clinical, hemodynamic, histologic and prognostic characteristics. J. Am. Coll. Cardiol. 1986, 8, 52–56. [Google Scholar] [CrossRef]
- Osterman-Pla, A.D.; López-Cepero, R.; Jiménez, L.; Romaguera, J.; Aranda, J. Peripartum Cardiomyopathy: Experience at a Tertiary Care Center in Puerto Rico. Puerto Rico Health Sci. J. 2016, 35, 224–227. [Google Scholar]
- Pandit, V.; Shetty, S.; Kumar, A.; Sagir, A. Incidence and outcome of peripartum cardiomyopathy from a tertiary hospital in South India. Trop. Dr. 2009, 39, 168–169. [Google Scholar] [CrossRef]
- Patel, H.; Berg, M.; Barasa, A.; Begley, C.; Schaufelberger, M. Symptoms in women with Peripartum Cardiomyopathy: A mixed method study. Midwifery 2016, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Ainuddin, J.; Jabbar, S.; Soomro, K.; Ali, A. Peripartum cardiomyopathy: Frequency and predictors and indicators of clinical outcome. J. Pak. Med. Assoc. 2016, 66, 1517–1521. [Google Scholar] [PubMed]
- Phan, D.; Duan, L.; Ng, A.; Shen, A.Y.; Lee, M.S. Characteristics and outcomes of pregnant women with cardiomyopathy stratified by etiologies: A population-based study. Int. J. Cardiol. 2020, 305, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Prameswari, H.P.; Dewi, T.I.; Hasan, M.; Martanto, E.; Aprami, T.M. Hypertension in pregnancy as the most influential risk factor for PPCM. Br. J. Cardiol. 2018, 25, 111–114. [Google Scholar]
- Prasad, G.S.; Bhupali, A.; Prasad, S.; Patil, A.N.; Deka, Y. Peripartum cardiomyopathy–case series. Indian Heart J. 2014, 66, 223–226. [Google Scholar] [CrossRef]
- Ravi Kiran, G.; RajKumar, C.; Chandrasekhar, P. Clinical and echocardiographic predictors of outcomes in patients with peripartum cardiomyopathy: A single centre, six month follow-up study. Indian Heart J. 2021, 73, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Rosman, L.; Salmoirago-Blotcher, E.; Cahill, J.; Wuensch, K.L.; Sears, S.F. Depression and health behaviors in women with Peri-partum Cardiomyopathy. Heart Lung 2017, 46, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Safirstein, J.G.; Ro, A.S.; Grandhi, S.; Wang, L.; Fett, J.D.; Staniloae, C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int. J. Cardiol. 2012, 154, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sagy, I.; Salman, A.A.; Kezerle, L.; Erez, O.; Yoel, I.; Barski, L. Peripartum cardiomyopathy is associated with increased uric acid concentrations: A population based study. Heart Lung 2017, 46, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Saltzberg, M.T.; Szymkiewicz, S.; Bianco, N.R. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J. Card. Fail. 2012, 18, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Samonte, V.I.; Ngalob, Q.G.; Mata, G.D.; Aherrera, J.A.; Reyes, E.; Punzalan, F.E. Clinical and echocardiographic profile and out-comes of peripartum cardiomyopathy: The Philippine General Hospital experience. Heart Asia 2013, 5, 245–249. [Google Scholar] [CrossRef]
