The Predictive Role of C-Reactive Protein, Leukocyte Cell Count, and Soluble Urokinase Plasminogen Activator Receptor for Pulmonary Sequelae in Hospitalized COVID-19 Survivors: A Prospective Single-Center Cohort Study
Abstract
Highlights
- What is already known on this topic?
- There is an urgent clinical need for biomarkers to predict pulmonary deterioration among acutely admitted patients with COVID-19. Patients with COVID-19 present with a wide range of clinical outcomes, from mild illness to respiratory failure. Identifying patients who are at risk of deterioration early on can guide clinical management, resource allocation, and targeted interventions.
- What does this study add?
- This study shows that the most commonly affected pulmonary function parameter during follow-up was DLCO impairment. Among the biomarkers studied, soluble urokinase Plasminogen Activator Receptor (suPAR) at admittance demonstrated the strongest correlation with DLCO impairment, and a low suPAR cut-off value showed the highest negative predictive value (NPV) for DLCO impairment.
- How might this study affect research, practice, or policy?
- This study could assist physicians in reducing the number of patients requiring follow-up at pulmonary outpatient clinics, particularly due to the high negative predictive value (NPV) of the biomarkers in forecasting DLCO impairment. This could potentially be of benefit for individual patients and, at the same time, alleviate the pressure on the healthcare system.
Abstract
1. Background
2. Methods
2.1. Study Design and Setting
2.2. Patients
2.3. Variables and Measurements
2.4. Patients and Public Involvement
2.5. Patient Consent for Publication
2.6. Statistics
3. Results
Biomarker Correlation with DLCO
4. Discussion
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Oancea, C.; Pescariu, S.A.; Pop, G.N. Factors Influencing the Evolution of Pulmonary Hypertension in Previously Healthy Subjects Recovering from a SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 5272. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Vejen, M.; Hansen, E.F.; Al-Jarah, B.N.I.; Jensen, C.; Thaning, P.; Jeschke, K.N.; Ulrik, C.S. Hospital admission for COVID-19 pneumonitis—Long-term impairment in quality of life and lung function. Eur. Clin. Respir. J. 2022, 9, 2024735. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, I.G.; da Silva, R.M.C.; Hetzel, G.M.; da Silva Viana, G.; Guimarães, A.R.; Folador, L.; Brentano, V.B.; Garcia, T.S.; Ribeiro, S.P.; de Tarso Roth Dalcin, P.; et al. Impact of impaired pulmonary function on clinical outcomes in survivors of severe COVID-19 without pre-existing respiratory disease. J. Bras. Pneumol. 2023, e20220452. [Google Scholar] [CrossRef]