- Shah, M.; Ram, P.; Lo, K.B.; Patnaik, S.; Patel, B.; Tripathi, B.; Patil, S.; Lu, M.; Jorde, U.P.; Figueredo, V.M. Etiologies, Predictors, and Economic Impact of 30-Day Readmissions Among Patients with Peripartum Cardiomyopathy. Am. J. Cardiol. 2018, 122, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Shani, H.; Kuperstein, R.; Berlin, A.; Arad, M.; Goldenberg, I.; Simchen, M.J. Peripartum cardiomyopathy—Risk factors, character-istics and long-term follow-up. J. Perinat. Med. 2015, 43, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Azibani, F.; Baard, J.; Osman, A.; Zühlke, L.; Lachmann, A.; Libhaber, E.; Chin, A.; Ntsekhe, M.; Soma-Pillay, P.; et al. Reducing late maternal death due to cardiovascular disease—A pragmatic pilot study. Int. J. Cardiol. 2018, 272, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Förster, O.; Libhaber, E.; Fett, J.D.; Sundstrom, J.B.; Hilfiker-Kleiner, D.; Ansari, A.A. Peripartum cardiomyopathy: In-flammatory markers as predictors of outcome in 100 prospectively studied patients. Eur. Heart J. 2006, 27, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Kagiyama, N.; Hasselberg, N.E.; Blauwet, L.A.; Briller, J.; Cooper, L.; Fett, J.D.; Hsich, E.; Wells, G.; McNamara, D.; et al. Global Left Ventricular Strain at Presentation Is Associated with Subsequent Recovery in Patients with Peripartum Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.A.; Shadmani, A.K. Clinical Spectrum and Outcome of Patients with Peri-Partum Cardiomyo-Pathy. Pak. Heart J. 2019, 52, 302–306. [Google Scholar]
- Tremblay-Gravel, M.; Marquis-Gravel, G.; Avram, R.; Desplantie, O.; Ducharme, A.; Bibas, L.; Pacheco, C.; Couture, E.; Simard, F.; Poulin, A.; et al. The effect of bromocriptine on left ventricular functional recovery in peripartum cardiomyopathy: Insights from the BRO-HF retrospective cohort study. ESC Heart Fail. 2019, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vettori, D.V.; Rohde, L.E.; Clausell, N. Asymptomatic left ventricular dysfunction in puerperal women: An echocardiographic-based study. Int. J. Cardiol. 2011, 149, 353–357. [Google Scholar] [CrossRef]
- Whitehead, S.J.; Berg, C.J.; Chang, J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991–1997. Obstet. Gynecol. 2003, 102, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Witlin, A.G.; Mabie, W.C.; Sibai, B.M. Peripartum cardiomyopathy: Anonymous diagnosis. Am. J. Obstet. Gynecol. 1997, 176, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Chen, T.H.; Yeh, J.K.; Wu, M.; Lu, C.H.; Chen, S.W.; Wu, K.P.; Cheng, C.W.; Chang, C.H.; Hung, K.C.; et al. Clinical outcomes of peripartum cardiomyopathy: A 15-year nationwide population-based study in Asia. Medicine 2017, 96, e8374. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.I.; Moon, J.Y.; Shim, M.; Yang, P.S.; Kang, S.H.; Kim, S.H.; Kim, W.J.; Sung, J.H.; Kim, I.J.; Lim, S.W.; et al. Clinical features differentiating Takotsubo cardiomyopathy in the peripartum period from peripartum cardiomyopathy. Heart Vessel. 2020, 35, 665–671. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W. Acute kidney injury predicts poor left ventricular function for patients with peripartum cardiomyopathy. BMC Cardiovasc. Disord. 2021, 21, 205. [Google Scholar] [CrossRef]
- Sliwa, K.; Skudicky, D.; Bergemann, A.; Candy, G.; Puren, A.; Sareli, P. Peripartum cardiomyopathy: Analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J. Am. Coll. Cardiol. 2000, 35, 701–705. [Google Scholar] [CrossRef]
- Elkayam, U.; Akhter, M.W.; Singh, H.; Khan, S.; Bitar, F.; Hameed, A.; Shotan, A. Pregnancy- associated cardiomyopathy: Clinical char-acteristics and a comparison between early and late presentation. Circulation 2005, 111, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Modi, K.A.; Illum, S.; Jariatul, K.; Caldito, G.; Reddy, P.C. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am. J. Obstet. Gynecol. 2009, 201, 171.e1–171.e5. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, L.M.; Williams, K.; Davis, D.R.; Tang, A.S.; Lemery, R.; Green, M.S.; Gollob, M.H.; Haddad, H.; Birnie, D.H. Frequency of peripartum cardiomyopathy. Am. J. Cardiol. 2006, 97, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J. Am. Coll. Cardiol. 2011, 58, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Gentry, M.B.; Dias, J.K.; Luis, A.; Patel, R.; Thornton, J.; Reed, G.L. African American women have a higher risk for developing peripartum cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 654–659. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Garg, J.; Palaniswamy, C.; Pandey, A.; Ahmad, H.; Frishman, W.H.; Lanier, G. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: Findings from the Nationwide Inpatient Sample. J. Cardiovasc. Med. 2016, 17, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.S.; Khan, S.S.; Sandhu, G.K.; Jorgensen, M.B.; Parikh, N.; Hsu, J.-W.Y.; Shen, A.Y.-J. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am. J. Cardiol. 2007, 100, 302–304. [Google Scholar] [CrossRef]

| Study | Year | Country | No. of Patients | Age (yrs), Mean ± sd | PE (%) | HDP (%) | Twins or Multiples (%) | Multiparous, n (%) or Mean ± sd |
|---|---|---|---|---|---|---|---|---|
| Abbas et al. [26] | 2021 | Pakistan | 71 | 29.8 ± 6.8 | 44 (62) | NA | NA | 57 (80.3) |
| Afana et al. [27] | 2016 | USA NIS database | 1337 | 35+, n (%): 314 (23.5) | 366 (27.4) | 715 (53.5) | 96 (7.2) | |
| Akil et al. [28] | 2016 | Turkey | 58 | 31.5 ± 6.3 | 11 (21.6) | 14 (27.5) | 7 (13.7) | 38 (73.1) |
| Amos et al. [29] | 2006 | USA | 55 | 29 ± 6 | 25 (46) | 31 (56) | NA | NA |