- Blanco, J.-R.; Cobos-Ceballos, M.-J.; Navarro, F.; Sanjoaquin, I.; de las Revillas, F.A.; Bernal, E.; Buzon-Martin, L.; Viribay, M.; Romero, L.; Espejo-Perez, S.; et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin. Microbiol. Infect. 2021, 27, 892–896. [Google Scholar] [CrossRef]
- D’agnillo, F.; Walters, K.-A.; Xiao, Y.; Sheng, Z.-M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef]
- Pellicori, P.; Doolub, G.; Wong, C.M.; Lee, K.S.; Mangion, K.; Ahmad, M.; Berry, C.; Squire, I.; Lambiase, P.D.; Lyon, A.; et al. COVID-19 and its cardiovascular effects: A systematic review of prevalence studies. Cochrane Database Syst. Rev. [Internet] 2021, 2022, CD013879. [Google Scholar]
- Camporota, L.; Cronin, J.N.; Busana, M.; Gattinoni, L.; Formenti, F. Pathophysiology of coronavirus-19 disease acute lung injury. Curr. Opin. Crit. Care 2022, 28, 9–16. [Google Scholar] [CrossRef]
- The Lille COVID-19 ICU and Anatomopathology Group; Copin, M.C.; Parmentier, E.; Duburcq, T.; Poissy, J.; Mathieu, D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020, 46, 1124–1126. [Google Scholar]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in Patients with COVID-19 at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, G.T.; Halldorsson, A.B.; Jonsson, H.M.; Eythorsson, E.; Sigurdardottir, S.E.; Hardardottir, H.; Gudmundsson, G.; Hansdottir, S. Respiratory function and CT abnormalities among survivors of COVID-19 pneumonia: A nationwide follow-up study. BMJ Open Respir. Res. 2022, 9, e001347. [Google Scholar] [CrossRef]
- Balbi, M.; Conti, C.; Imeri, G.; Caroli, A.; Surace, A.; Corsi, A.; Mercanzin, E.; Arrigoni, A.; Villa, G.; Di Marco, F.; et al. Post-discharge chest CT findings and pulmonary function tests in severe Patients with COVID-19. Eur. J. Radiol. 2021, 138, 109676. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A.M.; McIntosh, M.J.; Kooner, H.K.; Abdelrazek, M.; Albert, M.S.; Dhaliwal, I.; Nicholson, J.M.; Ouriadov, A.; Svenningsen, S.; Parraga, G. Longitudinal follow-up of postacute COVID-19 syndrome: DLCO, quality-of-life and MRI pulmonary gas-exchange abnormalities. Thorax 2023, 78, 418–421. [Google Scholar] [CrossRef]
- Altintas, I.; Eugen-Olsen, J.; Seppälä, S.; Tingleff, J.; Stauning, M.A.; El Caidi, N.O.; Elmajdoubi, S.; Gamst-Jensen, H.; Lindstrøm, M.B.; Rasmussen, L.J.H.; et al. suPAR Cut-Offs for Risk Stratification in Patients With Symptoms of COVID-19. Biomark. Insights 2021, 16, 117727192110346. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, K.M.; Watson, R.O.; Schneider, J.M.; Neff, C.P.; Yamada, E.; Zhang, M.; Campbell, T.B.; Falta, M.T.; Jolley, S.E.; Fontenot, A.P.; et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLOS Pathog. 2022, 18, e1010359. [Google Scholar] [CrossRef]
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.; Idzikowski, E.; et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 2021, 54, 797–814.e6. [Google Scholar] [CrossRef]