| Arnaout et al. [30] | 2019 | USA | 558 | NA | 146 (26.1) | 198 (35.4) | NA | NA |
| Aroney et al. [31] | 1986 | Australia | 5 | 28.8 ± 5.5 | 1 (20) | NA | 2 (40) | 4 (80) |
| Arora et al. [32] | 2014 | USA | 10 | 28 ± 6 | 7 (70) | 8 (80) | 1 (10) | 8 (80) |
| Azibani et al. [33] | 2020 | Germany and S. Africa | 151 | 31 ± 6 | NA | 37 (26) | NA | NA |
| Barasa et al. [16] | 2018 | Sweden | 24 | 34.2 ± 5.0 | 14 (58.3) | NA | 5 (20.8) | 1.6 ± 0.7 |
| Barbosa et al. [34] | 2012 | Brazil | 9 | 29.7 ± 7.9 | 4 (44) | NA | NA | NA |
| Behrens et al. [35] | 2019 | Denmark | 126 | 35+, n (%): 37 (29.4) | 34 (27) | 39 (31.0) | 10 (7.9) | 69 (54.8) |
| Bernstein et al. [36] | 2001 | USA | 23 | 30.9 ± 4.6 | NA | 8 (35) | 4 (17) | 16 (70) |
| Binu et al. [37] | 2020 | India | 54 | 25.5 | NA | 27 (50) | 7 (13.0) | 29 (35.2) |
| Biteker et al. [38] | 2011 | Turkey | 24 | 26.6 ± 5.5 | NA | 4 (16.7) | 2 (8.3) | 1.7 ± 0.8 |
| Biteker et al. [39] | 2020 | Turkey | 52 | 28.0 ± 5.3 | NA | 8 (15.4) | 2 (3.8) | 2.6 ± 1.0 |
| Bortnick et al. [40] | 2021 | USA | 53 | 31 ± 7 | 10 (18.9) | 11 (20.8) | NA | 2 ± 1.3 |
| Briasoulis et al. [41] | 2016 | USA | 61 | 29 | NA | 20 (32.8) | NA | 45 (73.7) |
| Carvalho et al. [42] | 1989 | USA | 19 | 25.9 ± 7.2 | NA | 5 (26.3) | NA | 10 (52.6) |
| Chang et al. [43] | 2017 | Taiwan | 512 | 35+, n (%): 93 (18.16) | NA | 102 (19.92) | 38 (7.42) | NA |
| Chapa et al. [44] | 2005 | USA | 32 | 27 ± 6 | 5 (15.6) | NA | 4 (12.5) | Median (range): 2 (1–6) |
| Cho et al. [45] | 2021 | USA | 320 | 30.5 ± 7.0 | 95 (29.7) | 125 (39.1) | 34 (10.6) | 177 (55.3) |
| Codsi et al. [46] | 2018 | USA | 25 | Median (range): 26 (15–37) | 9 (36.0) | 15 (60) | 4 (16.0) | Median (range): 0 (0–3) |
| Cuenza et al. [47] | 2016 | Philippines | 39 | 28.4 ± 6.9 | NA | 13 (33) | 1 (0.25) | 16 (41) |
| Cunningham et al. [48] | 1986 | USA | 28 | 28.5 ± 6.5 | 22 (78.6) | NA | NA | 2.2 ± 2.3 |
| Davis et al. [49] | 2019 | USA | 100 | 30 ± 6 | NA | 45 (45) | 19 (19.0) | Median (range): 2 (1–6) (1–6) |
| Davis et al. [50] | 2021 | USA | 651 | 30+, n (%): 299 (45.9) | 167 (26) | NA | 70 (11) | NA |
| Dhesi et al. [51] | 2017 | Canada database | 194 | 30.4 ± 6.6 | NA | 58 (29.9) | 16 (8.2) | 76 (39.2) |
| Douglass et al. [52] | 2021 | USA | 48 | 28 ± 7 | NA | 27 (56.3) | 8 (17) | Median (range): 1 (0–2.5) |
| Duran et al. [53] | 2008 | Turkey | 33 | 32 ± 7 | NA | 4 (12.1) | 2 (6) | Median (range): 3 (1–7) |
| Ekizler et al. [54] | 2019 | Turkey | 64 | 29.2 ± 6 | NA | 10 (15.6) | NA | NA |
| Elkayam et al. [55] | 2019 | USA | 15 | 30 ± 7.3 | 1/6 (16.7) | NA | NA | 6/10 (60) |
| Ersboll et al. [56] | 2017 | Denmark | 61 | 31.7 ± 6.3 | NA | 33 (54) | 5 (8.2) | 29 (47.5) |
| Farhan et al. [57] | 2021 | Iraq | 64 | 32.1 ± 6.8 | NA | 30/58 (51.7) | NA | 49/61 (80.3) |