- Mancilla-Ceballos, R.; Milne, K.M.; Guenette, J.A.; Cortes-Telles, A. Inflammation associated with lung function abnormalities in COVID-19 survivors. BMC Pulm. Med. 2023, 23, 235. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Cotes, J.E.; Chinn, D.J.; Quanjer, P.H.; Roca, J.; Yernault, J.C. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 41–52. [Google Scholar] [CrossRef]
- Stauning, M.A.; Altintas, I.; Kallemose, T.; Eugen-Olsen, J.; Lindstrøm, M.B.; Rasmussen, L.J.H.; Gamst-Jensen, H.; Nehlin, J.O.; Andersen, O.; Tingleff, J. Soluble Urokinase Plasminogen Activator Receptor as a Decision Marker for Early Discharge of Patients with COVID-19 Symptoms in the Emergency Department. J. Emerg. Med. 2021, 61, 298–313. [Google Scholar] [CrossRef]
- Fortini, A.; Torrigiani, A.; Sbaragli, S.; Forte, A.L.; Crociani, A.; Cecchini, P.; Bruni, G.I.; Faraone, A. COVID-19: Persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection 2021, 49, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.Y. Respiratory Structure and Function. In Goldman’s Cecil Medicine [Internet]; Elsevier: Amsterdam, The Netherlands, 2012; pp. 523–527. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9781437716047000853 (accessed on 24 June 2024).
- Bernstein, R.J.; Ford, R.L.; Clausen, J.L.; Moser, K.M. Membrane Diffusion and Capillary Blood Volume in Chronic Thromboembolic Pulmonary Hypertension. Chest 1996, 110, 1430–1436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maiese, A.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Autopsy findings in COVID-19-related deaths: A literature review. Forensic Sci. Med. Pathol. 2021, 17, 279–296. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.-M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Hakansson, K.E.J.; Rasmussen, L.J.H.; Godtfredsen, N.S.; Tupper, O.D.; Eugen-Olsen, J.; Kallemose, T.; Andersen, O.; Ulrik, C.S. The biomarkers suPAR and blood eosinophils are associated with hospital readmissions and mortality in asthma—A retrospective cohort study. Respir. Res. 2019, 20, 258. [Google Scholar] [CrossRef]
- Håkansson, K.E.J.; Ulrik, C.S.; Godtfredsen, N.S.; Kallemose, T.; Andersen, O.; Eugen-Olsen, J.; Marsaa, K.; Rasmussen, L.J.H. High suPAR and Low Blood Eosinophil Count are Risk Factors for Hospital Readmission and Mortality in Patients with COPD. Int. J. Chron. Obstruct. Pulmón. Dis. 2020, 15, 733–743. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Ekbom, E.; Frithiof, R.; Emilsson Öi Larson Im Lipcsey, M.; Rubertsson, S.; Wallin, E.; Janson, C.; Hultström, M.; Malinovschi, A. Impaired diffusing capacity for carbon monoxide is common in critically ill Patients with COVID-19 at four months post-discharge. Respir. Med. 2021, 182, 106394. [Google Scholar]
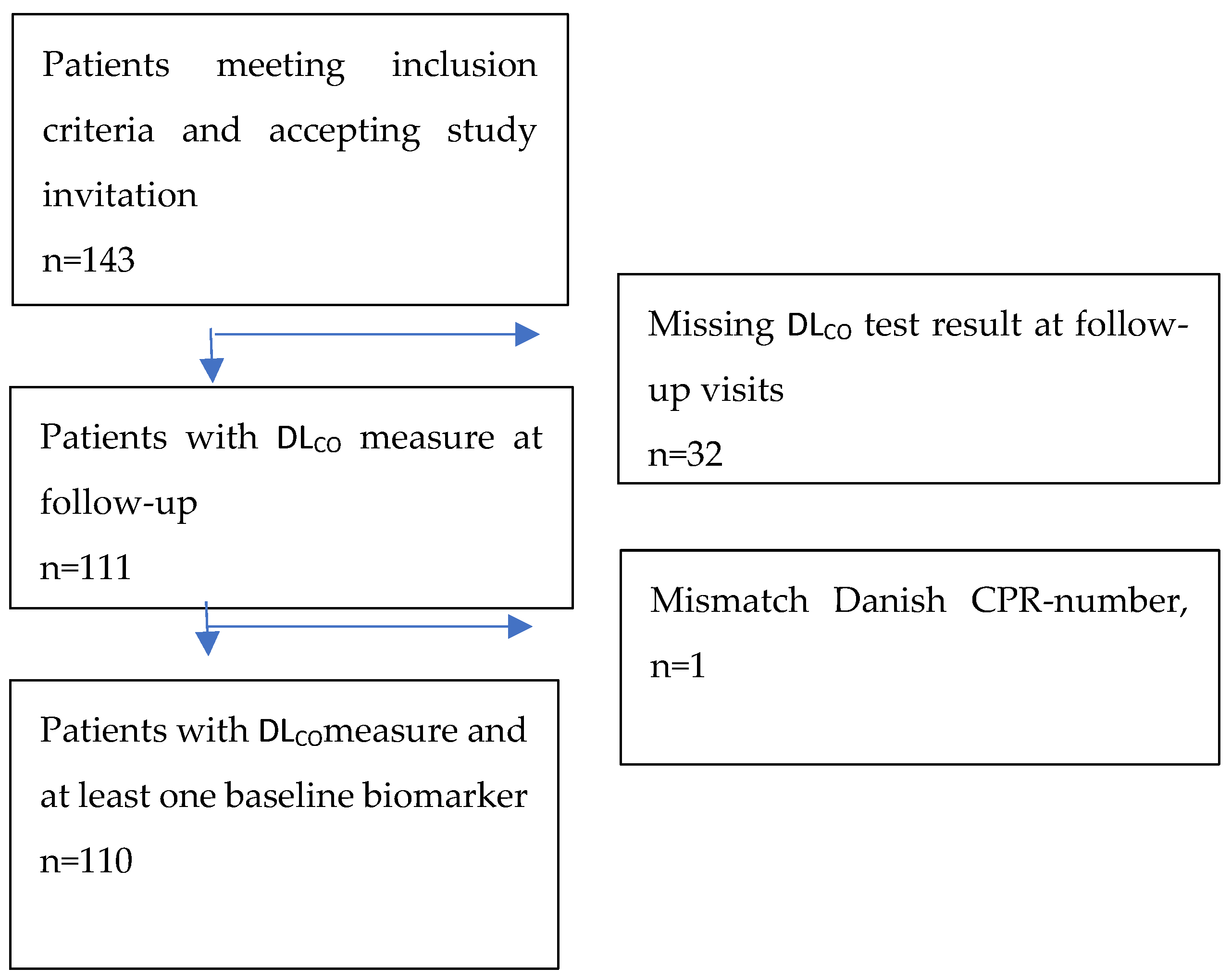
| Emergency Department: Variable (Median, IQR) | Level | Total (n = 110) | DLCO < 80% (n = 50) | DLCO > 80% (n = 60) | Age < 60 Years (n = 51) | Age > 60 Years (n = 59) |