| Felker et al. [58] | 2000 | USA | 42 | 29 ± 6 | NA | 11 (26) | 5 (12) | 22 (53) |
| Fett et al. [6] | 2005 | Haiti | 98 | Mean (range): 32.2 (16–50) | 4 (4) | 15 (15) | NA | 74 (75.5) |
| Ford et al. [59] | 2000 | USA | 11 | 28 ± 5 | NA | 3 (27.3) | 0 (0) | NA |
| Gambahaya et al. [60] | 2017 | Zimbabwe | 43 | 27.9 ± 6.0 | NA | 15 (34.9) | 3 (7) | 28 (65.1) |
| Goland et al. [61] | 2011 | USA | 187 | 30 ± 6 | NA | 75/184 (41) | 34/184 (18) | 2.7 ± 2 |
| Goland et al. [62] | 2016 | Israel | 41 | 35 ± 6 | 5 (17) | 10 (34) | 7 (24) | 2.3 ± 1.2 |
| Goli et al. [63] | 2021 | Multinational | 469 | NA | NA | 36.5% | NA | NA |
| Guldbrandt Hauge et al. [64] | 2018 | Denmark | 61 | NA | NA | 33 (54.1) | NA | 29 (47.5) |
| Gunderson et al. [9] | 2011 | USA | 110 | 35+, n (%): 38 (34.5) | 13 (11.8) | 46 (41.8) | 9 (8.2) | 67 (60.9) |
| Gürkan et al. [65] | 2017 | Turkey | 40 | 30 ± 5.9 | NA | 10 (25) | 1 (2.5) | 26 (65) |
| Haghikia et al. [66] | 2013 | Germany | 115 | 34 ± 6 | NA | 50/112 (45) | 17 (15) | 2 (0–9) |
| Haghikia et al. [67] | 2015 | Germany | 34 | 34 ± 5 | 4 (12) | NA | NA | Median (range): 1 (1–4) |
| Harper et al. [68] | 2012 | USA | 85 | 35+, n (%): 27 (31.8%) | 21/79 (26.6) | 41/79 (52) | 7/79 (8.9) | NA |
| Hasan et al. [69] | 2010 | Pakistan | 32 | 32 ± 3 | 6(18.75) | NA | NA | 23 (71.8) |
| Hassan et al. [70] | 2019 | Pakistan | 60 | 30.1 ± 5.4 | 6 (10) | NA | 15 (25) | 86.7 |
| Hilfiker-Kleiner et al. [71] | 2017 | Germany | 63 | NA | 13 (20.6) | NA | NA | NA |
| Horgan et al. [72] | 2013 | Republic of Ireland | 12 | Mean (range): 34.7 (28–41) | 9 (75) | NA | 2 (17) | 9 (75) |
| Horne et al. * [73] | 2011 | USA | 71 | GWA study: 29.8 ± 5.8 Replication study: 30.7 ± 6.6 | 18 (25.3) | NA | NA | NA |
| Huang et al. [74] | 2010 | China | 82 | 29.5 ± 6.4 | NA | 11 (13.4) | NA | 28 (34.1) |
| Huisman et al. [75] | 2013 | Netherlands | 17 | NA | NA | 10 (58.8) | NA | NA |
| Irizarry et al. * [76] | 2017 | USA UPHS database | 220 | 29.5 ± 6.6 | NA | 80/180 (44.4) | 28 (13) | 109 (49.5) |
| Isogai et al. [77] | 2017 | Japan | 283 | 32.7 ± 7.5 | 48 (17) | 53 (18.7) | NA | NA |
| Jackson et al. * [17] | 2021 | European PPCM Registry | 735 | no HDP: 30.3 ± 6.4 HDP: 31.7 ± 5.7 PE: 30.7 ± 6.6 | 184 (25) | 283 (38.5) | 33 (4.5) | 362 (48.1) |
| Johansson et al. [78] | 2021 | Sweden | 22 | NA | 14 (63.6) | NA | NA | NA |
| Kamiya et al. [79] | 2011 | Japan | 102 | HDP: 33.8 ± 4.2 no HDP: 31.9 ± 4.1 | 29(28.4) | 42 (41.2) | 15 (14.7) | 1.62 ± 1.17 I 1.67 ± 0.78 |
| Kao et al. [80] | 2013 | USA database | 535 | 30+, n (%): 276 (51.6) | 157 (29.3) | NA | 60 (11.2) | NA |
| Karaye et al. * [7] | 2020 | Nigeria Registry | 406 | 28.6 ± 7.2 | 64 (15.8) | NA | 59 (14.5) | 289 (71.2) |
| Karaye et al. [81] | 2016 | Nigeria | 54 | 26.6 ± 6.7 | NA | 25 (46.3) | NA | NA |
| Kim et al. [82] | 2020 | Korea | 21 | 32 ± 4.9 | 5 (23.8) | NA | 8 (38.1) | 7 (35) |
| Kolte et al. * [8] | 2014 | USA database | 34219 | 30.3 ± 7 | 3867 (11.3) | 5714 (16.7) | 701 (2) | 115 (0.3) |
| Laghari et al. [83] | 2013 | Pakistan | 45 | 27.4 ± 6.1 | 4 (8.8) | NA | 3 (6.6) | 20 (44.4) |
| Lee et al. [84] | 2018 | Korea | 795 | 32.1 ± 4.3 | 127 (16) | NA | 52 (6.5) | 356 (44.8) |
| Lewey et al. [20] | 2020 | USA | 220 | No HDP: 30.4 ± 5.8 HDP: 28 ± 7.2 | NA | 76/175 (43.4) | 22 (10) | NA |
| Li et al. [85] | 2016 | China | 71 | 28 ± 6 | 29 (40.1) | 38 (54) | NA | 14(19.7) |
| Lim et al. [86] | 2013 | China | 11 | 32.3 ± 5.7 | 1 (9) | 5 (45.5) | 1 (9) | NA |
| Lindley et al. [87] | 2017 | USA | 39 | No PE: 29.3 ± 5.9 PE: 27.4 ± 7.4 | 17 (43.6) | NA | NA | No PE: 3.1 ± 1.9 PE: 2.6 ± 2.2 |
| Ma et al. [88] | 2019 | China | 80 | 29.2 ± 4.3 | 16 (20) | 45 (56.2) | 24 (30) | 52 (65) |
| Mahowald et al. [89] | 2019 | USA | 59 | 29.5 ± 6.8 | 13 (22) | 21 (36) | NA | NA |
| Malhame et al. [90] | 2019 | Canada database | 949 | NA | 283 (32.4) | 358 (37.7) | NA | NA |
| Masoomi et al. [91] | 2018 | USA | 568 | Mean (95% CI): 30.0 (29.3– 30.6) | 165 (29) | 276 (48.6) | 38 (6.7) | NA |
| McNamara et al. [92] | 2015 | North America | 100 | 30 ± 6 | NA | 45 (45) | NA | 2.2 ± 1.3 |
| Midei et al. [93] | 1990 | USA | 18 | 28 ± 1 | NA | NA | NA | 11 (61.1) |
| Moulig et al. [94] | 2019 | Germany | 67 | 34 ± 5 | 9 (13.0) | 15 (22.0) | 13 (19.0) | 1.7 ± 1 |
| O’Connell et al. [95] | 1986 | USA | 14 | 28.7 ± 5.7 | NA | 4 (29) | 2 (14) | 3 (21) |
| Osterman-Pla et al. [96] | 2016 | Puerto Rico | 12 | 27 ± 8 | 6 (50.0) | NA | 2 (16.7) | 2.6 ± 1.6 |
| Pandit et al. [97] | 2009 | India | 9 | 28.5 ± 2.5 | NA | 1 (11.1) | NA | 6 (66.7) |
| Patel et al. [98] | 2016 | Sweden | 19 | 37 ± 5.8 | 13 (68) | 14 (74) | 4 (21) | NA |
| Perveen et al. [99] | 2016 | Pakistan | 22 | 30, n (%): 12 (54.5) | 2 (9.1) | 18 (81.8) | 2 (9.1) | 8 (36.4) |
| Phan et al. [100] | 2020 | USA | 333 | Median (IQR): 33.2 (29.4– 36.9) | 37 (11.1) | NA | NA | 259 (77.8) |
| Pillarisetti et al. [19] | 2014 | USA | 100 | 30 ± 6.5 | 36 (36) | 69 (69) | NA | 61 (61) |
| Prameswari et al. [101] | 2018 | Indonesia | 96 | Median (IQR): 30.5 (13) | NA | 55 (57.3) | 8 (8.3) | 60 (62.5) |
| Prasad et al. [102] | 2014 | India | 16 | 25.25 | 4 (25) | 10 (62.5) | NA | 7 (43.7) |
| Ravi Kiran et al. [103] | 2021 | India | 43 | 25.4 ± 2.9 | NA | 8 (18.6) | NA | 1.4 ± 0.8 |
| Rosman et al. [104] | 2017 | USA | 177 | 30.6 ± 5.5 | 56 (37.6) | 87 (49.2) | NA | 2.7 ± 1.7 |
| Safirstein et al. [105] | 2012 | USA | 55 | 31.7 ± 5.68 | NA | 23 (41.8) | 8 (14.5) | 28 (50.9) |
| Sagy et al. [106] | 2017 | Israel | 42 | 30.8 ± 7 | 14 (33.3) | 1 (2.4) | 4.5 (1–7) | |
| Saltzberg et al. [107] | 2012 | USA | 107 | 31.2 ± 6.3 | NA | 42 (39) | 15% | 66.2% |
| Samonte et al. [108] | 2013 | Philippines | 9 | 29.3 ± 8.7 | 7 (78) | NA | 1 (6.2) | 6 (66.6) |