|---|---|---|---|---|---|---|
| Age (years) | 61.5 (50.0:73.0) | 65.0 (54.2:75.0) | 56.5 (48.8:68.2) | 49.0 (43.5:54.0) | 72.0 (65.0:79.0) | |
| BMI | 27.2 (23.7:30.5) | 25.0 (23.0:29.6) | 27.8 (24.9:31.0) | 27.7 (24.8:30.8) | 26.2 (23.3:29.6) | |
| Sex | Female | 64 (58.2%) | 24 (48%) | 40 (66.7%) | 32 (62.7%) | 32 (54.2%) |
| CRP (mg/mL) (n = 72) | 55.0 (28.2:100.0) | 55.0 (27.0:100.0) | 55.0 (33.5:97.0) | 55.0 (27.5:105.0) | 55.0 (30.5:93.5) | |
| Leukocyte Cell Count (n = 72) | 6.4 (4.9:8.4) | 6.4 (5.2:8.4) | 6.3 (4.9:8.1) | 6.4 (5.2:9.3) | 6.2 (4.4:7.8) | |
| suPAR (ng/mL) (n = 66) | 5.1 (3.7:6.1) | 5.4 (4.4:6.7) | 4.6 (3.5:6.0) | 5.0 (3.6:6.0) | 5.1 (4.2:6.4) | |
| Hypertension (n) | 48 (43.6%) | 21 (42%) | 27 (45%) | 15 (29.4%) | 33 (55.9%) | |
| Diabetes (n) | 26 (23.6%) | 12 (24%) | 14 (23.3%) | 13 (25.5%) | 13 (22%) | |
| Congestive Heart Failure (n) | 12 (10.9%) | 6 (12%) | 6 (10%) | 1 (2%) | 11 (18.6%) | |
| Atrial Fibrillation (n) | 7 (6.4%) | 4 (8%) | 3 (5%) | 0 (0%) | 7 (11.9%) | |
| Chronic Obstructive Pulmonary Disease (n) | 7 (6.4%) | 5 (10%) | 2 (3.3%) | 1 (2%) | 6 (10.2%) | |
| Asthma (n) | 10 (9.1%) | 5 (10%) | 5 (8.3%) | 3 (5.9%) | 7 (11.9%) | |
| Smoking (n) | Current smoker | 3 (2.8%) | 2 (4.1%) | 1 (1.7%) | 1 (2%) | 2 (3.4%) |
| Never smoker | 58 (53.7%) | 22 (44.9%) | 36 (61%) | 29 (59.2%) | 29 (49.2%) | |
| Former smoker | 47 (43.5%) | 25 (51%) | 22 (37.3%) | 19 (38.8%) | 28 (47.5%) | |
| Tobacco Use (packyears) | 18.0 (5.0:33.5) | 25.0 (15.0:43.8) | 10.0 (3.2:22.2) | 10.0 (3.2:15.0) | 25.0 (9.8:43.8) |
| Respiratory Outpatient Clinic | ||
|---|---|---|
| Variable | Mean (SD) | |
| FEV1 (%) | 90.4 (19.7) | |
| FVC (%) | 94.3 (17.6) | |
| DLCO (%) | 78.9 (20.5) | |
| TLC (%) | 87.6 (14.5) | |
| Number (% of total) | ||
| FEV1 n (%) (n = 109) | <80% predicted | 26 (23.9%) |
| >80% predicted | 83 (76.1%) | |
| FVC, n (%) (n = 109) | <80% predicted | 17 (15.6%) |
| >80% predicted | 92 (84.4%) | |
| FEV1/FVC, n (%) (n = 109) | <0.70 | 15 (13.8) |
| ≥0.70 | 94 (86.2) | |
| TLC, n (%) (n = 110) | <80% predicted | 31 (28.2%) |
| >80% predicted | 79 (71.8%) | |
| DLCO, n (%) (n = 110) | <80% predicted | 50 (45.5%) |
| >80% predicted | 60 (54.5%) |
| Variable (Units) | Cut-Off | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| CRP (mg/L), baseline | ≥50 (low) (50–100, >100) vs. (<50) | 0.52 (0.37:0.66) | 0.45 (0.32:0.58) | 0.44 (0.31:0.58) | 0.53 (0.38:0.67) |
| >100 (high) (>100) vs. (<50, 50–100) | 0.22 (0.12:0.36) | 0.80 (0.68:0.89) | 0.48 (0.27:0.69) | 0.55 (0.44:0.66) | |
| Leukocyte cell counts, baseline (×109/L) | ≥3.5 (low) | 0.88 (0.76:0.95) | 0.07 (0.02:0.16) | 0.44 (0.34: 0.54) | 0.40 (0.12:0.74) |
| >8.8 (high) | 0.18 (0.09:0.31) | 0.77 (0.64:0.87) | 0.39 (0.20: 0.61) | 0.53 (0.42:0.64) | |