| Shah et al. * [109] | 2018 | USA NRD database | 6880 | 31.0 ± 6.8 | 1947 (28.3) | 2243 (32.6) | 1.7% | 0.9% |
| Shani et al. [110] | 2015 | Israel | 36 | 33.5 ± 6 | NA | 13 (38.9) | 12 (33.3) | 13 (36.1) |
| Sliwa et al. [111] | 2018 | Africa | 269 | 28.6 ± 5.9 | 5 (1.8) | 18 (6.7) | 7 (3) | 224 (83.3) |
| Sliwa et al. [112] | 2006 | USA | 100 | 31.6 ± 6.6 | NA | 2(2) | NA | NA |
| Sugahara et al. [113] | 2019 | USA | 90 | Median (IQR): 31 (25–34) | NA | 39 (43) | NA | NA |
| Sultan et al. [114] | 2019 | Pakistan | 32 | 27.4 ± 5.8 | 1 (3.1) | 10 (31.1) | NA | 26 (81.2) |
| Tremblay-Gravel et al. [115] | 2019 | Canada | 76 | NA | 20 (26.3) | 31 (40.8) | 8 (10.5) | 40 (52.6) |
| Vettori et al. [116] | 2011 | Brazil | 6 | 26.5 ± 7.1 | 3 (50) | NA | 1 (16.7) | 1 (16.7) |
| Whitehead et al. [117] | 2003 | USA | 171 | NA | NA | 25 (15) | NA | NA |
| Witlin et al. [118] | 1997 | USA | 9 | 33 ± 6.9 | 2 (22.2) | NA | NA | NA |
| Wu et al. [119] | 2017 | Taiwan | 742 | 30.5 ± 5.7 | 146 (19.7) | NA | NA | 55 (7.4) |
| Yang et al. [120] | 2020 | Korea | 21 | 33 ± 5 | 6 (29) | 16 (77) | 5 (24) | 5 (24) |
| Zhu et al. [121] | 2021 | China | 60 | 30 ± 5 | 7 (11.7) | 12 (20) | NA | 36 (60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biljic-Erski, A.; Rajovic, N.; Pavlovic, V.; Bukumiric, Z.; Rakic, A.; Rovcanin, M.; Stulic, J.; Anicic, R.; Kocic, J.; Cumic, J.; et al. Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy: A Meta-Analysis of Prevalence and Impact on Left Ventricular Function and Mortality. J. Clin. Med. 2025, 14, 1721. https://doi.org/10.3390/jcm14051721
Biljic-Erski A, Rajovic N, Pavlovic V, Bukumiric Z, Rakic A, Rovcanin M, Stulic J, Anicic R, Kocic J, Cumic J, et al. Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy: A Meta-Analysis of Prevalence and Impact on Left Ventricular Function and Mortality. Journal of Clinical Medicine. 2025; 14(5):1721. https://doi.org/10.3390/jcm14051721
Chicago/Turabian StyleBiljic-Erski, Aleksandar, Nina Rajovic, Vedrana Pavlovic, Zoran Bukumiric, Aleksandar Rakic, Marija Rovcanin, Jelena Stulic, Radomir Anicic, Jovana Kocic, Jelena Cumic, and et al. 2025. "Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy: A Meta-Analysis of Prevalence and Impact on Left Ventricular Function and Mortality" Journal of Clinical Medicine 14, no. 5: 1721. https://doi.org/10.3390/jcm14051721
APA StyleBiljic-Erski, A., Rajovic, N., Pavlovic, V., Bukumiric, Z., Rakic, A., Rovcanin, M., Stulic, J., Anicic, R., Kocic, J., Cumic, J., Markovic, K., Zdravkovic, D., Stanisavljevic, D., Masic, S., Milic, N., & Dimitrijevic, D. (2025). Hypertensive Disorders of Pregnancy and Peripartum Cardiomyopathy: A Meta-Analysis of Prevalence and Impact on Left Ventricular Function and Mortality. Journal of Clinical Medicine, 14(5), 1721. https://doi.org/10.3390/jcm14051721