| suPAR (ng/mL), baseline | ≥4 (low) | 0.84 (0.67:0.95) | 0.38 (0.22:0.56) | 0.56 (0.41:0.71) | 0.72 (0.47:0.90) |
| >6 (high) | 0.31 (0.16:0.50) | 0.76 (0.59:0.89) | 0.56 (0.31:0.78) | 0.54 (0.39:0.69) | |
| Combination (threshold probability) | 0.97 (0.84:1.00) | 0.41 (0.25:0.59) | 0.61 (0.46:0.74) | 0.93 (0.68:1.00) |
| Variable (Units) | Level | DLCO < 80% | DLCO ≥ 80% | Total (%) |
|---|---|---|---|---|
| CRP (mg/L) | <50 (low) | 24 (48) | 27 (45) | 51 (46.4) |
| 50–100 (middle) | 15 (30) | 21 (35) | 36 (32.7) | |
| >100 (high) | 11 (22) | 12 (20) | 23 (20.9) | |
| Leukocytes (×109/L) | <3.5 (low) | 6 (12) | 4 (6.7) | 10 (9.1) |
| 3.5–8.8 (middle) | 35 (70) | 42 (70) | 77 (70) | |
| >8.8 (high) | 9 (18) | 14 (23.3) | 23 (20.9) | |
| suPAR (ng/mL) | <4 (low) | 5 (15.6) | 13 (38.2) | 18 (27.3) |
| 4–6 (middle) | 17 (53.1) | 13 (38.2) | 30 (45.5) | |
| >6 (high) | 10 (31.2) | 8 (23.5) | 18 (27.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altintas, I.; Kallemose, T.; Lindstrøm, M.B.; Parvaiz, I.; Rokkedal, I.; Rasmussen, L.J.; Iversen, K.K.; Eugen-Olsen, J.; Iversen, K.K.; Hansen, E.F.; et al. The Predictive Role of C-Reactive Protein, Leukocyte Cell Count, and Soluble Urokinase Plasminogen Activator Receptor for Pulmonary Sequelae in Hospitalized COVID-19 Survivors: A Prospective Single-Center Cohort Study. J. Clin. Med. 2025, 14, 1717. https://doi.org/10.3390/jcm14051717
Altintas I, Kallemose T, Lindstrøm MB, Parvaiz I, Rokkedal I, Rasmussen LJ, Iversen KK, Eugen-Olsen J, Iversen KK, Hansen EF, et al. The Predictive Role of C-Reactive Protein, Leukocyte Cell Count, and Soluble Urokinase Plasminogen Activator Receptor for Pulmonary Sequelae in Hospitalized COVID-19 Survivors: A Prospective Single-Center Cohort Study. Journal of Clinical Medicine. 2025; 14(5):1717. https://doi.org/10.3390/jcm14051717
Chicago/Turabian StyleAltintas, Izzet, Thomas Kallemose, Mette Bendtz Lindstrøm, Imran Parvaiz, Iben Rokkedal, Lene Juel Rasmussen, Katrine Kjær Iversen, Jesper Eugen-Olsen, Kasper Karmark Iversen, Ejvind Frausing Hansen, and et al. 2025. "The Predictive Role of C-Reactive Protein, Leukocyte Cell Count, and Soluble Urokinase Plasminogen Activator Receptor for Pulmonary Sequelae in Hospitalized COVID-19 Survivors: A Prospective Single-Center Cohort Study" Journal of Clinical Medicine 14, no. 5: 1717. https://doi.org/10.3390/jcm14051717
APA StyleAltintas, I., Kallemose, T., Lindstrøm, M. B., Parvaiz, I., Rokkedal, I., Rasmussen, L. J., Iversen, K. K., Eugen-Olsen, J., Iversen, K. K., Hansen, E. F., Ulrik, C. S., Nehlin, J. O., & Andersen, O. (2025). The Predictive Role of C-Reactive Protein, Leukocyte Cell Count, and Soluble Urokinase Plasminogen Activator Receptor for Pulmonary Sequelae in Hospitalized COVID-19 Survivors: A Prospective Single-Center Cohort Study. Journal of Clinical Medicine, 14(5), 1717. https://doi.org/10.3390/jcm14051717








